?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was carried out to determinate phenolic, flavonoid content, and antioxidant capacity in methanolic extract from three Alperujo varieties. Alperujo Barnea showed the highest concentration of phenols and flavonoid. The greater hydroxytyrosol content was obtained in the same extract (4.93 ± 0.37 µg/mg extract), whereas the greater tyrosol content (0.23 ± 0.012 µg/mg extract) was found in Arbequina extract. These results were correlated with the greatest radical scavenging and the highest inhibition of lipoperoxidation process observed in Barnea extract (IC50 of 27.9 ± 1.04 µg/mL; IC50 22.8 ± 3.5 µg/mL, respectively). In spite of differences, alperujo extracts exhibited notable antioxidant capacities.
INTRODUCTION
The olive oil industry generates large amounts and variety of waste according to the mechanical extraction systems used. With the “system of three-phase centrifugation” to separate the oil from the vegetable water and the solid phase of the olive oil (orujo), it is estimated that for every 1000 kg of olive processed under this system, approximately 210 kg of olive oil, 1 to 1.6 m3 of vegetable water and 550 kg of orujo are generated.[Citation1,Citation2]
The main drawback of this system is the generation, in a short period of time, of large amounts of fluids highly polluting products of water added in the different steps of production process.[Citation2] In order to reduce the environmental impact, different methods have been proposed for extracting olive oil. However, the “system of two-phase centrifugation” has persisted, which greatly reduces the generation of liquid waste and pollutant load,[Citation3,Citation4] producing only a solid and wet by-product called “alperujo or alpeorujo.” Moreover, it is estimated that for every 1000 kg of olive processed under this methodology, 200 kg of olive oil is generated, 0.2 m3 of liquid is waste and 800 kg of alperujo is obtained.[Citation2] Although is called “eco-system,” it generates residues (alperujo) that have a deleterious effect on the structural stability of the soil, adversely affecting the germination processes, plant growth, and microbial activity due to the presence of phenolic, organic, and fatty acids compounds.[Citation5,Citation6] However, it is known that phenolic compounds have strong antioxidant properties, and the presence in these residues can make them an economic source of natural antioxidants,[Citation4,Citation7] with potential uses in the food industry for humans and animals. On the other hand, from a public health point-of-view, compounds derived from alperujo may help to protect the body against oxidative damage caused by oxidizing agents involved in the etiology of a variety of chronic diseases such as diabetes, atherosclerosis, and neurodegenerative diseases all of them highly prevalent throughout the world.[Citation8–Citation13]
According to the Food Health Regulations (DTO. 977/96) of the Ministry of Health, Chile; food or food produce is any substance or mixture of substances intended for human consumption, including drinks and all the ingredients and additives of such substances. In food production, safety must be checked from the microbiological perspective and potential toxic substances.[Citation14]
Alperujo corresponds to a derivative of processed extra virgin olive oil product where only pressure and centrifugation is applied and, therefore, it could be a raw material suitable for inclusion in processed foods. However, to be used as food it would be required chemical, physical, or biological treatment to transform it in a safety food product, according to conditions obtained. Compared to olive oil, alperujo is a great source of phenolic compounds reaching average values of 8680 µg/g, unlike 50–1200 µg/g of olive oil.[Citation15] This fact can be explained due to the apolar owned olive oil and polar alperujo, generating the latter drag phenols during the olive oil extraction. Since the alperujo is the main waste produced by the oil industry and there is no demand for this derivative and its accumulation results in a soil contamination, it is necessary to find a new use to convert it into a useful industrial product. Knowing the chemical characterization and biological properties of the alperujo extracts, is essential to enhance the benefits of this residue, so that they can be used as a basis for the food and pharmaceutical industry to help improving the health of the population. In the present study, we characterized the phenolic compounds and evaluated the antioxidant capacity of three different alperujo extracts from Region del Maule, Chile, in order to establish its potential use as an industrial product
MATERIALS AND METHODS
Collection and Pre-Treatment of Alperujo
Three varieties of alperujo (Arbequina, Barnea, and Frantoio) were obtained between May and August 2011 from “Olivares de Quepu” company, Maule Region, Chile and were stored in hermetic bags at –20ºC until the time of use.
Alperujo Extracts
To remove the remaining oil in the alperujo, an extraction with petroleum ether was performed. Briefly, 500 g of alperujo were mixed with petroleum ether (Merck) and then it was sonicated for 15 min at 40 KHz at room temperature. Finally the mixture was filtered and the solvent was discarded. The alperujo free from oil, obtained from the extraction with petroleum ether was used for the preparation of a methanolic extract. Approximately, 450 g of different varieties were mixed with 1.5 L of methanol (Merck) and then they were filtered to obtain the soluble phenolic compounds. The filtrate was concentrated on a rotary evaporator (Heidolph Laborota 4001) at 50 rpm and a temperature of 40°C and finally lyophilized (Labconco Equilab) and stored at –20°C until use.
Total Phenolic Content (TPC)
The TPC of the extracts was determined according to the Folin–Ciocalteu method.[Citation16] Briefly, 20 µL of extracts (100 µg/mL, water/methanol) or standard (gallic acid), were mixed with 1.58 mL of distilled water and 100 µL of Folin–Ciocalteu reagent, the reaction mixture was pre-incubated for 8 min and then 300 µL of sodium carbonate 20%, was added. Finally, each tube was incubated for 2 h at room temperature and the absorbance was obtained in a spectrophotometer (Thermo Spectronic Genesys 10 UV) at a wavelength of 765 nm, the TPC was expressed as gallic acid equivalents (GAE) in milligrams per gram of fresh alperujo.
Total Flavonoid Content (TFC)
The TFC were determined spectrophotometrically using the method reported by Zhishen et al.,[Citation17] based on the formation of a flavonoid-aluminum complex. Briefly, 0.5 mL of extracts (100 µg/mL) or standard (quercetin) were mixed with 2 mL of distilled water and 0.15 mL of sodium nitrate (NaNO3, 5%). After 6 min of incubation, 0.15 mL of aluminum chloride (AlCl3, 10%) were added and allowed to incubate for another 6 min, after which, 2.0 mL of sodium hydroxide (NaOH, 4%) were added to the mixture. Water was added to achieve a final volume of 5 mL, and the solution was incubated for another 15 min. The absorbance was obtained in a spectrophotometer (Thermo Spectronic Genesys 10 UV) at a wavelength of 510 nm. The results were reported as quercetin equivalents (QE) in milligrams per g of fresh alperujo.
Tyrosol and Hydroxytyrosol Content
An aqueous mixture from Arbequina, Barnea, and Frantoio varieties was used. Alperujo extracts (100 mg) were re-dissolved in 1 mL of methanol: water (1:1), then sonicated for 30 min (ultrasound-sonicator, Elma, BioLogics Inc., Virginia, USA). Fifty microliters of each calibrator (Tyrosol (2-(4-Hydroxy-phenyl)ethanol) and Trihydroxityrosol (3-Hydroxytyrosol); Sigma Aldrich Co.), controls and samples were mixed with 50 µL of an internal standard (Primidone, 20 µg/mL in methanol, Laboratorio Chile S.A, Chile). Each sample was vortexed for 10 s and impregnated on a roll cellulose (Whatman filter paper 3.5 × 7 cm) that was contained in a 5 mL glass tube. Then, 1 mL of ethyl acetate was added to each tube and vortexed for 10 s. This step was repeated until getting 3 mL of ethyl acetate and then was evaporated at 40°C until dryness was reached.
High-performance liquid chromatography (HPLC) analysis was performed using a HPLC/ultraviolet (UV) Agilent 1100 (Agilent Technologies, CA, USA). The separation of phenolic compounds was performed on a Zorbax XDB C18 (4.6 × 30 mm; 3.5 µm particle size, Agilent Technologies, CA, USA). All analysis were performed using an isocratic mobile phase (water:methanol:acetonitrile, 70:24:6; pH 7.0). The dry residue was re-dissolved in 75 µL of mobile phase. The injection volume was 10 µL at a flow rate of 1 mL/min. The UV absorbance of the eluent was measured at 280 nm. Phenolic concentrations were calculated by linear last-squares regression analysis of calibration curves of each compound, using height ratio between sample and the internal standard.
Determination of Antioxidant Activity
Free radical scavenging assay 2,2-diphenyl-1-picrylhydrazyl (DPPH)
The scavenging activity of the extract and mixtures were estimated using DPPH as the free radical model according to the method adapted from Brand-Williams et al.[Citation18] For each extract, standard solutions were prepared at a final concentration of 5 mg/mL (methanol) and then were diluted to obtain solutions of 600, 300, 150, and 3 µg/mL. The assay consisted on 0.75 mL of each extract solutions and 1.5 mL of a methanolic solution of DPPH (20 mg/dL; Sigma-Aldrich).
The samples were incubated 15 min at room temperature and the absorbance was measured at 517 nm (Thermo Spectronic 10 Genesys UV). To transform the absorbance in a percentage of antioxidant activity (% AA) the following formula was used.
where sample Ab, is the absorbance of the extract or mixture, blank Ab is the absorbance of the blank sample, and the control Ab is the absorbance of the DPPH reagent. Ascorbic acid (AA) was used as the reference compound.
Low density lipoprotein (LDL) isolation from human plasma
Peripheral venous blood was collected from normal volunteers according to a protocol approved by the Universidad de Talca in tubes containing ethylenediaminetetraacetic acid (EDTA) (1 mg/mL), the plasma was isolated by centrifugation at 3500 rpm for 15 min at 20ºC. The LDL was isolated by differential ultracentrifugation, according to the method of Galle and Wanner[Citation19] and the protein concentration was determined using the Lowry assay.[Citation20]
Formation of conjugated dienes
The degree of LDL oxidation was measured with respect to formation of conjugated dienes by monitoring the change in absorbance at 2 min intervals at 234 nm (Genesys 10 UV spectrophotometer).[Citation21] The LDL (30 μg/mL) was incubated with Cu2+ 10 μM, in absence (control) or presence of the extracts (25 μg/mL) in NaCl 0.15 M, for 6 h at 25ºC. The concentration of conjugated dienes in the samples was calculated by using a molar extinction coefficient of 2.95 × 104 M–1 cm–1, data were expressed as median ± standard deviation (SD; n = 3). AA was used as a reference antioxidant.
Red blood cell lipoperoxidation
The erythrocyte lipid peroxidation assay was carried out as described by De Azevedo et al.[Citation22] For this purpose, blood was collected from normal volunteers according to a protocol approved by the Universidad de Talca. The blood was centrifuged and the clear plasma and buffy coat layers were discarded, the red cells suspension was washed three times with cold phosphate-buffered saline (PBS) pH 7.4.
The assay consisted in a suspension of 50% hematocrit in PBS (100 µL), mixed with 20 µL of the different concentrations (100, 50, and 10 µg/mL, final volume) of each extract and 0.78 mL of PBS; the mixture was incubated for 10 min at 37ºC.
After the incubation, 100 µL of tert-butylhydroperoxide (t-BHP, 1 mM) were aggregated and incubated for 30 min at 37ºC, then 100 µL of Triton X-100 (1%, v/v) and 2 mL of tiobarbituric acid/trichloro acetic acid/hydrochloric acid (TBA/TCA/HCl) were added and blended for 5 min. The reaction tubes were then centrifugated for 5 min at 3500 rpm, the supernatant obtained was incubated for 15 min at 90ºC and the absorbance was measured at 535 nm (Thermo Spectronic 10 Genesys UV). The results were expressed as the percent of inhibition compared with the control (100% lipoperoxidation).
Statistical Analysis
Each measurement was performed three times. All the data is expressed by mean ± SD. The statistical analysis t-test and ANOVA was used as appropriate with the software SPSS 15.0 (Statistical Product and Service Solutions). The statistical significance level was set up at p < 0.05.
RESULTS
TPC
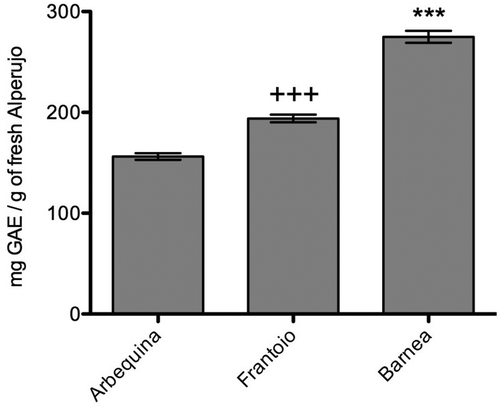
The TPC of the different extract of alperujo studied were determined spectrophotometrically using gallic acid as standard. Among the different alperujo extracts, the highest concentration of phenols (275.0 ± 5.9 mg/g of fresh alperujo) were obtained in the Barnea variety, whereas the extract of Arbequina showed the minor phenolic content with a concentration of 156.3 ± 3.3 mg/g of fresh alperujo ().
TFC
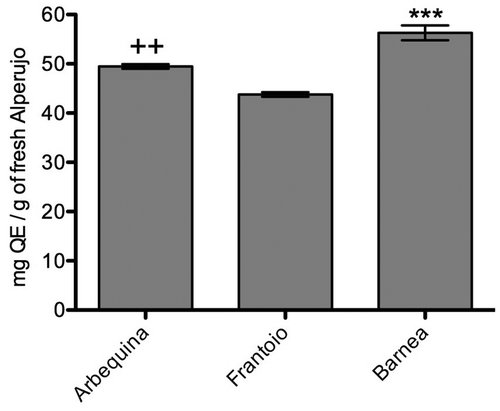
The alperujo extract of the variety Barnea showed the highest concentration of flavonoids (56.3 ± 1.5 mg QE/g of fresh alperujo) in correlation with the phenolic content, while the alperujo extracts of the varieties Arbequina and Frantoio showed a different behavior (), being the alperujo extract of the variety Frantoio which showed the lowest concentration of flavonoids (43.8 ± 0.5 mg QE/g of fresh alperujo).
Hydroxytyrosol and Tyrosol Content of the Alperujo Extract
The content of Hydroxytyrosol and Tyrosol was measured by HPLC using (Tyrosol (2-(4-Hydroxy-phenyl)ethanol) and Trihydroxityrosol (3-Hydroxytyrosol); Sigma Aldrich Co.) as calibrators (). The greater content of hydroxytyrosol was found in the alperujo extract of Barnea (4.93 ± 0.37 µg/mg of extract), whereas the greater content of tyrosol (0.23 ± 0.012 µg/mg of extract) belongs to the alperujo extract of Arbequina.
TABLE 1 Hydroxytyrosol and tyrosol content in alperujo extracts
Antioxidant activity
The free radical antioxidant activity (DPPH) of the different extracts showed that the alperujo extract of the variety Barnea has the best antioxidant activity with an IC50 of 27.9 ± 1.04 µg/mL, while the varieties Arbequina and Frantoio had an IC50 of 41.1 ± 1.1 and 48.7 ± 1.4 µg/mL respectively (). The red blood cells lipoperoxidation inhibition showed a similar behavior than the DPPH assay, with the alperujo extract of Barnea with the highest inhibition of the lipoperoxidation (IC50 22.8 ± 3.5 µg/mL) followed by the extracts of Frantoio and Arbequina (32.8 ± 3.1 and 57.3 ± 4.1 µg/mL, respectively; ).
TABLE 2 Antioxidant properties in alperujo extracts
Inhibition of the LDL oxidation
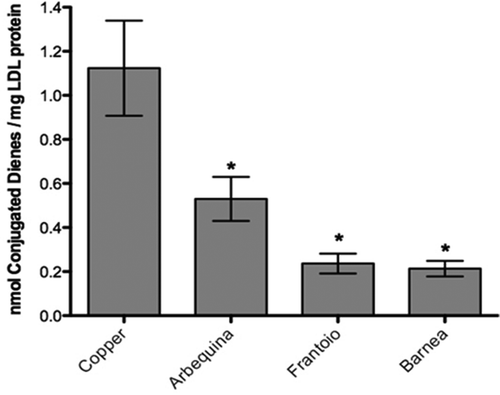
The inhibition of the LDL oxidation as a result of the antioxidant property of the different extracts of alperujo (25 µg/mL), was studied through the conjugated dienes production at 234 nm. The alperujo extracts of the varieties Barnea and Frantoio showed the highest inhibition of the LDL oxidation followed by the Arbequina variety (), with a statistically significant difference (p < 0.05) compared with the positive control (copper 10 µM).
DISCUSSION
In this study, the antioxidant profile of three varieties of alperujo obtained in the Maule region in 2011 was determined through measurement of phenolics, total flavonoids, DPPH radical inactivation, inhibition of lipoperoxidation in red blood cells, and LDL oxidation. It is important to highline that this study is one of the first in this area of research, because when we search in different databases, we found limited information about the chemical profile of alperujo.
Using the Folin–Ciocalteu method[Citation16] and the Aluminum Chloride method,[Citation17] it was showed that alperujo Barnea is the strain that has a higher concentration of phenolic compounds (275.0 ± 5.9 mg/g of fresh alperujo) being almost twice the concentration that Arbequina exhibits. This amount is comparable to the one shown by several extracts from plants with medicinal properties and that have been used against chronic diseases in which oxidative stress plays an important role.[Citation23,Citation24]
Comparing our results with the phenolic compounds levels in whole olives, from a cultivar Frantoio in Australia reported by Goldsmith et al.,[Citation25] it is possible to infer that they have a lower concentration than the one found in the Frantoio alperujo exposed here, albeit in their liquid waste obtained from the olive oil process exhibit more than double found by us (data not shown). Moreover, the analysis of phenolic compounds and flavonoids made in extra virgin oil obtained from Arbequina cultivar in Tunisia,[Citation26] exhibited a lower concentration than Arbequina extract of our study, showing that the antioxidant potential is mainly within the waste of the process.
Regarding the amount of total flavonoids, the Barnea variety shown the highest concentration (56.3 ± 1.5 mg QE/g of fresh alperujo) and Frantoio variety the lowest (43.8 ± 0.5 mg GAE/g of fresh alperujo), these results were approximately an 50% of that found in methanolic extracts of waste of Frantoio variety olive obtained in Ovalle, Chile,[Citation27] extracted in similar conditions but in different geographic areas, which in our country is an important consideration, because Chile covers a wide varieties of climates, from arid (desert) to Mediterranean with great differences in temperatures between day and night that could be affect this chemical composition.
One of the most studied phenolic compounds in olive fruits is the Trihydroxityrosol (3-Hydroxytyrosol),[Citation2,Citation28] an o-diphenol having the ability to donate an electron hydroxyl in ortho position and the subsequent formation of stable intramolecular hydrogen bonds phenoxylic with the radical.[Citation29] In the present work, the concentration of tyrosol (2-(4-Hydroxy-phenyl) ethanol) and Trihydroxityrosol (3-Hydroxytyrosol) was measured in the three varieties of alperujo by HPLC/UV, and according to the results, the variety Barnea has averaged 4.93 µg/mg Trihydroxityrosol, twice that observed in Arbequina. However, when the amount of tyrosol is evaluated, the results were reversed as has the highest concentration in Arbequina. In a comparative study on the quality of olive oil from cultivars Barnea, Frantoio, and Arbequina from five different geographical regions of Chile,[Citation30] they don’t found significant differences between concentration of Trihydroxityrosol in variety Barnea and Frantoio which is similar to our study that was made in alperujo extracts. In relationship to Arbequina variety it had the lowest concentration in olive oil and that was similar to our results in alperujo extracts.
The antioxidant activity of the three varieties was also determined through inhibition of DPPH radical, red cell lipid peroxidation and LDL oxidation which led to determine that Barnea presented the best antioxidant activity (, ). This correlates with what Soleas et al.[Citation31] proposed in the sense that the content of phenolic compounds is directly related to the antioxidant capacity of the matrix studied. Deiana et al.[Citation32] reported that the Trihydroxityrosol is able to inhibit the oxidation of LDL in vitro, which agrees with the results obtained here, since the variety Barnea and Frantoio significantly reduced the formation of conjugated dienes and they were the varieties with the higher concentration of Trihydroxityrosol.
Tyrosol and hydroxityrosol present biological activity, being a possible target to prevent many diseases. For example, tyrosol and hydroxityrosol have a neuroprotective effect against Aβ-induced toxicity in neuroblastoma N2a cells, given the properties to prevent Alzheimer disease.[Citation33] It has been shown an antiproliferative effect in human cancer cells,[Citation34] a protective effect on lipoperoxidation in tubular epithelial cells, preventing renal injuries,[Citation35] and also exhibit antimicrobial and anti-inflammatory activities.[Citation36] This information could be important because our results highlights the potential contribution of olive oil waste for human use in medical therapeutically or nutrition benefits. Given the importance that has been attributed to the oxidation of LDL in the atherosclerosis process,[Citation37,Citation38] it is interesting to analyze, the possibility of therapeutic use of the phenolic antioxidant extracts as an industrial waste—alperujo—in diseases where oxidative stress plays a crucial role.
CONCLUSION
In this study, we found differences between three varieties of alperujo extracts. Alperujo Barnea showed the highest concentration of phenols and flavonoids. The greater hydroxytyrosol content was obtained in the same extract (4.93 ± 0.37 µg/mg extract), whereas the greater tyrosol content (0.23 ± 0.012 µg/mg extract) was found in Arbequina extract. These results were correlated with the greatest radical scavenging and the highest inhibition of lipoperoxidation process observed in Barnea extract (IC50 of 27.9 ± 1.04 µg/mL; IC50 22.8 ± 3.5 µg/mL, respectively). In spite of differences, alperujo extracts exhibited notable antioxidant capacities. Considering all the above, it can be concluded that extracts of alperujo of Maule Region of Chile, have a high antioxidant potential, highlighting the Barnea alperujo variety. However, further studies are required to assess the cytotoxicity of the extracts and bioavailability of the compounds in an in vivo model and establish their true therapeutic potential.
REFERENCES
- Sierra, J.; Marti, E.; Montserrat, G.; Cruanas, R.; Garau, M.A. Characterisation and Evolution of a Soil Affected by Olive Oil Mill Wastewater Disposal. The Science of the Total Environment 2001, 279, 207–214.
- Alburquerque, J.A.; Gonzalvez, J.; Garcia, D.; Cegarra, J. Agrochemical Characterisation of “Alperujo”, a Solid By-Product of the Two-Phase Centrifugation Method for Olive Oil Extraction. Bioresource Technology 2004, 91, 195–200.
- Moreno, R.; Benítez, E.; Melgar, R.; Polo, A.; Gómez, M.; Nogales, R. Vermicomposting As An Alternative for Reusing By-Products from the Olive Oil Industry. Fresenius Environmental Bulletin 2000, 9, 1–8.
- Lesage-Meessen, L.; Navarro, D.; Maunier, S.; Sigoillot, J.C.; Lorquin, J.; Delattre, M.; Simon, J.L.; Asther, M.; Labat, M. Simple Phenolic Content in Olive Oil Residues As a Function of Extraction Systems. Food Chemistry 2001, 75, 501–507.
- González, M.D.; Moreno, E.; Quevedo-Sarmiento, J.; Ramos-Cormenzana, A. Studies on Antibacterial Activity of Waste Waters from Olive Oil Mills (Alpechin): Inhibitory Activity of Phenolic And Fatty Acids. Chemosphere 1990, 20, 423–432.
- Riffaldi, R.; Levi-Minzi, R.; Saviozzi, A.; Vanni, G.; Scagnozzi, A. Effect of the Disposal of Sludge from Olive Processing on Some Soil Characteristics: Laboratory Experiments. Water, Air, and Soil Pollution 1993, 69, 257–264.
- Galli, C.; Visioli, F. Antioxidant and Other Activities of Phenolics in Olives/Olive Oil, Typical Components of the Mediterranean Diet. Lipids 1999, 34, S23–S26.
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose Toxicity in Beta-Cells: Type 2 Diabetes, Good Radicals Gone Bad, and the Glutathione Connection. Diabetes 2003, 52, 581–587.
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative Stress and the Use of Antioxidants in Diabetes: Linking Basic Science to Clinical Practice. Cardiovascular Diabetology 2005, 4, 5.
- Waris, G.; Ahsan, H. Reactive Oxygen Species: Role in the Development of Cancer and Various Chronic Conditions. Journal of Carcinogenesis 2006, 5, 14.
- Robertson, R.P.; Harmon, J.S. Pancreatic Islet Beta-Cell and Oxidative Stress: The Importance of Glutathione Peroxidase. FEBS Letters 2007, 581, 3743–3748.
- Chang, Y.C.; Chuang, L.M. The Role of Oxidative Stress in the Pathogenesis of Type 2 Diabetes: From Molecular Mechanism to Clinical Implication. American Journal of Translational Research 2010, 2, 316–331.
- Drews, G.; Krippeit-Drews, P.; Dufer, M. Oxidative Stress and Beta-Cell Dysfunction. Pflugers Archiv: European Journal of Physiology 2010, 460, 703–718.
- Ministerio de Salud. Reglamento Sanitario de los Alimentos (dto. 977/96 (d.Of. 13.05.97).
- Priego-Capote, F.; Ruiz-Jimenez, J.; de Castro, M.D. Fast Separation and Determination of Phenolic Compounds by Capillary Electrophoresis-Diode Array Detection. Application to the Characterisation of Alperujo After Ultrasound-Assisted Extraction. Journal of Chromatography. A 2004, 1045, 239–246.
- Singleton, V.L.; Joseph, A. Colorimetry of total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chemistry 1999, 64, 555–559.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Science and Technology 1995, 28, 25–30.
- Galle, J.; Wanner, C. Oxidized LDL and LP(A): Preparation, Modification, and Analysis: Methods in Molecular Biology. In Free Radical and Antioxidants Protocols; Armstrong, D.; Ed; Humana Press Inc.: Totowa, NJ, 1998; 108, 119–130.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. The Journal of Biological Chemistry 1951, 193, 265–275.
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous Monitoring of in Vitro Oxidation of Human Low Density Lipoprotein. Free Radical Research Communications 1989, 6, 67–75.
- de Azevedo, A.D.; Prisco, J.T.; Enéas-Filho, J.; Abreu, C.E.; Gomes-Filho, E. Effect of Salt Stress on Antioxidative Enzymes and Lipid Peroxidation in Leaves and Roots of Salt-Tolerant and Salt-Sensitive Maize Genotypes. Environmental and Experimental Botany 2006, 56, 87–94.
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant Activities, Total Phenolics, and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber Officinale Roscoe). Molecules 2010, 15, 4324–4333.
- Spiridon, I.; Bodirlau, R.; Teaca, C.-A. Total Phenolic Content and Antioxidant Activity of Plants Used in Traditional Romanian Herbal Medicine. Open Life Sciences 2011, 6, 388.
- Goldsmith, C.; Stathopoulos, C.; Golding, J.; Roach, P. Fate of the Phenolic Compounds During Olive Oil Production with the Traditional Press Method. International Food Research Journal 2014, 21, 101–109.
- Chtourou, M.; Gargouri, B.; Jaber, H.; Abdelhedi, R.; Bouaziz, M. Comparative Study of Olive Oil Quality from Chemlali Sfax Versus Arbequina Cultivated in Tunisia. European Journal of Lipid Science and Technology 2013, 115, 631–640.
- Uribe, E.; Lemus-Mondaca, R.; Pasten, A.; Astudillo, S.; Vega-Gálvez, A.; Puente-Díaz, L.; Di Scala, K. Dehydrated Olive-Waste Cake As a Source of High Value-Added Bioproduct: Drying Kinetics, Physicochemical Properties, and Bioactive Compounds. Chilean Journal of Agricultural Research 2014, 74, 293–301.
- Fernandez-Bolaños, J.; Rodriguez, G.; Rodriguez, R.; Heredia, A.; Guillen, R.; Jimenez, A. Production in Large Quantities of Highly Purified Hydroxytyrosol from Liquid-Solid Waste of Two-Phase Olive Oil Processing Or “Alperujo.” Journal of Agricultural and Food Chemistry 2002, 50, 6804–6811.
- Fernandez-Bolaños, J.; Lopez, O.; Fernandez-Bolaños, J.; Rodriguez-Gutierrez, G. Hydroxytyrosol and Derivatives: Isolation, Synthesis, and Biological Properties. Current Organic Chemistry 2008, 22, 442–463.
- Garcia-Gonzalez, D.L.; Romero, N.; Aparicio, R. Comparative Study of Virgin Olive Oil Quality from Single Varieties Cultivated in Chile and Spain. Journal of Agricultural and Food Chemistry 2010, 58, 12899–12905.
- Soleas, G.; Tomlinson, G.; Diamandis, E.; Goldberg, D. Relative Contributions of Polyphenolic Constituents to the Antioxidant Status of Wines: Development of a Predictive Model. Journal of Agricultural and Food Chemistry 1997, 45, 3995–4003.
- Aruoma, O.I.; Deiana, M.; Jenner, A.; Halliwell, B.; Kaur, H.; Banni, S.; Corongiu, F.P.; Dessí, M.A.; Aeschbach, R. Effect of Hydroxytyrosol Found in Extra Virgin Olive Oil on Oxidative DNA Damage and on Low-Density Lipoprotein Oxidation. Journal of Agricultural and Food Chemistry 1998, 46, 5181–5187.
- St-Laurent-Thibault, C.; Arseneault, M.; Longpre, F.; Ramassamy, C. Tyrosol and Hydroxytyrosol Two Main Components of Olive Oil, Protect N2a Cells Against Amyloid-β-Induced Toxicity. Involvement of the NF-κB Signaling. Current Alzheimer Research 2011, 8 (5), 543–551.
- Fuggetta, M.P.; Cottarelli, A.; Lanzilli, G.; Tricarico, M.; Bernini, R. In Vitro Antitumor Activity of Olive Oil Tyrosol and Hydroxytyrosol and their Methyl Carbonate Derivatives. Medicinal and Aromatic Plant Science and Biotechnology 2012, 6 (2), 25–30.
- Loru, D.; Incani, A.; Deiana, M.; Corona, G.; Atzeri, A.; Melis, M.P.; Rosa, A.; Dessì, M.A. et al. Protective Effect of Hydroxytyrosol and Tyrosol Against Oxidative Stress in Kidney Cells. Toxicology and Industrial Health 2009, 25(4–5), 301–310.
- Fernández-Bolaños, J.G.; López, Ó.; López-García, M.Á.; Marset, A. Biological Properties of Hydroxytyrosol and Its Derivatives. In Olive Oil - Constituents, Quality, Health Properties and Bioconversions; Boskou, D.; Ed.; InTech: Rijeka, Croatia, 2012; 375–396..
- Witztum, J.L.; Steinberg, D. Role of Oxidized Low Density Lipoprotein in Atherogenesis. The Journal of Clinical Investigation 1991, 88, 1785–1792.
- Li, D.; Mehta, J.L. Oxidized LDL, A Critical Factor in Atherogenesis. Cardiovascular Research 2005, 68, 353–354.