?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Coconut is grown in tropical and subtropical areas worldwide. The endosperm (water and meat) is consumed and processed in different forms. This study investigated the antioxidant activities and identified the phenolic compounds existing in the water and meat of coconut fruits at three different maturity stages, i.e., 180, 190, and 225 days after pollination from two planting areas in Thailand. Total phenolic content and antioxidant activity indices increased as the coconut matured from 180 to 190 days after pollination and then decreased or remained unchanged at 225 days after pollination. Catechin and salicylic acid were the major phenolic compounds found in the water, while gallic, caffeic, salicylic, and p-coumaric acids were found in the meat. The fat content of the meat increased significantly with maturity stage. Medium chain fatty acids profiles were also analyzed. The results are important for producers, processors, and consumers to realize an optimal quality and functionality of coconut water and meat when used for specific purposes.
INTRODUCTION
Coconut (Cocos nucifera Linn.) is produced worldwide, with a global production of about 56 million tons (MT) per year and is one of the important agricultural commodities in Thailand with an annual production of 1.1 MT.[Citation1] The aromatic coconut cv. Nam Hom is of the utmost importance to Thailand exportation. It is a favorite because of its pleasurable scent and sweet smell that comes from various fruit parts, such as the coconut water and meat.[Citation2] It is used at various maturity stages for different commercial purposes. Young coconut or green coconut with tender meat is usually consumed as fresh and processed into burnt aromatic coconut, coconut water, young coconut in jelly and coconut ice cream; while mature meat is used for an extraction of virgin coconut oil and a production of dehydrated coconut. Fresh coconut water is a traditional, refreshing drink in Southeast Asia and Latin American countries, and recent reports indicate that packaged ready-to-drink coconut water is gaining much interest from consumers in other countries as a natural drink for hydration[Citation3] even though scientific data for its effectiveness is scarce.[Citation4] The main composition, mineral contents and aromatic compounds of coconut water from several cultivars have been reviewed.[Citation5,Citation6] Normally, coconut water contains about 5–8% of total soluble solids (TSS), of which the majority are sugars (3–7%). Other minor components are amino acids and minerals. Recently, various health benefits of coconut water have been reported. Young coconut water (6 months), which contains estrogen-like compounds, might be used to prevent Alzheimer’s disease in menopausal women.[Citation7] Mature coconut water (12 months) also showed hypoglycemic effect and reduced oxidative stress in rats.[Citation8]
Phenolic compounds are important phytochemicals that exhibit several bioactive properties including antioxidant activity. Quantification and identification of phenolic compounds in different kinds of fruits and vegetables and their processed products have been reported in many studies. Also, the determination of total phenolic content (TPC) by Folin–Ciocalteu’s reagent was widely performed for preliminary screening of potential high antioxidant sources. The antioxidant activity of coconut water, however, has been reported by only a few studies.[Citation9,Citation10] Identification of phenolic containing compounds was only reported.[Citation11] For coconut meat, fat components are of interest for food scientists and industries. It is well-known that triacylglycerols present in coconut contain mainly lauric and other medium chain fatty acids (MCFA).[Citation12] These medium chain triacylglycerols (MCT) show a potential use as a functional food ingredient.[Citation13,Citation14]
To date, the TPC, antioxidant activities, and MCFA profiles of coconut water and meat have been under-reported in literature, especially with respect to different maturity stages. This information can be useful for selecting appropriate harvesting period of coconut crops for specific purposes. Therefore, the present study provides results focused on these analyses for a well-known aromatic coconut crop variety “Nam Hom” grown in Thailand.
MATERIALS AND METHODS
Chemicals and Reagents
Gallic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), Folin–Ciocalteu’s reagent, sodium methoxide, standard fatty acid methyl esters (FAMEs; caprylate, caprate, laurate, myristate, and palmitate), standard phenolic compounds (salicylic, 4-hydroxybenzoic, syringic, m-coumaric, p-coumaric, gallic and caffeic acids, and catechin), N,O-bis(trimethylsilyl) acetamide (BSA), trimethylchlorosilane (TMCS), and pyridine were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC) grade methanol and isopropanol were purchased from Merck (Darmstadt, Germany). Chloroform was purchased from RCI Labscan (Bangkok, Thailand). Acetic acid and hexane were purchased from Mallinckrodt Baker (Phillipsburg, NJ, USA). Sodium carbonate, potassium persulfate, and potassium chloride were obtained from Ajax Chemicals (NSW, Australia).
Sample Preparation
Thai aromatic coconuts cv. Nam Hom of three different maturity stages, i.e., 180, 190, and 225 days after pollination (DAP), were purchased from the same areas and same local producers in two major planting areas in Thailand, i.e., Ban Phaeo, Samut Sakhon province (13° 35′ 26″ N, 100° 6′ 28″ E) and Damnoen Saduak, Ratchaburi province (13° 31′ 6″ N, 99° 57′ 18″ E). Those three selected maturity stages are covered the harvesting maturity for commercial uses of aromatic coconut (approximately 6–7 months). The coconuts at 180 DAP contained jelly-like meat with a transparent characteristic and a thin layer on the soft eye, whereas at 190 DAP, the meat of the whole fruit became rather thick, more tender with light cream color, whereas the end near the stem has a small portion of transparent pulp which corresponds to the most preferable stage for fresh consumption. The last maturity stage of aromatic coconut used in this study was 225 DAP, at which point the meat was thick and hard without transparent pulp. The two selected planting areas are similar in type of soil (sandy loam), irrigation method (furrow irrigation), and amount of rainfall. The distance between the two areas are about 34 km.
Thirty fruits from each planting area and maturity stage were harvested in October 2008, January 2009, and July 2009. For each lot, all coconuts were manually dehusked after which the coconut water and meat were separated. The coconut water and meat from 30 fruits were pooled and stored at –18°C for subsequent analyses. Chemical properties of the water were analyzed using pH meter (PHM 210, Radiometer Analytical, Villeurbanne Cedex, France) for pH, hand refractometer (2110-W06, Atago, Tokyo, Japan) for TSS, and titration with 0.1 N NaOH for titratable acidity. The meat thickness was measured by a vernier calliper. Moisture, crude protein, crude fat, crude fiber, and ash contents of the meat were determined using hot air oven drying, Kjeldahl method, Soxhlet method, acid-base hydrolysis, and dry ashing, respectively according to the AOAC International methods.[Citation15]
TPC, Antioxidant Activities, and Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
Extraction
Extraction of the phenolic compounds from the coconut meat was performed according to Maisuthisakul et al.[Citation16] with some modifications. Thawed coconut meat (20 g) was ground in a food processor. The ground sample was combined with methanol (100 mL) and the mixture was shaken at room temperature for 3 h using an orbital shaker at 90 rpm. The mixture was filtered through Whatman no. 4 paper and the liquid extract was evaporated under vacuum at 50°C using a rotary evaporator (Rotavapor R–114, Buchi, Switzerland). The concentrated extract was volumetrically adjusted with methanol to 10 mL in a volumetric flask for further analyses. Similarly for coconut water, the liquid was thawed and was mixed with methanol (1:5 v/v) and the extraction steps were followed as previously described. All extractions were performed in triplicate.
TPC determination
The methanolic extracts of coconut meat and water were determined for TPC using Folin–Ciocalteu’s reagent.[Citation17] The extract (0.2 mL) was mixed with 10% v/v Folin–Ciocalteu’s reagent (1 mL) and allowed to react for 3 min. Sodium carbonate (7.5 % w/v, 0.8 mL) was added to the mixture and allowed to react at room temperature for 2 h. The absorbance at 765 nm was measured using a spectrophotometer (Genesys 20, Spectronic, USA). Gallic acid was used for standard calibration and TPC were expressed as mg gallic acid equivalents (GAE) per 100 g fresh meat or 100 mL water.
DPPH assay
DPPH assay was performed as described by Brand-Williams et al.[Citation18] Diluted extract (0.1 mL) was mixed with 6 × 10–5 M DPPH in methanol (3.9 mL). The mixture was kept at room temperature in the dark for 2 h. Absorbance was measured at 515 nm and the DPPH radical scavenging activity was calculated as:
where As and A0 are the absorbance of DPPH solutions with and without the sample, respectively.
ABTS assay
ABTS assay was performed according to Re et al.[Citation19] The ABTS reagent (7.0 mM) was mixed with potassium persulfate (2.45 mM) in the ratio of 2:1 v/v and kept in the dark at room temperature for 12 h. Before the analysis, the reagent was diluted with 95% ethanol to adjust the absorbance at 734 nm to 0.7 ± 0.02. The ABTS•+ reagent (4 mL) and the extract (40 µL) were mixed. Absorbance at 734 nm was recorded after 6 min. Trolox solution (1 mM) was used for calculating the Trolox equivalent antioxidant capacity (TEAC) from:
GC–MS Analysis of Phenolic Compounds
Phenolic compounds were converted to trimethylsilyl derivatives with BSA for GC–MS analysis.[Citation20] Five milliliters of the methanolic extract was mixed with 50 μL of pyridine at 60°C for 10 min. The mixture was combined with BSA (500 μL) and TCMS (200 μL) and kept for another 60 min. GC–MS analysis was performed using a HP 6890 GC with a HP 5973 MS (Agilent, Santa Clara, CA, USA). Separation was accomplished on HP5 column (30 m × 0.25 mm i.d., 0.25 μm film thickness) using helium at 1.5 mL/min as the carrier gas. The injector temperature was set at 240°C, whereas column temperature was 90°C for 1 min, then increased to 240 at 20°C/min, kept constant for 10 min, then increased to 280 at 20°C/min and finally kept constant for 5 min. Samples (1 μL) were injected using a splitless mode. Authentic standards of phenolic compounds were used for peak identification.
MCFA Profile of Coconut Meat
Oil extraction and purification
Coconut oil extraction was conducted using the modified Folch procedure as described by Christie.[Citation21] Coconut meat (10 g) was dissolved with chloroform-methanol (2:1 v/v) in a continuous shaker at 90 rpm for 12 h. The coconut residue was separated by filtration through Whatman no. 4 paper and the solvent was then removed using a rotary evaporator under vacuum at 50°C. The oil residue at this point was considered as crude oil.
The coconut crude oil was purified by the procedure of Christie.[Citation21] Crude oil was mixed with chloroform-methanol (2:1 v/v, 30 mL) and shaken continuously for 3 min. The mixture was washed with 8 mL of potassium chloride solution (0.88% w/v) in a measuring cylinder. The mixture was shaken thoroughly before allowing to settle. The upper aqueous layer was drawn off by pipette. Methanol-potassium chloride solution (1:1 v/v, 10 mL) was added twice to the lower layer for washing. The mixture was filtered before the solvent was removed using a rotary evaporator. The purified oil was stored at –18°C for fatty acids methylation.
Fatty acids methylation
The purified oil (10 mg) was dissolved with hexane (5 mL) in a capped test tube and sodium methoxide in methanol (0.5 M, 0.1 mL) was added. The mixture was shaken for 5 min at room temperature. Glacial acetic acid (10 µL) and anhydrous calcium chloride (2 g) were added to the mixture. After 1 h, the mixture was centrifuged at 5000 rpm for 5 min to precipitate the drying agent. The supernatant was taken for subsequent GC analysis.
Gas chromatography-flame ionization detector (GC-FID)
The FAMEs were analyzed by a Shimadzu GC 2010 (Kyoto, Japan) equipped with a flame ionization detector (FID). The esters were chromatographically separated in an Alltech AT-WAX capillary column (50 m × 0.25 mm i.d., 0.2 µm film thickness; Grace, Deerfield, IL, USA). The oven temperature was kept at 120°C for 3 min, followed by an increase at 5°C/min to 210°C that was held for 15 min. Helium was used as the carrier gas at a flow rate of 0.4 mL/min. The injector and detector temperatures were maintained at 210°C. Ten microliters of sample was injected in a split mode (1:10). A comparison of the retention time of FAMEs with authentic standards was made to facilitate identification.
Statistical Analyses
To evaluate effects of maturity stage and origin of coconut, analysis of variance (ANOVA) for 3 × 2 factorial in randomized complete block design (RCBD) using three different harvesting dates of samples as blocks, followed by the least significant difference (LSD) test were performed using PASW Statistics 18 (IBM, NY, USA). Simple main effects were analyzed if significant interaction between the two factors was observed.[Citation22]
RESULTS AND DISCUSSION
Chemical Compositions and Physical Properties
Some basic chemical compositions and physical properties of coconut water and meat from the two planting areas are shown in . TSS and TA of the coconut water slightly decreased at 225 DAP. These were in agreement with previously reported values in literature.[Citation23] For the coconut meat, thickness and fat content increased significantly with the maturity stages. Data in are important for selection the proper maturity for different commercial coconut utilizations. For example, coconut water is usually consumed as natural beverage, therefore, the coconut water at 190 DAP would give moderate acidity and adequate sweetness while the meat from 225 DAP is suitable for producing coconut virgin oil due to the considerably higher amount of fat compared with other maturities.
TABLE 1 Chemical and physical properties of coconut water and meat at different maturity stages (n = 3)
TPC and Antioxidant Activity
TPC and two antioxidant activity indices (DPPH and ABTS) for coconut water and meat are shown in . Aromatic coconuts from these two important planting areas of Thailand showed no significant differences in antioxidant activities (p > 0.05), while significant differences could be found for the TPC depending on the maturity, both in the water and meat. Overall, the coconut meat contained higher TPC and antioxidant activities wet basis than the water. Leong and Shui[Citation10] also reported that the ABTS radical scavenging activity (per g fresh weight [FW]) of meat was about four times higher than that of water for coconut samples purchased from local market in Malaysia. In this study, it was found that during the maturation of fruit, TPC significantly increased at 190 DAP and then slightly decreased at 225 DAP. The TPC values were in ranges of 5.18–7.17 mg GAE/100 mL and 6.28–10.01 mg GAE/100 g for the water and meat, respectively. These values were in agreement with data from a recent report,[Citation23] even though they were relatively low compared with those from other well-known high phenolic content fruit juices, for example, pomegranate (400–1600 mg GAE/100 mL),[Citation24] apple (70 mg GAE/100 g),[Citation25] and white and red grapes (25.4–38.9 and 140.7 to 224.6 mg GAE/100 mL).[Citation26]
TABLE 2 Total phenolic content and antioxidant capacity as measured by DPPH and ABTS assays (n = 3)
Both free radical scavenging activity indices increased until 190 DAP and remained unchanged at 225 DAP. The TEAC values were in ranges of 2.98–4.55 µM TE/mL and 4.00–7.69 µM TE/g FW for the water and meat, respectively. These values were also lower than those of some other fruits, for example, blueberry fruits (~30 µM TE/g), and juice (~10 µM TE/mL).[Citation27] Leong and Shui[Citation10] reported that the antioxidant activities of coconut water and meat were classified as “low” based on ABTS values.
Phenolic Compounds
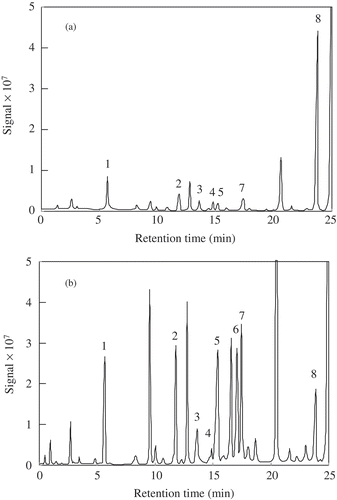
Identification of phenolic compounds in coconut water and meat is presented in . Based on the chromatographic peaks of eight identified compounds, the phenolic components found in the water samples were catechin and salicylic acid, while gallic, caffeic, salicylic, and p-coumaric acids were the main phenolic compounds found in the meat (). Overall, the amounts of the phenolic compounds found in the water were less than in those of the meat, which was in agreement with the TPC and antioxidant activities described earlier. Identification of phenolic compound in coconut water was scarcely reported. The presence of (+)-catechin and (−)-epicatechin in coconut water with concentrations of 0.344 and 0.242 μg/mL, respectively, was recently reported by Chang and Wu.[Citation11] Similar phenolic compounds were identified from a methanolic extract of coconut oil. Seneviratne et al.[Citation28] found only gallic, syringic acids, and (−)-epigallocatechin in coconut oil extracted under cold conditions, while gallic, caffeic, syringic, p-hydroxybenzoic, ferulic acids, (−)-epigallocatechin, (+)-epicatechin, and (+)-catechin were found in coconut oil extracted under heated conditions. Gallic and caffeic acids, as well as catechin, are phenolic compounds with relatively high antioxidant properties[Citation29] found in many fruits and vegetables. These compounds should contribute considerably to the antioxidant activities of coconut water and meat. Other major antioxidants in coconut water and meat were not reported in literature. Leong and Shui[Citation10] reported that only small amount of ascorbic acid was found in coconut water and meat (0.7 and 0.9 mg/100 g, respectively). Salicylic acid was also detected in the water. It is considered as a phytohormone found in coconut water.[Citation30]
Fat Content and MCFA Profile of Coconut Meat
Because the water and meat are part of the endosperm tissues of the fruit, accumulation of the fat during maturation of coconut is expected. The crude fat contents of coconut meat increased with the maturity as shown in . Since the fat content in coconut water is generally low, no attempt was made to analyze the value in this study. However, increase in fat content in coconut water was reported by Jackson et al.[Citation31] for some varieties in Jamaica, which were in the range of 1–2% during maturity stages of 7–10 months.
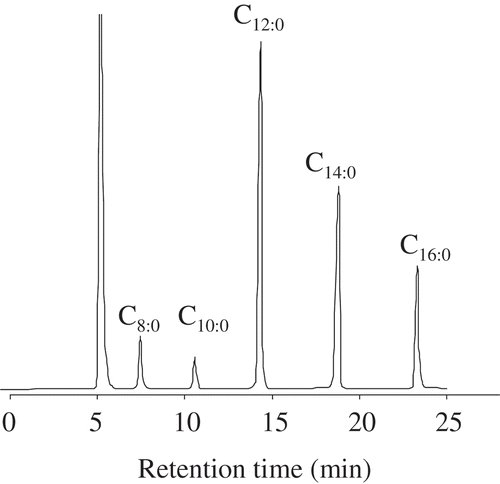
Even though there is some disagreement about the consumption of coconut oil due to its high content of saturated fatty acids, several recent reports on the health beneficial of this oil, specifically virgin coconut oil, have been published in the last decade.[Citation13] In addition, it should be noted that coconut meat and coconut milk are used in many tradition cuisines for people in South and Southeast Asia. Lately, MCT as functional ingredients have received more interest from nutritionists. A typical GC-FID chromatogram of FAMEs found in coconut meat is shown in . The major fatty acids in coconut were lauric, myristic, and palmitic acids (). The percentages of these fatty acids were in agreement with those present in extracted coconut oil.[Citation13] The content of all fatty acids also increased in later maturity stages.
TABLE 3 Phenolic compounds of coconut meat and water at different maturity stages from Samut Sakhon (n = 3)
TABLE 4 Medium-chain fatty acids (MCFAs) contents of coconut meat at different maturity stages (n = 3)
CONCLUSIONS
This study reported several chemical changes during the maturation of coconut which should be important for producers, manufacturers, and consumers to select an appropriate quality and functionality of coconut water and meat for specific purposes.
FUNDING
This research was supported by the Research and Development Institute, Silpakorn University, Thailand (SURDI 53/02/03). Busarakorn Mahayothee gratefully acknowledges the Invitation Professorship from the Food Security Center, Universität Hohenheim, Germany supported by German Academic Exchange Service (DAAD) and the Federal Ministry for Economic Cooperation and Development (BMZ).
Additional information
Funding
REFERENCES
- FAO. Food and Agricultural Commodities Production; FAO: Rome, 2012.
- Jangchud, K.; Puchakawimol, P.; Jangchud, A. Quality Changes of Burnt Aromatic Coconut During 28-Day Storage in Different Packages. LWT–Food Science and Technology 2007, 40, 1232–1239.
- Prades, A.; Dornier, M.; Diop, N.; Pain, J.-P. Coconut Water Preservation and Processing: A Review. Fruits 2012, 67, 157–171.
- Saat, M.; Singh, R.; Sirisinghe, R.G.; Nawawi, M. Rehydration After Exercise with Fresh Young Coconut Water, Carbohydrate-Electrolyte Beverage, and Plain Water. Journal of Physiological Anthropology Human Science 2002, 21, 93–104.
- Prades, A.; Dornier, M.; Diop, N.; Pain, J.-P. Coconut Water Uses, Composition, and Properties: A Review. Fruits 2012, 67, 87–107.
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos Nucifera L.) Water. Molecules 2009, 14, 5144–5164.
- Radenahmad, N.; Saleh, F.; Sawangjaroen, K.; Vongvatcharanon, U.; Subhadhirasakul, P.; Rundorn, W.; Withyachumnarnkul, B.; Connor, J.R. Young Coconut Juice, a Potential Therapeutic Agent That Could Significantly Reduce Some Pathologies Associated with Alzheimer’s Disease: Novel Findings. British Journal of Nutrition 2011, 105, 738–746.
- Preetha, P.P.; Devi, V.G.; Rajamohan, T. Hypoglycemic and Antioxidant Potential of Coconut Water in Experimental Diabetes. Food & Function 2012, 3, 753–757.
- Mantena, S.K.; Jagadish; Badduri, S.R.; Siripurapu, K.B.; Unnikrishnan, M.K. In Vitro Evaluation of Antioxidant Properties of Cocos Nucifera, Linn. Water. Food/Nahrung 2003, 47, 126–131.
- Leong, L.P.; Shui, G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chemistry 2002, 76, 69–75.
- Chang, C.-L.; Wu, R.-T. Quantification of (+)-Catechin and (-)-Epicatechin in Coconut Water by LC-MS. Food Chemistry 2011, 126, 710–717.
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. Journal of the American Oil Chemists’ Society 2009, 86, 301–307.
- Marina, A.M.; Che Man, Y.B.; Amin, I. Virgin Coconut Oil: Emerging Functional Food Oil. Trends in Food Science & Technology 2009, 20, 481–487.
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-Chain Triglycerides. International Dairy Journal 2006, 16, 1374–1382.
- AOAC. Official Methods of Analysis of AOAC International, 17th Ed.; AOAC International: Rockville, MD, 2000.
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of Phenolic Content and Free Radical-Scavenging Capacity of Some Thai Indigenous Plants. Food Chemistry 2007, 100, 1409–1418.
- Zadernowski, R.; Czaplicki, S.; Naczk, M. Phenolic acid Profiles of Mangosteen Fruits (Garcinia Mangostana). Food Chemistry 2009, 112, 685–689.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensmittel Wissenschaft und Technologie 1995, 28, 25–30.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying An Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic Acid Profiles in Some Small Berries. Journal of Agricultural and Food Chemistry 2005, 53, 2118–2124.
- Christie, W.W. Gas Chromatography and Lipids: A Practical Guide; The Oily Press: Bridgewater, UK, 1989; 320.
- Kuehl, R.O. Design of Experiment: Statistical Principles of Research Design and Analysis, 2nd Ed.; Duxbury Press: Pacific Grove, CA, 2000; 666.
- Tan, T.-C.; Cheng, L.-H.; Bhat, R.; Rusul, G.; Easa, A.M. Composition, Physicochemical Properties, and Thermal Inactivation Kinetics of Polyphenol Oxidase and Peroxidase from Coconut (Cocos nucifera) Water Obtained from Immature, Mature, and Overly Mature Coconut. Food Chemistry 2014, 142, 121–128.
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Thermal Stability of Anthocyanins and Colourless Phenolics in Pomegranate (Punica granatum L.) Juices and Model Solutions. Food Chemistry 2013, 138, 1800–1809.
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication As a Potential Quality Enhancement Technique of Apple Juice. Ultrasonics Sonochemistry 2014, 21, 984–990.
- Bosanek, C.A.; Silliman, K.; Kirk, L.L.; Frankel, E.N. Total Phenolic Content and Antioxidant Potential of Commercial Grape Juice. Journal of the American Dietetic Association 1996, 96, A35.
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flรดres, S.H.; Rios, A.D.O.; de Jong, E.V. Cold Storage of Blueberry (Vaccinium spp.) Fruits and Juice: Anthocyanin Stability and Antioxidant Activity. Journal of Food Composition and Analysis 2014, 33, 111–116.
- Seneviratne, K.N.; Hapuarachchi, C.D.; Ekanayake, S. Comparison of the Phenolic-Dependent Antioxidant Properties of Coconut Oil Extracted Under Cold and Hot Conditions. Food Chemistry 2009, 114, 1444–1449.
- Khuwijitjaru, P.; Plernjit, J.; Suaylam, B.; Samuhaseneetoo, S.; Pongsawatmanit, R.; Adachi, S. Stability of Some Phenolic Compounds in Subcritical Water and Radical Scavenging Activity of Their Degradation Products. The Canadian Journal of Chemical Engineering 2014, 92, 810–815.
- Wu, Y.; Hu, B. Simultaneous Determination of Several Phytohormones in Natural Coconut Juice by Hollow Fiber-Based Liquid-Liquid-Liquid Microextraction-High Performance Liquid Chromatography. Journal of Chromatography A 2009, 1216, 7657–7663.
- Jackson, J.C.; Gordon, A.; Wizzard, G.; McCook, K.; Rolle, R. Changes in Chemical Composition of Coconut (Cocos Nucifera) Water During Maturation of the Fruit. Journal of the Science of Food and Agriculture 2004, 84, 1049–1052.