Abstract
In this study, the effect of different whey protein concentrate coating formulations (with or without glycerol) on the storage of fillets over a period of 15 days at 4ºC was investigated. Fillets were conducted to microbiological, chemical, and sensory analyses. Difference in microbiological and chemical changes between samples was found to be significant (p < 0.05) during storage period. The Y and Z were preferred more by the panellists, while C samples received the lowest scores. According to the results of microbiological analyses, shelf life of fillets was estimated at 6, 9, 12, and 15 days for C, X, Y, and Z samples, respectively.
INTRODUCTION
Rainbow trout (Oncorhynchus mykiss) is a widespread cultured fish species all over the world[Citation1] and has a major economic importance in Turkey. Rainbow trout is one of the most widely farmed species with a total production in Turkey of 114.569 tons in 2012.[Citation2] Microorganisms can grow in the muscle tissue of aquatic products due to their high-nutrient content. So the conservation and handling of aquatic products are very important.[Citation3] Edible coatings are important for sensitive foods, such as seafood.[Citation4] It can be prepared from a wide variety of raw materials, including polysaccharides, proteins, and lipids.[Citation5] Coatings may be applied directly to the foodstuff,[Citation6,Citation7] or they may be made into films that are then used to coat the surface of the food.[Citation8] The action of these coatings as a barrier to the passage of oxygen and water, thereby slowing oxidation reactions and retaining moisture, is the main mechanism used by coatings to enhance quality and extend storage life.[Citation5,Citation9] A number of coating materials have been tested in an attempt to maintain quality and prolong shelf life of aquatic products.[Citation4,Citation6,Citation9–Citation16] In recent years, packaging research has focused more on biodegradable films, including films made from plant and animal edible protein sources, such as corn zein, wheat gluten, soy and peanut protein, cottonseed, albumin, gelatin, collagen, casein, and whey proteins.[Citation17,Citation18]
Whey proteins have exceptional nutritional value and functional properties.[Citation19–Citation21] Cheese whey, which is produced in large quantities as a by-product in the cheese making process, has excellent functional properties and is used to produce edible films and coatings. Utilization of whey excess in the form of whey protein concentrate (WPC) could effectively alleviate the whey disposal problem by the conversion of whey into value-added products, such as edible films and coatings.[Citation22] The use of edible films to release antimicrobial constituents in food packaging is a form of active packaging that could extend the shelf life of a product and provide microbial safety for consumers.[Citation23] It acts to reduce, inhibit, or retard the growth of pathogen microorganisms in packed foods and packaging materials.[Citation24] In order to control undesirable microorganisms on food surfaces, natural or synthetic antimicrobial agents can be incorporated into polymer coatings.[Citation25,Citation26]
To the best of the authors’ knowledge, there has been no study dealing with the effect of whey protein on rainbow trout. The aim of this work is to evaluate the effects of different WPC coating formulations on rainbow trout microbiological, chemical, and sensory parameters.
MATERIALS AND METHODS
Preparation of Fish Samples
Rainbow trout (O. mykiss; 250 ± 25 g) was obtained from the Ataturk University Agricultural College Fisheries Department’s rainbow trout breeding and research center. The fresh fish samples were carried to the laboratory and washed with tap water. A total of 48 fish samples were eviscerated, stored until rigor had resolved, and then filleted, yielding a total of 96 fillets. WPC was obtained from Armor Proteines (Saint-Brice en Coglès, France) while glycerol was purchased from Fisher Scientific Inc. (Fair Lawn, NJ). Following the storage process, fish samples were divided into four groups: control (C: without edible films), WPC (X: without any addition), WPC+glycerol (Y: 1:1), and WPC+glycerol (Z: 2:1).
Preparation of Whey Forming Solution
In the preparation of coating solution, a modified procedure of Rodriquez-Turienzo et al.[Citation14] was used where WPC (8% protein, w/w) was dispersed in deionized water (30 min) at room temperature (20°C). Then, glycerol was added to the solution. All solutions were mixed for 30 min and then heated in water bath (80°C for 30 min). In order to obtain an ideal coating and dipping process, each surface of the fish fillets was submerged into the solution for 1 min at ambient temperature between each submersion to dry each surface (15 s). The fish fillets were then packed in polyethylene bags (15 × 25 cm, composed of polyethylene/polyamide (PA/PE) at a thickness (3-seal bags GB 70) that allowed an O2 permeability of 40 cm3/(m2.day.atm) at 23°C, an N2 permeability of cm3/(m2.day.atm) at 23°C, CO2 permeability of 145 cm3/(m2.day.atm) at 23°C and a water vapor permeability of <3 g (m2.day.atm) at 23ºC). The trout samples were first analyzed at the end of the storage process and before coating. Other analyses were carried out at a 3-day intervals (that is, on days 0, 3, 6, 9, 12, and 15) and stored at 4°C by duplicating all the analyses.
Microbiological Analysis
Dried coating solution was removed from each fillet using sterile gloves and the sample groups were subjected to microbiological analysis. A sample (25 g) was taken from each trout fillet, transferred aseptically into a stomacher bag containing 225 mL of 0.1% peptone, water was added, and the mixture was homogenized for 60 s using a Stomacher (Lab Stomacher Blender 400-BA 7021 Sewardmedical, England) at room temperature. For microbial enumeration, 0.1 mL samples of serial dilutions (1:10, diluents, 0.1% peptone water) of fish homogenates were spread on petri dish of various agar materials. The numbers of total aerobic mesophilic bacteria (TAMB; Merck, Darmstadt, Germany; at 30 ± 1°C for 48 h), psychrotrophic bacteria (PCA; Merck, at 10ºC for 7 days), lactic acid bacteria (LAB in MRS; Merck, at 30°C for 48 h in anaerobic conditions), and Enterobacteriaceae (Violet Red Bile Agar, Oxoid) at 35 ± 2°C for 48 h were counted according to Gokalp et al.[Citation27]
Chemical Analysis
After the removal of coating materials, each of the samples was fully shredded. The moisture, protein, pH, lipid, and ash content were determined according to Gokalp et al.[Citation27] The moisture content of trout was determined by drying the fish meat in an oven at 105ºC. Crude protein content was calculated by converting the nitrogen content determined by Kjeldahl’s method. Lipid content was determined using the soxhelet system. Ash value was obtained by dry-ashing in a furnace at 525ºC for 18 h. The parameter of peroxide was measured conveniently using the method of Shantha and Decker.[Citation28] Total volatile basic nitrogen (TVB-N) was determined using the method described by Malle and Tao.[Citation29] Thiobarbituric acid reactive substan (TBARS) was determined according to the method described by Lemon[Citation30] and Kilic and Richards.[Citation31]
Sensory Evaluation
Eight panellists experienced in the sensorial evaluation of cooked rainbow trout assessed the samples on days 0, 3, 6, 9, 12, and 15 of storage considering the method described by Dikel[Citation32] and modifying the sensory criteria for the characteristics of trout. Samples were scored considering five sensorial features which are: appearance, taste, odor, firmness, and general acceptability as 1–3 (spoiled), 4–6 (good), and 7–9 (excellent).
Statistical Analysis
The experimental design consisted of completely randomized design in a factorial arrangement: three treatments of rainbow trout (C, X, Y, and Z), four storage time (0, 3, 6, 9, 12, and 15 days) and two replicates. All statistical calculations were performed using SAS Statistical Software (SPSS 17.0; Chicago, IL, USA).[Citation33] Duncan’s multiple range tests and variance analysis were used to evaluate the significance level (p < 0.05) for statistical differences.
RESULTS AND DISCUSSION
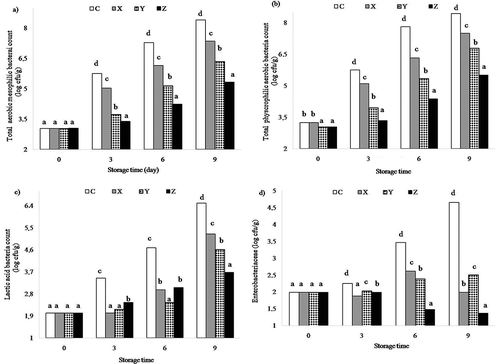
The results of the microbiological analysis carried out on trout samples are given in . Microbiological counts in fillets were found to be significant (p < 0.05) during storage time. The results revealed that the TAMB counts of C samples were higher than the others. As can be seen in , total number of mesophilic aerobic bacteria in samples was discovered to range in fillets from 3.04 to 10.36 log cfu/g. Can and Coban[Citation16] found mesophilic bacteria counts to be between 4.3 and 7.3 log cfu/g in uncoated rainbow trout on the 9th day while it was between 3.8 and 5.9 log cfu/g in coating with zein on the 15th day. Mohan et al.[Citation27] stated that the highest counts of psychotropic occurred on a day when it was uncoated while the lowest counts occurred when it was coated with chitosan film of Sardinella samples at the end of storage. The total number of psychotropic bacteria in samples was determined to range in fillets from 3.04 to 10.47 log cfu/g. The inhibiting effect, which was influenced by the number of psychotropic bacteria, on uncoated samples was higher than that of coated samples of fillets during storage. Jeon et al.[Citation35] reported that in cod and herring samples coated with chitosan films, the counts of total psychotropic bacteria decreased on the 12th day compared to the control group. Such results found in previous studies are consistent with the present study. The initial LAB (Fig. 1c) of rainbow trout fillets was 2.02 log cfu/g. At the end of the storage period, levels 8.65, 7.88, 6.81, and 5.95 log cfu/g were reached for samples C, X, Y, and Z, respectively. These results were found to be consistent with the values found in previous studies.[Citation36] Can and Coban[Citation16] observed LAB counts to be between 1.5 and 3.8 log cfu/g in uncoated rainbow trout on 9th day while it was between 1.3 and 1.5 log cfu/g in coating with zein on the 15th day. The treatment and storage processes were found to significantly affect Enterobacteriaceae (p < 0.05) in rainbow trout samples, in the present study, it is between 1.37 and 5.63 log cfu/g. Microbiological analyses showed that samples coated with different WPC coating formulations (with or without glycerol) exhibited a decrease on Enterobacteriaceae compared to control after 15 days of storage while X (1.88–3.81 log cfu/g) presented higher Enterobacteriaceae than the sample Y (1.99–2.81 log cfu/g) and Z (1.37–1.99 log cfu/g) at the end of storage time. Also, a significant difference was observed in coated sample as compared to the non-coated samples. Alak et al.[Citation37] found that Enterobacteriaceae bacteria counts were higher in the control group than those of the chitosan and modified atmosphere packaging (MAP) groups; MAP statistically demonstrated lower counts of Enterobacteriaceae as compared to the control group (p < 0.05) and chitosan film had the best inhibition in all groups. These results were similar to ours.
FIGURE 1 The changes in microbiology characteristics rainbow trout samples (log cfu/g); a) Total aerobic mesophilic bacteria; b) total physcrophilic aerobic bacteria; c) lactic acid bacteria (in MRS); d) Enterobacteriaceae.
Chemical Evaluation
Chemical composition of the trout samples is given in . Chemical changes in samples were found to be statistically significant (p < 0.05) during storage time. Moisture of the samples was found to change between 64.04 and 70.56%. This increase was higher for the trout samples without coating. These results also showed that the coating process of different WPC formulations (with or without glycerol) might have delayed moisture losses compared to control. Lu et al.[Citation38] indicated that the alginate calcium coatings on the fish fillets acted effectively as water vapor barriers during the entire storage period. In the present study, the lipid content in all the trout samples increased with increasing storage time (p < 0.05). The lowest lipid content was observed in sample C (7.61 %) while the highest was observed in sample Y (12.29%). Hasanzati Rostami et al.[Citation12] found that lipid content in all Kilka samples decreased during the storage period and significant difference was found in coated samples as compared to the non-coated samples.
TABLE 1 The changes in chemical characteristics of rainbow trout samples during storage
Our data demonstrated that the protein values found in the samples were between 18.55 and 23.31. According to Hasanzati Rostami et al.,[Citation12] no significant difference (p > 0.05) was observed in protein content between the samples (coated with whey protein film and uncoated Kilka). The pH value ranged from 6.22 to 6.69 for control sample and 6.26 to 6.50 for X, 6.24 to 6.48 for Y, and 6.14 to 6.47 for Z. Dikel[Citation32] stated that the pH ranged from 6.38 and 6.50 in coated samples (15% gelatin + 0.5% chitosan and 15% gelatin + 1% chitosan) while in uncoated sea bream (Sparus aurata L.), fillets pH was found to be 6.60 on 6th day of storage. Turan[Citation39] reported that a significant difference was observed among pH values in rainbow trout samples coating with chitosan. These data are consistent with the present study. As can be seen in , ash content in the samples increased with increasing storage time (p < 0.05). Rodriguez-Turienzo et al.[Citation14] emphasized that fresh salmon showed lower dry matter content than frozen atlantic salmon coated with protein-based whey (Salmo salar) and the authors explained that ash values of fresh fishes were also lower, probably due to the loss of moisture during frozen storage. The initial (day 0) TVB-N values () of rainbow trout fillets were 12.41 mg/100 g. C and X rainbow trout fillets exceeded the value of 25 mg/100 g with an upper acceptable limit TVBN value of 25 mg N/100 for rainbow trout as suggested by the International Commission on Microbiological Specifications for Foods (ICMSF)[Citation40] at day 9 and 15 of storage, respectively. This limit was not exceeded throughout the storage period of Y and Z. TVB-N value was found to increase in all samples during storage. This increase is related to the activity of spoilage bacteria and endogenous enzymes.[Citation15,Citation41,Citation42] Can and Coban[Citation16] reported TVB-N values to be between 5.8 and 42 mg/100 g in uncoated rainbow trout on the 9th day while it was between 5.8 and 16 mg/100 g in coating with zein on the 15th day. These findings are consistent with the findings in the present study. The values of TVB-N were found to increase in non-coated and coated with sodium alginate-based edible samples of bream (Megalobrama amblycephala) during storage in Song et al.[Citation15] Initial TBARS value () was 1.08 µmol malondialdehyde (MDA)/kg. At the end of the storage period, TBARS values 9.20, 8.30, 6.89 and 5.50 µmol MDA/kg were recorded for treatments C, X, Y, and Z, respectively. Jeon et al.[Citation35] have reported lower TBARS contents over storage in chitosan-coated herring and cod samples than in uncoated samples. Whey protein films and coatings have proven to be excellent oxygen barriers.[Citation12,Citation43] The peroxide ranged from 2.02 to 3.92 meq O2/kg lipid for the control samples, 2.06 to 4.32 meq O2/kg lipid for X, 1.82 to 3.23 meq O2/kg lipid for Y, and 1.76 to 3.28 meq O2/kg lipid for Z. The peroxide value was significant (p < 0.05) during storage time. Hasanzati Rostami et al.[Citation12] found that the peroxide value of all coated samples was lower than that of uncoated samples during storage, indicating that coating can reduce lipid oxidation.
Sensory Evaluation
The results of the sensory evaluation of trout samples on a scale of 1 (poor) to 9 (excellent) are shown in . A significant difference (p < 0.05) was found among samples for appearance, taste, odor, firmness, and general acceptability. Y and Z fish samples scored higher than the treatment fish (p < 0.05). From the data, we learned that all treatments maintained better sensory quality for the fish samples than those of the untreated samples. Samples Y and Z were mostly preferred by the panellists. Sample C was given the lowest scores by the panellists. The results were in accordance with the findings of Song et al.[Citation15] who found that the shelf life of untreated bream was 21 days according to sensory score, and that the fish with alginate calcium coating were still considered to be acceptable. Dikel[Citation32] reported lower score for appearance, taste, odor, firmness, and general acceptability scores of sea bream compared to control samples and those coated with chitosan and gelatin.
TABLE 2 The changes in sensory characteristics of rainbow trout samples during storage
CONCLUSIONS
Coating of trout with edible film composed of different WPC coating formulations (with or without glycerol) showed reducing effect on the microbial growth and extended the shelf life of the products. The results also indicated that the use of whey coating together with glycerol (2:1) can suppress microbial growth during the storage period without any adverse effects on fish quality. During the storage process of treatment samples, the values of TVB-N and TBARS determined the effect of the edible film coating on the rate and extent of product quantitatively in fish. In terms of sensorial evaluation, panellists gave the highest scores mostly to group Z. These conclusions were supported by the results from microbiological and chemical quality analyses. According to the results of TAMB analyses, the shelf life of rainbow trout fillets was estimated at day 9, 12, and 15 for C, X, Y, and Z respectively.
REFERENCES
- Emre, Y.; Kurum, V. Havuz ve Kafeslerde Alabalık Yetiştiriciliği Teknikleri [The Techniques of Breeding Trout in Ponds and Cages]. Minpa Matbaacılık Tic. Ltd. Şti.: Ankara, Turkey, 1998; 202.
- Australian Fisheries Statistics. Australian Bureau of Agricultural and Resources Economics and Sciences, 2012.
- Babadogan, G. Fisheries Sector Research; IGEME: Ankara, Turkey, 1998; 124.
- Kilincceker, O.; Dogan, I.S.; Kucukoner, E. Effect of Edible Coatings on the Quality of Frozen Fish Fillets. LWT–Food Science and Technology 2009, 42, 868–873.
- Gennadios, A.; Hanna, M.A.; Kurth, L.B. Application of Edible Coatings on Meats, Poultry, and Seafoods: A Review. LWT–Food Science and Technology 1997, 30, 337–350.
- Sathivel, S. Chitosan and Protein Coatings Affect Yield, Moisture Loss, and Lipid Oxidation of Pink Salmon (Oncorhynchus Gorbuscha) Fillets During Frozen Storage. Journal of Food Science 2005, 70, E455–E459.
- Stuchell, Y.M.; Krochta, J.M. Edible Coating on Frozen King Salmon: Effect of Whey Protein Isolate and Acetylated Monoglycerides on Moisture Loss and Lipid Oxidation. Journal of Food Science 1995, 60, 28–31.
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial and Antioxidant Effects of Milk Protein-Based Film Containing Essential Oils for the Preservation of Whole Beef Muscle. Journal of Agricultural and Food Chemistry 2004, 52, 5598–5605.
- Gómez-Estaca, J.; Montero, P.; Giménez, B.; Gómez-Guillén, M.C. Effect of Functional Edible Films and High Pressure Processing on Microbial and Oxidative Spoilage in Cold-Smoked Sardine (Sardina Pilchardus) Food Chemistry 2007, 105, 511–520.
- Min, S.; Rumsey, T.R.; Krochta, J.M. Diffusion of the Antimicrobial Lysozyme from a Whey Protein Coating on Smoked Salmon. Journal of Food Engineering 2008, 84, 39–47.
- Kilincceker, O.; Kurt, S. The Sensory Quality of Pearl Mullet (Chalcalburnus Tarichi) Fillets Coated with Different Coating Materials. Turkish Journal of Fisheries and Aquatic Sciences 2010, 10, 471–476.
- Hasanzati Rostami, A.; Motallebi, A.A.; Khanipour, A.A.; Soltani, M.; Khanedan, N. Effect of Whey Protein Coating on Physico-Chemical Properties of Gutted Kilka During Frozen Storage. Iranian Journal of Fisheries Sciences 2010, 9, 412–421.
- Lin, L.S.; Wang, B.J.; Weng, Y.M. Quality Preservation of Commercial Fish Balls with Antimicrobial Zein Coatings. Journal of Food Quality 2011, 34, 81–87.
- Rodriguez-Turienzo, L.; Cobos, A.; Moreno, V.; Caride, A.; Vieites, J.M.; Diaz, O. Whey Protein-Based Coatings on Frozen Atlantic Salmon (Salmo Salar): Influence of the Plasticiser and the Moment of Coating on Quality Preservation. Food Chemistry 2011, 128, 187–194.
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of Sodium Alginate-Based Edible Coating Containing Different Anti-Oxidants on Quality and Shelf Life of Refrigerated Bream (Megalobrama Amblycephala). Food Control 2011, 22, 608–615.
- Can, O.P.; Coban O.E. Vakum Paketlemenin ve Zein ile Kaplamanın Balık Filetolarının Kalite Kriterleri Üzerine Etkilerinin Karşılaştırılması [The Effect of Compared Quality Criteria Rainbow Trout with Vacuum Packaging and Coating with Zein]. Biyoloji Bilimleri Araştırma Dergisi [Journal of Biology Sciences Research] 2012, 5, 87–91 (in Turkish).
- Tharanathan, R.N. Biodegradable Films and Composite Coatings: Past, Present, and Future. Trends in Food Science and Technology 2003, 14, 71–78.
- Seydim, A.C.; Sarikus, G. Antimicrobial Activity of Whey Protein Based Edible Films Incorporated with Oregano, Rosemary, and Garlic Essential Oils. Food Research International 2006, 39, 639–644.
- Huffman, L.M. Processing Whey Protein for Use As a Food Ingredient. Food Technology 1996, 50, 49–52.
- Kinsella, J.E.; Morr, C.V. Milk Proteins: Physicochemical and Functional Properties. Critical Reviews in Food Science and Nutrition 1984, 21, 197–262.
- Ozdemir, M.; Floros, J.D. Optimization of Edible Whey Protein Films Containing Preservatives for Water Vapor Permeability, Water Solubility, and Sensory Characteristics. Journal of Food Engineering 2008, 86, 215–224.
- Javanmard, M. Biodegradable Whey Protein Edible Films As a New Biomaterials for Food and Drug Packaging. Iranian Journal of Pharmaceutical Sciences 2009, 5, 129–134.
- Rooney, M.L. Active Packaging in Polymer Films. In Active Food Packaging; Rooney, M.L.; Ed.; Blackie Academic & Professional: Glasgow, UK, 1995; 1–37.
- Vermeiren, L.; Devlieghere, F.; Van Beest, M.; De Kruiif, N.; Debevere, J. Developments in the Active Packaging of Food. Trends in Food Science and Technology 1999, 10, 77–86.
- Appendini, P.; Hotchkiss, J.H. Review of Antimicrobial Food Packaging. Innovative Food Science and Emerging Technologies 2002, 3, 113–126.
- Pérez, L.M.; Balagué, C.E.; Rubiolo, A.C.; Verdini, R.A. Evaluation of the Biocide Properties of Whey-Protein Edible Films with Potassium Sorbate to Control Non-O157 Shiga Toxin Producing Escherichia Coli. Procedia Food Science 2011, 1, 203–209.
- Gokalp, H.Y.; Kaya, M.; Zorba, O.; Tulek, Y. Et ve Ürünlerinde Kalite Kontrolü ve Laboratuar Uygulama Kılavuzu [Quality Control and Laboratory Practice Guidelines in Meat and Meat Products], Atatürk Üniversitesi Ziraat Fakültesi Yay. No: 786. Ziraat Fak. Yay. No: 320. Ders Kitapları Serisi No: 70. Erzurum 1999; 309–339.
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods, or Determination of Peroxide Values of Food Lipids. Journal of AOAC International 1994, 77, 421–424.
- Malle, P.; Tao, S.H. Rapid Quantitative Determination of Trimethylamine Using Steam Distillation. Journal of Food Protection 1987, 50, 756–760.
- Lemon, D.W. An Improved TBA Test for Rancidity. Environment Canada. Fisheries and Marine Service: Halifax, NS, Canada, 1975.
- Kilic, B.; Richards, M.P. Lipid Oxidation in Poultry Döner Kebab: Pro‐Oxidative and Anti‐Oxidative Factors. Journal of Food Science 2003, 68, 686–689.
- Dikel, C. Effect of Gelatin Coating Incorporated with Chitosan on the Chemical, Physical, Microbiological, and Sensory Quality of Sea Bream (Sparus Aurata L., 1758) Fillets. University of Cukurova, The Institute of Natural and Applied Sciences, Department of Fishing and Processing Technology, Master Thesis, Adana, Turkey. 2012.
- SAS, SAS/STAT Software. Changes and Enhancements Through Release 6.12; SAS Institute Inc.: Cary, NC, 1998.
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Gopal, T.S. Effect of Chitosan Edible Coating on the Quality of Double Filleted Indian Oil Sardine (Sardinella Longiceps) During Chilled Storage. Food Hydrocolloids 2011, 26, 167–174.
- Jeon, Y.J.; Kamil, J.Y.; Shahidi, F. Chitosan as an Edible Invisible Film for Quality Preservation of Herring and Atlantic Cod. Journal of Agricultural and Food Chemistry 2002, 50, 5167–5178.
- Alparslan, Y.; Baygar, T.; Baygar, T.; Hasanhocaoglu, H.; Metin, C. Effects of Gelatin-Based Edible Films Enriched with Laurel Essential Oil on the Quality of Rainbow Trout (Oncorhynchus Mykiss) Fillets During Refrigerated Storage. Food Technology and Biotechnology 2014, 52, 325–333.
- Alak, G.; Hisar, A.S.; Hisar, O.; Kaban, G.; Kaya, M. Microbiological and Chemical Properties of Bonito Fish (Sarda Sarda) Fillets Packaged with Chitosan Film, Modified Atmosphere, and Vacuum. Journal of the Faculty of Veterinary Medicine, University of Kafkas 2010, 16, 73–80.
- Lu, F.; Liu, D.; Ye, X.; Wei, Y.; Liu, F. Alginate-Calcium Coating Incorporating Nisin and EDTA Maintains the Quality of Fresh Northern Snakehead (Channa Argus) Fillets Stored at 4°C. Journal of the Science of Food and Agriculture 2009, 89, 848–854.
- Turan, M. Effects of Chitosan Glazing on the Quality of Rainbow Trout Fillets (Oncorhynchus Mykiss) During Frozen Storage. Ankara University Graduate School of Natural and Applied Sciences Department of Food Engineering Master Thesis, Ankara, Turkey, 2011.
- ICMSF. Microorganisms in Foods-I: Their Significance and Methods of Enumeration, 2nd Ed; International Commission on Microbiological Specifications for Foods. University of Toronto Press: Canada, 1992.
- Kyrana, V.R.; Lougovois, V.P.; Valsamis, D.S. Assessment of Shelf-Life of Maricultured Gilthead Sea Bream (Sparus Aurata) Stored in Ice. International Journal of Food Science and Technology 1997, 32, 339–347.
- Vareltzis, K.; Koufidis, D.; Gavriilidou, E.; Papavergou, E.; Vasiliadou, S. Effectiveness of a Natural Rosemary (Rosmarinus Officinalis) Extract on the Stability of Filleted and Minced Fish During Frozen Storage. Zeitschriftfür Lebensmitteluntersuchung und-Forschung A 1997, 205, 93–96.
- Franssen, L.R.; Rumsey, T.R.; Krotcha, J.M. Whey Protein Film Composition Effects on Potassium Sorbate and Natamycin Diffusion. Journal of Food Science 2004, 69, 347–351.
