ABSTRACT
The aim of this study was to examine the antioxidant activity of pennyroyal (Mentha piperita) extracts of sunflower oil as a replacement for synthetic antioxidant. This study was performed in two stages. In the current study, it was proven that the examined pennyroyal was in all ways similar to Mentha piperita sequence and its blast performance at the National Center for Biotechnology Information (NCBI). The anti-oxidation activities of Mentha piperita extracts were identified using 2,2-diphenyl-1-picrylhydrazyl and total phenolic content. The protective effect of extracts on the stabilization of sunflower oil was tested and compared with tertiary butyl hydroquinone by measuring peroxide values (y1), p-anisidine (y2), and rancimat value (y3) during storage for 4 weeks at 60°C (Schaal test).The results showed that the antioxidant activity of Mentha piperita extracts (500 ppm) was equal with tertiary butyl hydroquinone effect (100 ppm) at 60°C. In the next step, the sunflower oil containing Mentha piperita extracts (500 ppm) and tertiary butyl hydroquinone (100 ppm) as two blocks were tested to determinate the optimum condition of both high temperature (x1:180–220°C) and time (x2: 5–15 min) variables on oxidative stability. The optimum conditions were optimized using response surface methodology. The analysis of variance result showed that temperature and time had significant effects on peroxide, p-anisidine, and rancimat values (p < 0.01). The optimal condition for the examined parameters was related to a sunflower oil treatment containing Mentha piperita extracts (500 ppm) at 180°C and 5 min (with the desirability of 92.1%).
Introduction
In recent years, a great deal of research has been devoted to the replacement of chemical materials with natural ones to eliminate or reduce chemical and synthetic constituents in food. Hence, many efforts have been made to find natural antioxidants from herbal sources. Lipid oxidation during food storage and processing not only causes the loss of nutritional and digestive quality of food, it also releases oxidized products such as free radicals. Free radicals can cause many diseases, especially cancer, in biological systems as well as the reduction of sensory indexes of food.[Citation1] Antioxidants are constituents that effectively prevent lipids oxidation.[Citation2,Citation3] Currently, synthetic antioxidants (tertiary butyl hydroquinone [TBHQ]) are used for deferring lipid oxidation in industry, but due to the bad nutritional and carcinogenic effects of these constituents, as well as consumers’ tendency toward using natural compounds, use of natural antioxidants has received far more attention.[Citation4] One of the best sources of natural antioxidants is the phenolic constituents found in herbal samples.[Citation5] The Labiatae family includes about 220 different kinds and 3,300 species which are widely used for various purposes.[Citation6] Plants belonging to Labiatae are rich in polyphenolic constituents, and many of them are known for their antioxidant qualities.[Citation7] Mentha is a member of this family and a flora plant of Iran which contains six species.[Citation8] The species of Mentha are generally known as “na’na” and “pooneh” in Iran, and are mainly used as herbal tea, flavoring, and herbs.[Citation9] Several methods have been introduced for examining lipid oxidation so far, but no test is useful for all the oxidation stages and none of them is useful for fat and food by themselves. At best, a test can control and determine only one or few of physical changes in oxidation. Therefore, it is best to apply a combination of several methods to evaluate oxidation. The aim of this study was to evaluate the antioxidation effect of pennyroyal (Mentha Peperita) extract on sunflower oil (SFO) oxidation at low and high temperatures.
Materials and Methods
Plant Material
Pennyroyal, the plant being used in this experiment, was collected in spring during blossoming. It came from Khoy, West Azerbaijan and the genomic DNA was extracted. The primers were designed by Oligo 7 software to amplify ribulose-1, 5-bisphosphate carboxylase/oxygenase large subunit (rbcL gene). The primer sequence was: MRbsL: 5’-GTG TTA AAG AGT ACA AAT TGAC-3’MRbsLR: 5’-AG CAT TCA AGT AA T GCC CTT TG -3’. PCR reactions were performed in an automatic thermal cycler (Bio-Rad, USA) under the following conditions: Initial denaturation at 94°C for 5 min, 35 cycles of 94°C for 60 s, 53°C for 60 s, and 72°C for 90 s, and final extension at 72°C for 10 min and held at 4°C. Polymerase chain reaction (PCR) production was sequenced at Macrogene, Korea and blasted in the National Center for Biotechnology Information (NCBI) and then the species of Mentha piperita was identified.[Citation10]
SFO and Chemicals
The decolorized and refined SFO, which was completely free from antioxidant was obtained from the oil extraction factory of Piranshar city. According to the information from the factory, this oil contains 62.3% linoleic acid (18: 2 n-6) and 24.74% oleic acid (18: n-90). All reagents used in this study were analytical grade and were mostly obtained from Merck (Darmstadt, Germany).
Preparing of Methanol Extract
The leaves of Mentha were separated and dried in shadow under natural conditions. The extracts were obtained by maceration of dry plant material (100 g) in 400 mL of methanol for 24 h in a dark place at room temperature. The macerates were filtered under a vacuum through a Buckner funnel with filter paper (Whatman #4), while the methanol extract was dried in a rotary evaporator (IKA RV10DS99). The extract was smoothed by dimethyl sulfoxide (DMSO; 10 mL; 100%) and kept in a glass bottle at 4°C for later examination.[Citation11]
Measuring Total Phenolic Content of the Extract
The total amount of phenolic constituents in pennyroyal extract was examined through colorimetric method using a Folin–Ciocalteu reagent. Measurements were carried out in triplicate and calculated based on a calibration curve of gallic acid. The contents of total phenols were expressed as µg gallic acid equivalent (GAE).g–Citation1dry weight (DW).[Citation12]
Free Radical-Scavenging Activity (DPPH)
The examined extract’s potential to combine with hydrogen or electron donation was tested by its potential for decolorizing 2,2-diphenyl-1-picrylhydrazyl solution (DPPH), a stable free radical that is neutralized in the presence of antioxidants. This solution changes to dark purple in ethanol, but if this radical is neutralized, the color intensity reduces and changes to light yellow. As a result, the reduction of light absorbance will be relative to the antioxidant potential of the sample. For performing the examination, 600 µL of DPPH were dispensed into five test tubes; then 20, 40, 60, 80, and 100 mL of the extract were added to the respective test-tubes, and the volume of all the tubes was increased to six using methanol. The sixth test tube was left as a blank. All samples were read after being kept at room temperature for 15 min at 517 nm.[Citation11] The scavenging stages were computed by below formula:
Antioxidative Activities of the Extract in SFO Emulsion
The methanol extract of Mentha piperita (100, 500, and 1000 ppm) and TBHQ (100 ppm) were added to the refined SFO as shown in . Oil samples were placed in oven at 60°C for a given time (28 days; Schaal test). The stability of the oil samples was evaluated by a rancimat test, peroxide value and p-anisidine values at the end of every 7 days. In the second stage, based on the results of Schaal test, the best natural antioxidant was selected from among the other ones and a synthetic antioxidant (TBHQ) with acceptable concentration (100 ppm) was added to the SFO. The effect of the temperature (X1) and the time (X2) were also examined. The tests of peroxide, p-anisidine, and rancimat values were measured for high temperature.
Table 1. The mixture used for examining anti-oxidative treatments.
Oil Stability Analysis
The peroxide value was measured in line with Shantha et al. and the p-anisidine value was determined according to Smouse’s study.[Citation13,Citation14] The stability of oils and fats against oxidation was determined by the rancimat index. The Metrohm rancimat instrument was used to perform the experiment.[Citation15]
Statistical Analysis
In this study, in the first stage of research (low temperature), the statistical analysis of data obtained from the Schaal test was performed as a factorial experiment on the basis of a completely randomized design by SPSS version 20. Duncan’s multiple range (p ≤ 0.05) was then used for the mean comparison and all the graphs were drawn by EXCEL2007. In the second stage of research, response surface methodology (RSM) with a central composite design (CCD) was employed to optimize the best combination of antioxidant in two blocks to produce SFO in high temperature. The independent variables were temperature (X1) and time (X2).The range and central point value of the variables are shown in . After the experiment was completed, the model was fitted to the data.
Table 2. Independent variables and their coded and actual values used for optimization.
Design-Expert software (version 7) was used to analyze data. Three-dimensional surface plots of the independent variables were drawn to illustrate the best combination based on the regression analysis.[Citation16,Citation17]
Results and Discussions
Pennyroyal Sequence and Blast by PCR
The examined Pennyroyal sequence and blast was 100% similar to the Mentha piperita recorded at NCBI in terms of molecular taxonomy with a large subset gene of Rubisco enzyme. The result of PCR is shown in . PCR was performed and after that the quality of extracted DNA was confirmed by primer design for amplifying gene span sequence ITS1-5.8S-ITS2. Finally, after purification, the product of PCR was sent to Macrogene in Korea, to be sequenced. Analysis of the sequence obtained was performed by its comparison with recorded sequences of the given genome span of Mentha at NCBI. Primer was designed based on the gene sequence of a large subset of Rubisco enzyme by Oligo7.[Citation18]
Phenolic Extraction Constituents and Antioxidant Capacity
Antioxidant activities in plant extracts are related to their phenolic contents, which change depending on genetic and environmental factors, as well as on post-harvest processing conditions, so it is necessary to determine the antioxidant capacity of phenolics. In this study, phenolic contents in the Mentha piperita extracts ranged from 87.127 to 100.01 μg GAE/g and the antioxidant capacity shown by IC50 was 0.303 μg /mL. This result was consistent with the results obtained by Kamkar et al.[Citation19] Furthermore, the study by Luximun-Ramma et al.[Citation20] showed a linear correlation between antioxidant capacity and the phenolic contents of extracts of plant, fruits, and beverages. Sugihara et al.[Citation21] and Spencer[Citation22] also suggested that flavonoids have the potential to scavenge hydroxyl radicals, superoxide anions, and lipid peroxyl radicals.
Antioxidant Activity in Oil Under Storage Conditions
Qualitative parameters of the oil treated with natural and synthetic antioxidants during 28 days of warming up at 60°C
SFO with more than 86% unsaturated fatty acids was exposed so that it was combined with oxygen. The results of the measured parameters for the stability of the oil at 60°C showed that there was a significant difference between treatments within 4 weeks of incubation (p < 0.01).
Peroxide value
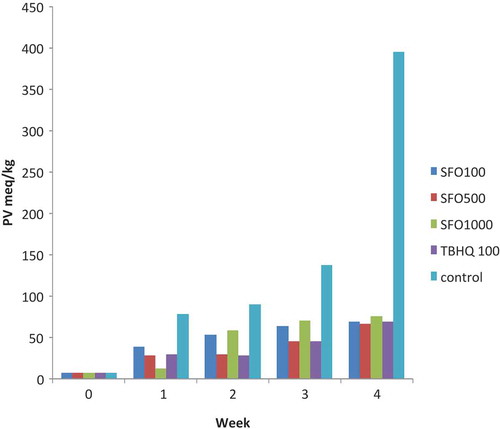
As shown in , during 4 weeks of keeping the oil at 60°C, the highest level of change of peroxide value was related to the control sample, where the rate of increase was slower in the first week but sharpened in the following weeks. In the first week of experiment, among treated samples, the lowest level of peroxide was related to the sample of SFO1000. In the following weeks, the increase in peroxide value (SFO1000) can be related to poeroxidation characteristics being produced in a high concentration of anti-oxidant.[Citation23,Citation24] Nevertheless, in the following weeks, treatments of T100 and SFO500 showed a greater effect on deferring the oxidation rather than other treatments, both of whose influence was almost the same.
In similar studies, the antioxidant effect of hydrous, ethanol, and methanol extracts of Iranian Mentha pulegium was examined on soybean oil in different concentrations (0, 200, 400, and 800 ppm). Results of a Schaal test showed that the peroxide value of the extracts was lower in comparison to the control sample and an extract of Mentha pulegium was comparable to the synthetic antioxidant of BHT extract (0.02 ppm) after 35 days at 65°C.[Citation25] Moreover, in another study, the protective effects of Eriobotrya japonica (Lindl) fruit peel extracts were tested in stabilization of soybean oil and compared to TBHQ by measuring the peroxide values during storage (65 days at 25°C). Results revealed that the fruit peel extract (400 ppm) showed stronger antioxidant activity, but the highest effect was observed in TBHQ.[Citation26] It can, therefore, be suggested that the effects of SFO500 and TBHQ100 in our study were similar to the above studies in the stabilization of antioxidant.
Para-anisidine value
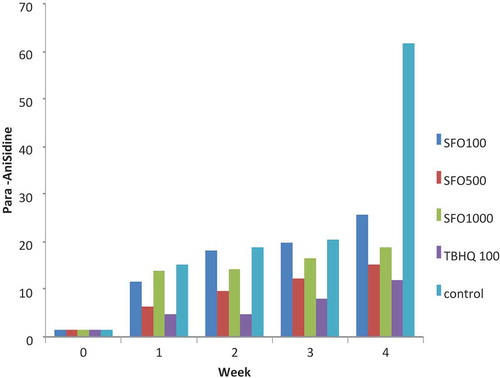
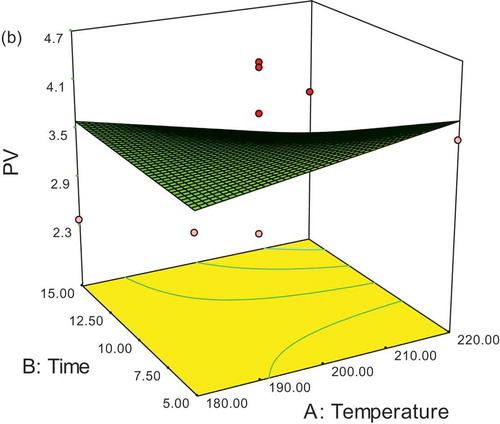
As shown in , the longer the storage time (28 days at 65°C) of the samples in oxidation conditions, the higher the values of p-anisidine in all examined treatments. The highest anisidine value was related to the control sample (oil without antioxidant), and the lowest p-anisidine was related to TBHQ in the weeks that the experiment was conducted.
Rancimat Analysis
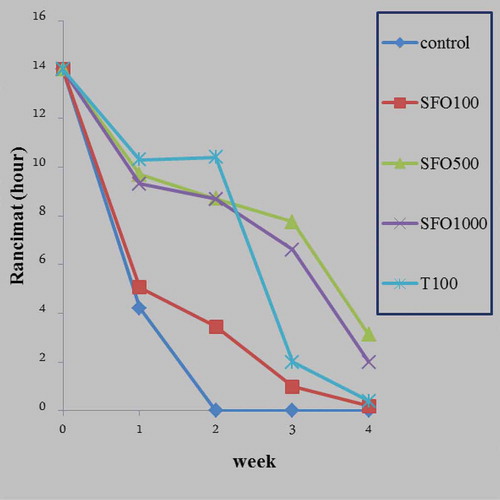
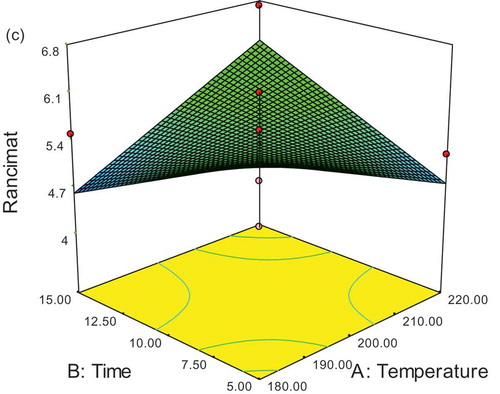
As shown in , the rancimat analysis was performed at 110°C and the induction period (h) was examined for SFOs both with and without Mentha extracts (100, 500, and 1000 ppm) and TBHQ (100 ppm). In all examined treatments, the longer the storage time of samples, the lower the rancimat value. A treatment containing synthetic antioxidant had the highest resistance in comparison with other treatments by the end of the second week. The control sample had also lost all its resistance by the end of the second week. The extract of Mentha Peperita (500 ppm) had 3 h of oxidative stability at the end of 4 weeks storage at 60°C. However, the other samples had no resistance at the end of 4 weeks.
As mentioned previously, and in accordance with to various stability indicators, it can be concluded that a the extract of Mentha Peperita (500 ppm) acted equal to synthetic TBHQ (100 ppm) in stabilizing SFO during the storage period, and was even more effective than synthetic antioxidant and other concentrations at the end of the final week. The reason for this can be related to both the high and suitable quantities of existing phenolic constituents in the extract (500 ppm) and thymol and carvacrol as a monoterpenoid phenol.[Citation27]
Response Surface Optimization of SFO Data Containing SFO500 and TBHQ100
In the present study, the relationship between the responses and the variables were identified by using a CCD in two blocks. Further, the heating conditions of the antioxidant activities were optimized. The data for each block, containing 13 runs, based on the experiment design, are shown in . The lowest peroxide and anisidine value and the highest rancimal value of each block is highlighted in . Based on these responses, these data were analyzed and they were optimized to obtain the fitted model.
Table 3. Central composite design with experimental and predicted values for peroxide, anisidine, and racimat value.
Models
In , a linear model was fitted for any responses. The results of analysis of variance (ANOVA) showed that the model was significant for all variables except for ranciment (SFO500). The significant contribution of each coefficient was determined by p-value of F-test (p < 0.05). The fitness of the model was generally assessed based on lack-of-fit test (p > 0.05). The quality of the fit model can be determined based on the coefficient of determination (RCitation2).
Table 4. Results of ANOVA and sunflower oil modeling containing antioxidant in peroxide, anisidine, and rancimat value.
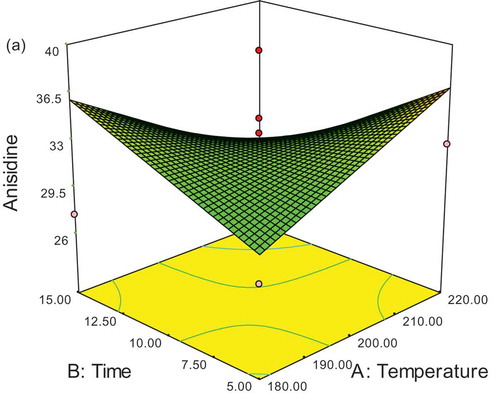
Results of the optimization of SFO containing two natural and synthetic antioxidants being computed by Expert Design software are shown in and . Therefore, because of the lowest values of peroxide and anisidine and the highest values of rancimat, the optimized sample was related to a Mentha piperita extract sample (500 ppm) at 180°C for a duration of 5 min and desirability of 92.1%. The 3D response surface as a function of time and temperature is shown in plot (, , and ).
Table 5. Optimization results of TBHQ100.
Table 6. Optimization Results of SFO-500.
Figure 5a. Response surface plotting of the effect of temperature and time on change of para-anasidine during frying after adding antioxidant (SFO 500 ppm).
Conclusion
The results of peroxide, p-anisidine and rancimat value in SFO by adding the natural antioxidant of Mentha piperita extract and synthetic antioxidant of TBHQ showed that natural antioxidant of Mentha piperita has a significant effect on the stability of SFO. Therefore, Mentha piperita is a natural antioxidant that has a great potential for replacing synthetic antioxidants. The extract could be introduced as a natural antioxidant in food following the successful conclusion of further tests.
Funding
The current article is extracted from a research project funded and supported by the Islamic Azad University of Tabriz and the support of that organization is greatly appreciated.
Additional information
Funding
References
- Lagha-Benamrouche, S.; Madani, K. Phenolic Contents and Antioxidant Activity of Orange Varieties (Citrus Sinensis L. and Citrus Aurantium L.) Cultivated in Algeria: Peels and Leaves. Industrial Crops and Products 2013, 50, 723–730.
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of Volatitle Components in Basil (Ocimumbasilicum L.) and Thyme Leaves (Thymusvulgaris L.) and Their Antioxidant Properties. Food Chemistry 2005, 91, 131–139.
- Cha, D.S.; Eun, J.S.; Jeon, H. Anti-Inflammatory and Antinociceptive Properties of the Leaves of Eriobotrya Japonica. Journal of Ethnopharmacology 2011, 134(2), 305–312.
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants During Accelerated Storage. Food Chemistry 2010, 118(3), 656–662.
- Yilmaz, Y.; Göksel, Z.; Erdoğan, S.S.; Öztürk, A.; Atak, A.; Özer, C. Antioxidant Activity and Phenolic Content of Seed, Skin, and Pulp Parts of 22 Grape (Vitisvinifera L.) Cultivars (4 Common and 18 Registered or Candidate for Registration). Journal of Food Processing and Preservation 2015, 39(6), 1682–1691.
- Ozgen, U.; Mavi, A.; Terzi, Z.; Yildirim, A.; Coskun, M.; Houghton, P.J. Antioxidant Properties of Some Medicinal Lamiaceae (Labiatae) Species. Pharmaceutical Biology 2006, 44, 107–112.
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the Antioxidant Potentials of Six Salvia Species from Turkey. Food Chemistry 2006, 95, 200–204.
- Mozaffarian, V. A Dictionary of Iranian Plant Names; Farhang Moaser Publishers: Tehran, Iran.
- Nickavar, B.; Alinaghi, A.; Kamalinejad, M. Evaluation of the Antioxidant Properties of Five Mentha Species. Iranian Journal of Pharmaceutical Research 2008, 7(3), 203–209.
- http://blast.ncbi.nlm.nih.gov/Blast.cgi.
- Cemeroglu, B. Gida Analizler Teknolojisi. Dernegiyayinari 2010, 34, 657–694.
- Taghvaei, M.; Jafari, S.M.; Mahoonak, A.S.; Nikoo, A.M.; Rahmanian, N.; Hajitabar, J.; Meshginfar, N. The Effect of Natural Antioxidants Extracted from Plant and Animal Resources on the Oxidative Stability of Soybean Oil. LWT–Food Science and Technology 2014, 56(1), 124–130.
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. Journal of AOAC International 1994, 77(2), 421–424.
- Smouse, T.H. Methods to Assess Oil Quality and Stability. In Factors Affecting Oil Quality and Stability; Warner, K.; Eskin, N.A.M.; Eds.; AOCS Press: Champaign, IL, 1994; 12.
- Farhoosh, R.; Tavassoli-Kafrani, M.H. Simultaneous Monitoring of the Conventional Qualitative Indicators During Frying of Sunflower Oil. Food Chemistry 2011, 125(1), 209–213.
- Atkinson, A.C.; Donev, A.N. Optimum Experimental Design. Oxford University Press 1992, 5, 132–189.
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 2nd ed., Wiley Pub Inc.: New York, NY, 2002; 51–83.
- Barzegari, A.; ZununiVahed, S.; Atashpaz, S.; Khani, S.; Omid, Y. Rapid and Simple Methodology for Isolation of High Quality Genomic DNA from Coniferous Tissues (Taxusbaccata). Molecular Biology Reports 2010, 37, 833–837.
- Kamkar, A.; JebelliJavan, A.; Asadi, F.; Kamalinejad, M. The Antioxidative Effect of Iranian Mentha Pulegium Extracts and Essential Oil in Sunflower Oil. Food and Chemical Toxicology 2010, 48, 1796–1800.
- Luximun-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruomai, O.I. Antioxidant Activities of Phenolic, Proanthocyanidin, and Flavonoid Components in Extracts of Cassia Fistula. Journal of Agricultural and Food Chemistry 2002, 50, 5042–5047.
- Sugihara, N.; Arakawa, T.; Ohnishi, M.; Furuno, K. Anti- and Pro-Oxidative Effects of Flavonoids on Metal-Induced Lipid Hydroperoxide-Dependent Lipid Peroxidation in Cultured Hepatocytes Loaded with Alpha-Linolenic Acid. Free Radical Biology & Medicine Journal 1999, 27, 1313–1323.
- Spencer, J.P. Flavonoids: Modulators of Brain Function? British Journal of Nutrition 2008, 99(E–S1), ES60–ES77.
- Ho, C.T.; Li, J.; Kuo, M.C. Flavor Chemistry of Selected Condiments and Spices Used in Chinese Foods. In Flavor and Chemistry of Etanic Foods; Shahidi, F.; Ho, C.T.; Ed.; Proceedings of the 5th Chemical Congress of North America, Canun: November 11–15, Kluwer Academic, Plenum Publisher: New York, NY, 1999; 55–76.
- Shahidi, F.; Wanasundara, P.K.J.P.D. Phenolic Antioxidants. Critical Reviews in Food Science and Nutrition 1992, 32, 67–103.
- Kamkar, A.; ShamseArdekani, M.R.; Shariatifar, N.; Misagi, A.; Mozaffari Nehjad, A.S.; Jamshidi, A.H. Antioxidative Effect of Iranian Pulicriagnaphalodes L. Extracts in Soybean Oil. South African Journal of Botany 2013, 85, 39–43.
- Delfanian, M.; Kenari, R.E.; Sahari, M.A. Antioxidant Activity of Loquat (Eriobotrya Japonica Lindl.) Fruit Peel and Pulp Extracts in Stabilization of Soybean Oil During Storage Conditions. International Journal of Food Properties 2015, 18(12), 2813–2824.
- Capecka, E.; Mareczek, A.; Leja, M. Antioxidant Activity of Fresh and Dry Herbs of Some Lamiaceae Species. Food Chemistry 2005, 93, 223–226.