Abstract
Over-fermented tempe, known as tempe semangit, is popular in Indonesian culture, especially in Java, as an umami seasoning in traditional foods. The objective of this study was to characterize taste-active compounds in water soluble extracts of over-fermented tempe. Over-fermented tempe was prepared from fresh tempe in which the fermentation was prolonged (0 to 96 h). Free amino acids in the water soluble extract were analyzed and characterized for their taste activity values. Water soluble extracts contained umami and bitter tasting free amino acids at relatively high concentrations. Their umami and bitter taste activity values were higher than the taste threshold concentration. Water soluble extract from 72 h over-fermentation had a higher umami taste activity value than bitter taste activity, exhibiting the highest umami taste dilution factor. The high-performance liquid chromatography profile of water soluble extract fractions obtained using Sephadex G-25 gel filtration chromatography demonstrated that fractions having higher umami taste intensity had more hydrophilic components than hydrophobic components.
INTRODUCTION
Tempe is an Indonesian traditional fermented soybean food which contains proteins that is comprised of approximately 40–50% of the total dry matter. Rhizopus mold hydrolized 65% of soybean proteins into amino acids and peptides during tempe fermentation.[Citation1,Citation2] Tempe’s tasty flavor is due to its high content of nitrogenous compounds.[Citation3]
Tempe is commonly consumed just after it is produced. This takes 24 to 48 h of fermentation. In Javanese cuisine, the tempe fermentation is intentionally prolonged to obtain tempe semangit and tempe bosok. Tempe semangit (slightly over-ripened tempe) has a shorter fermentation period, 48 to 72 h, whereas tempe bosok (over-ripened tempe) needs more than 72 h of fermentation.[Citation4] These types of tempe have a brown color, pungent odor, and soft texture compared to the white compact cake of fresh tempe. Indonesian people in Central Java have long used tempe semangit, mostly from 72 h of over-fermentation, as a seasoning in their cuisine to provide a distinctive flavor, odor, and taste.[Citation5] Most of taste components in these foods are water-soluble, including amino acids, peptides, nucleotides, organic acids and bases, and inorganic ions.[Citation6] Many studies showed that amino acids and peptides contribute to the taste of fermented food. In general, enzymatic hydrolysis of proteins gives a bitter taste, but some peptides can contribute to umami taste. Some research showed that free amino acids and the fraction of low molecular weight peptides in douchiba (a fermented food from China) are formed during the fermentative maturation process.[Citation7] Peptides isolated and characterized from cheese (Glu-Glu), fish protein hydrolisates (Glu-Gly-Ser),[Citation8,Citation9] and doenjang (low molecular acidic peptides)[Citation10] have been shown to have umami taste. Umami taste in fermented products could be formed due to the interaction between salt and free acidic amino acids.[Citation11] The aim of this study was to characterize the taste-active compounds in water-soluble extracts of over-fermented tempe prepared with several periods of over-fermentation.
MATERIAL AND METHODS
Over-Fermented Tempe
Fresh tempe was obtained from a tempe manufacturer, FreSoia Co., in Bekasi, West Java, Indonesia. The fermentation of fresh tempe was intentionally prolonged at 25–35°C and relative humidity (RH) of 70-75%. The periods of over-fermentation were 0, 24, 48, 72, and 96 h. Each over-fermented tempe was prepared in the same amount (600 g) as fresh tempe.
Water Soluble Extract (WSE) of Over-Fermented Tempe
WSEs were prepared to characterize the taste-active components in over-fermented tempe. Each over-fermented tempe was homogenized in 2400 mL of deionized water. The homogenates were heated to boiling, held for 10 min to inactivate microbial enzymes, and cooled at room temperature for 50 min. The mixtures were centrifuged at 2800 × g, 4°C for 30 min to separate the water soluble fraction from lipid in the upper layer and solid in the bottom in the centrifuge tube. The supernatant was filtered through cheese-cloth (80 and 150 mesh) and the filtrate was lyophilized.
Lyophilized WSE were analysed for their crude proteins, total acids, total sugars, pH, free amino acids, and taste activity values. Crude proteins were quantified using the Kjeldahl method.[Citation12] Total acid was determined by titrating 0.25 g of dried WSE dissolved in 25 mL of deionized water, with 0.10 N NaOH and using 0.1% phenolphthalein in ethanol as an indicator.[Citation12] Total sugars were quantified using the Anthrone method[Citation13] employing a ultraviolet-visible (UV-Vis) 160 spectrophotometer (Shimadzu, Japan). Acidity was measured using a pH meter (Milwaukee M801, Romania). Amino acid composition was determined using a high-performance liquid chromatography (HPLC) method with o-phthalaldehyde (OPA) pre-column derivatization (Shimadzu, Japan). This was done after acid hydrolysis (6N HCl) for total amino acids, and without acid hydrolysis for free amino acid composition.[Citation14] Amino acid residues in the samples were determined by subtracting free amino acids from total amino acids. Taste activity values of amino acids contained in sample were calculated by dividing the corresponding concentrations with their taste thresholds. The compounds with taste activity values greater than 1 were considered as contributing to food taste.[Citation15]
Separation of WSE by Gel Filtration Chromatography
Separation of WSE was conducted to obtain the fractions which contributed to the umami taste of WSE. The resulting fractions were then characterized to determine their umami taste dilution (TD) factors. The separation was performed as described below. Freeze dried WSE was dissolved in 10 mL of deionized water, and transferred to a 50 mL volumetric flask. Deionized water was then added to achieve volume. The WSE solution was centrifuged at 9000 × g, 4°C for 30 min, and passed through filter paper (Whatman No. 42). Twenty-five milliliters of the solution was filtered using an ultra centrifugal 3 kDa filter tube (Ultracel-3K, Amicon Ultra Centrifugal Filters, Regenerated Cellulose 3000 MWCO, Millipore, Irlandia, Indonesia) at 4500 × g, 4ºC for 30 min. The solution was adjusted to a total volume 25 mL and placed into a glass column (2.0 × 90 cm) filled with Sephadex G-25 medium (Amersham Pharmacia, Biotech AB, Swedia, Indonesia). The flow rate of elution was 4.25 mL min–1 at room temperature with deionized water used as a mobile phase to allow sensory evaluation of the recovered fractions. Absorbance of eluates (7.5 mL each) was measured at UV 240 nm, and all eluates were collected in 3 to 4 fractions. The collected fractions were lyophilized, and then dissolved in 5 mL of deionized waterprior to do HPLC profiles and sensory analyses.
Reverse Phase (RP)-HPLC Profiles
The fractions obtained from gel chromatography were separated by RP-HPLC to obtain their HPLC profiles. Separation by RP-HPLC was performed using an Agilent 1200 Series HPLC (Agilent Technologists Co., USA) on a Zorbax C18 column (4.6 × 150 mm, 5 µm, Zorbax Eclipse XDB-C18, Agilent, USA). Solvent A was 99.9% acetonitrile (HPLC grade, Merck Co., Germany) and solvent B was 100% deionized water. A linear gradient for 20 min from 10 to 20% acetonitrile was applied at 0.4 mL min–1 and at room temperature. UV absorbance at 214 nm was monitored.
TD Analysis
A panel of 30 subjects from the Department of Food Science and Technology of Bogor Agricultural University, Bogor, Indonesia, was formed using a panelist selection process. The selection was conducted in three steps. Four people became sensory panelists. They were trained to respond the five basic tastes (bitter, salty, acid, sweet, and umami) at several concentrations. The sensory panelists evaluated the umami taste of WSE solutions (concentrated five times) and their fractions by TD analysis. The samples were stepwise diluted 1:1 with deionized water. The dilution factor followed a 2n factor design. The serial dilutions of each WSE solution and their fractions were then presented to the panelists. Each dilution was analyzed using a triangle test. The dilution at which a taste difference between the diluted fraction and two blanks (deionized water) could just be detected was defined as the TD factor.[Citation16]
Statistical Analysis
All five WSEs were evaluated in two replicates. The results were expressed as mean value ± standard deviation (SD). Total acid and total sugar were analyzed using one-way analysis of variance (ANOVA). Correlation analysis was done to determine the correlation between the taste activity ratio and its corresponding TD factor obtained from sensory analysis. The data was analysed using SPSS (version 17).
RESULTS AND DISCUSSION
Chemical Characterization of WSE
The crude protein in WSE increased simultaneously with the periods of fermentation (). This indicated that longer periods of over-fermentation gave higher concentrations of water soluble nitrogenous compounds. Hence, during over-fermentation, the microflora could hydrolyze soy proteins into smaller components having lower molecular weights which are more soluble in water.
TABLE 1 Parameters of water-soluble extracts of over-fermented tempe obtained from the same initial weight of fresh tempe
Total acids of WSE decreased during the periods of over-fermentation and exhibited significant differences (p < 0.05) for different periods of over-fermentation (). Water-soluble organic acids are produced during the tempe fermentation.[Citation1] Some organic acids, such as lactic acid, malic acid, fumaric acid, and propionic acid, are produced by Rhizopus.[Citation17] The decrease of total acids in WSE was related to the increase in pH value (). The increase of the pH value observed in over-fermented tempe could be due to the release of ammonia.[Citation18] Ammonia is formed by deamination of amino acids during fermentation as a result of the use of amino acids by bacteria for their carbon and energy.[Citation19] The increasing pH may have caused a higher hydrolysis activity of protein by proteases to produce free amino acids and small peptides. The optimum pH value for this condition was reported as 7.18.[Citation1]
The total sugars of WSE also decreased during over-fermentation and exhibited significant differences (p < 0.05) between different periods of over-fermentation (). During tempe fermentation, the presence of polygalacturonase, endocellulase, xylanase, and arabinase in tempe[Citation20] allows hydrolysis of polysaccharides into smaller carbohydrate molecules such as sucrose, raffinose, or stachyose. These sugars are also used for carbon and energy for microbial growth during fermentation,[Citation21] and this condition may have prolonged tempe fermentation. The maximum growth of Rhizopus in tempe was at 72 h of fermentation, which was 24 h of over-fermentation. Rhizopus, yeasts, and bacteria were also found.[Citation22] Moreover, some lactic acid bacteria can grow together with Rhizopus oligosporus, even though these bacteria started to grow after 10 h of fermentation and increased as the fermentation progressed. For example, Lactobacillus fermentum began significantly inhibiting the growth of Rhizopus oligosporus after 24 h of fermentation.[Citation23] It was found that lactic acid bacteria plays a role in the tempe fermentation and contributes to the safety of the product.[Citation24]
The composition of free amino acids and amino acid residues in WSEs are given in . It is notable that both free glutamic acid and glutamic acid residues were dominant in all WSE samples. Amino acid residues were higher than their corresponding free amino acids in all extracts.This means that peptides were more dominant. However, among the extracts, WSE after 72 h of over-fermentation had the highest concentration of amino acid residues, while WSE after 96 h of over-fermentation had the highest concentration of free amino acids.
TABLE 2 Composition of amino acids in water soluble extracts of over-fermented tempe obtained from 600 g of fresh tempe subjected to prolonged fermentation
Amino acids and peptides in fermented food contribute to its taste characteristics.[Citation25] In general, protein hydrolysis produces peptides which have a bitter taste. However, some peptides isolated and characterized from soy sauce, miso, and doenjang had an umami taste.[Citation26–Citation28] Glutamic acid has the highest concentration among the amino acids in fermented food and contributes as a taste-active compound.[Citation29] Peptides with hydrophilic amino acids (acidic), such as glutamic acid and glutamic residues, on the N terminus had an umami taste as did dipeptides which consisted of aspartic, threonine, serine, or glutamate.[Citation30,Citation31]
In some fermented soybean foods, the taste of the food can be predicted by its free amino acid composition.[Citation32] The intensity of umami taste can be determined by the concentrations of two acidic amino acids, aspartic and glutamic acids. The sweet taste was predicted by the concentrations of four amino acids, alanine, glycine, serine, and threonine. The bitter taste is indicated by the presence of eight amino acids, arginine, histidine, isoleucine, leucine, methionine, phenylalanine, valine, and tyrosine.[Citation33] Lysine is considered as amino acid without taste. In fish sauces from Asian countries, aspartic and glutamic acids gave the highest taste activity values and produced umami taste.[Citation34]
The amino acid composition showed that amino acids with bitter taste were in relatively higher concentrations in all WSE samples (). There was a relatively higher bitter taste activity in all WSE samples () as well, except in the WSE with 72 h of over-fermentation. This sample had higher taste activity values for umami than bitter taste. According to the results in , the WSE of over-fermented tempe was dominated by free amino acids which had higher umami taste activity than bitter. The taste activity values of those amino acids were greater than 1.0, especially umami and bitter tastes. This means that umami and bitter tastes affected the taste of over-fermented tempe. It can be inferred that the free amino acids contributed significant taste to over-fermented tempe. Moreover, 48 to 96 h of over-fermentation increased the umami and sweet tastes of over-fermented tempe more than bitter taste, as described in .
TABLE 3 Taste activity ratio of umami-sweetness to bitterness taste in water soluble extracts (50 mL) of over-fermented tempe obtained from 600 g of fresh tempe after prolonged fermentation
FIGURE 1 Taste characteristic A: and taste activity value B: of free amino acids in water soluble extract of over-fermented tempe obtained from 600 g of fresh tempe. Amino acids are grouped as follows: Asp and Glu for umami taste; Ala, Gly, Ser, and Thr for sweet taste; Arg, His, Ile, Leu, Met, Phe, Val, and Tyr for bitter taste; and Lys as tasteless. Taste activity values were calculated from the individual amino acid concentrations divided by their corresponding threshold values.
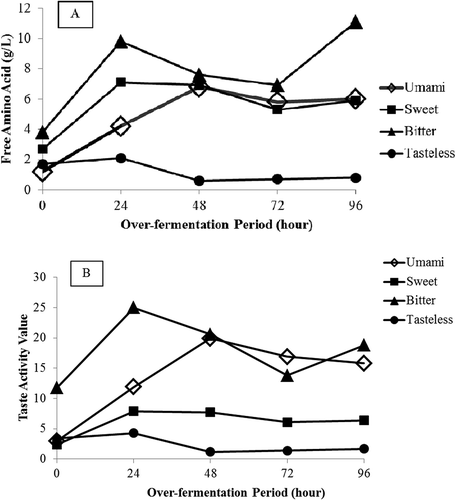
It is interesting to further examine the ratio between umami and sweet taste intensities to bitter taste intensity. The compounds with umami taste could be combined with sweet taste, because both of the tastes were desirable and somewhat different than bitter taste.[Citation35] WSE from 72 h of over-fermentation had the highest ratio of umami-sweet taste to bitter taste activity that was 1.6. This ratio is considered the maximum difference in umami taste intensity according to the sensory results. This correlated with the traditional practice found in Indonesia. Javanese people had prolonged fresh tempe fermentation for 3 days (72 h of over-fermentation) to obtain a tasty over-fermented tempe.
Umami TD Factor of WSE and Their Fractions
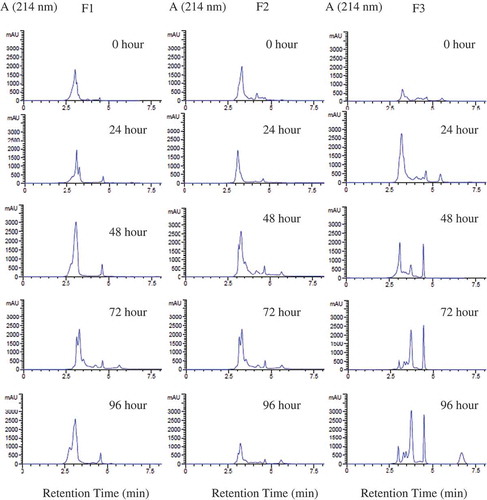
The WSE samples of over-fermented tempe were further divided into three to four fractions (F1 to F4) after separation using Sephadex G-25. The umami TD factors of WSE and their fractions are given in . The results showed that all WSE samples had umami taste but with different intensities. This reflected different dilution factors. WSE from 72 h of over-fermentation had the highest umami TD factor, 256. This is in accordance with the taste activity ratio presented in . According to statistical analysis, there was a positive correlation between the umami TD factor in and taste activity ratio in (r = 0.843 and p = 0.002). This indicated that umami taste in the WSE of over-fermented tempe could not be determined only by free amino acid content. The presence of other free amino acids needs to be considered. Those free amino acids with sweet and bitter taste are important when the ratio of umami and sweet taste activities was higher than the bitter taste activity. This phenomenon was first observed in the current study.
TABLE 4 Dilution factor of umami taste found in water-soluble extracts (WSE) of over-fermented tempe and their fractions. WSE was evaluated at 5 times concentrated
The highly intense of umami taste in WSE from 72 h over-fermentation could not be described by the individual umami taste intensities in WSE fractions, F1 to F3 (). Only F2 of the WSE had the highest umami taste intensity, therefore, the components in this fraction may describe the intense umami taste of the WSE. This research explained that the taste of WSE was provided by the presence of umami and sweet taste compounds, although at the same time the bitter taste compounds were also exist. However, the bitter taste activities were about two times lower than umami and sweet taste activities of other compounds
Fractions F1 may consist of peptides which had higher molecular weights than those of free amino acids, since Sephadex G-25 separates the sample based on the molecular weights of its components. Higher molecular weights are eluted first. Fractions F3 or F4 may contain more hydrophobic amino acids because the separation process of Sephadex G-25 allows interaction between hydrophobic sites of the sample and cross linking of epichlorohydrin and dextran in Sephadex’s matrix. Hydrophobic amino acids caused a less intense umami taste or lower dilution factors of fractions F3 and F4.
HPLC Profile
The results from the HPLC profiles of WSE are presented in . The HPLC performance by the RP-C18 column allowed separation of hydrophilic components, which appeared first, from the hydrophobic components eluted later. The HPLC profiles of F1 fractions were similar to F2 fractions, and could be distinguished from F3 fractions. It is clear from that F3 fractions had more hydrophobic components than F2 fractions. The concentration of hydrophobic components was higher as the fermentation time increased. F3 Fractions had lower umami taste intensities than other fractions, as presented in . However, it is possible that interaction between the components in fractions F1 to F3 provided for a higher umami taste intensities in the WSE. This has also been found in soy sauce.[Citation26]
CONCLUSIONS
The taste of over-fermented tempe was characterized as umami and bitter flavors. Over-fermentation for 72 h gave the highest umami taste intensity. This indicated why over-fermented tempe is commonly used in Indonesian cuisine. Free amino acids having umami and bitter tastes at a certain ratio may play a significant role in the intensity of umami taste. The HPLC profiles of WSE fractions indicated the presence of hydrophilic and hydrophobic components in WSE of over-fermented tempe. WSE fractions having a higher number of hydrophilic components had higher umami taste intensities.
ACKNOWLEDGMENTS
The authors gratefully thank Professor Yasuyuki Hashidoko of the Agricultural Faculty, Hokkaido University, Japan for his helpful advice.
FUNDING
This research was supported by grants funded by the Indonesian Ministry of National Education through the Competitive Funding called Hibah Kompetensi (HIKOM) 2012.
Additional information
Funding
REFERENCES
- Handoyo, T.; Morita, N. Structural and Functional Properties of Fermented Soybean (Tempeh) by Using Rhizopus Oligosporus. International Journal of Food Properties 2006, 9, 347–355.
- Higasa, S.; Negishi, Y.; Aoyagi, Y.; Sugahara, T. Changes in Free Amino Acids of Tempe During Preparation with Velvet Beans (Mucuna Pruriens). Journal of the Japanese Society for Food Science and Technology 1996, 43, 188–193.
- Nout, M.J.R.; Kiers, J.L. A Review. Tempe Fermentation, Innovation, and Functionality: Update into the Third Millenium. Journal of Applied Microbiology 2005, 98, 789–805.
- Shurleff, W.; Aoyagi, A. The Book of Tempeh. Harper and Row Publisher: New York, NY, 1979.
- Gunawan-Puteri, M.D.P.T.; Wijaya,C.H.; Mutukumira, A.N. The Utilization of Overriped Tempe (Tempe Semangit) As Indigenous Condiment. In 2nd Workshop on Food Safety Technologies and Innovations Applied to Food Safety. July 5–6, IATA-CSIC, Valencia, Spain. Food Safety Working Group of CIGR, Valencia, 2012; 134–153.
- Shah, A.K.M.A.; Ogasawara, M.; Egi, M.; Kurihara, H.; Takahashi, K. Identification and Sensory Evaluation of Flavour Enhancer in Japanese Traditional Dried Herring (Clupea Pallasii) Fillet. Food Chemistry 2010, 122, 249–253.
- Qin, L.; Ding, X. Formation of Taste and Odor Compounds During Preparation of Douchiba, a Chinese Traditional Soy-Fermented Appetizer. Food Biochemistry 2007, 31, 230–251.
- Gomez-Ruiz, J.A.; Taborda, G.; Amigo, L.; Ramos, R.; Molina, E. Sensory and Mass Spectrometric Analysis of the Peptidic Fraction Lower Than One Thousand Daltons in Manchego Cheese. Journal of Dairy Science 2007, 90, 4966–4973.
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste. In Flavor Chemistry Trends And Developments; Teranishi, R.; Buttery, R.G.; Shahidi, F.; Eds.; American Chemical Society: Washington, DC, 1989; 158–174.
- Rhyu, M.; Kim, E.Y. Umami Taste Characteristics of Water Extract of Doenjang, a Korean Soybean Paste: Low Molecular Acidic Peptides May Be a Possible Clue to the Taste. Food Chemistry 2011, 127, 1210–1215.
- Lioe, H.N.; Apriyantono, A.; Takara, K.; Wada, K.; Yasuda, M. Umami Taste Enhancement of MSG/Nacl Mixtures by Subthreshold L-α-Aromatic Amino Acids. Food Science 2005, 70, 401–405.
- AOAC. Official Methods of AOAC International, 18th ed.; Association of Official Analytical Communities: Gaithersburg, MD, 2005.
- Clegg, K.M. The Application of the Anthrone Reagent to the Estimation of Starch in Cereals. Journal of the Science of Food and Agriculture 1956, 7, 40–44.
- Harris, E.L.V. Amino Acid Analysis by Precolumn Derivatization, Vol. 3. In New Protein Techniques; Walker, J.M.; Ed.; Humana Press: Clifton, NJ, 1988; 33–47.
- Chen, D.W.; Zhang, M. Non-Volatile Taste Active Compounds in the Meat of Chinese Mitten Crab (Eriocheirsinensis). Food Chemistry 2007, 104, 1200–1205.
- Frank, O.; Ottinger, H.; Hofman, T. Characterization of An Intense Bitter-Tasting 1H,4H-Quiolizium-7-Olate by Application of the Taste Dilution Analysis, a Novel Bioassay for Screening and Identification of Taste-Active-Compunds in Foods. Journal of Agricultural and Food Chemistry 2001, 49, 231–238.
- Oda, Y.; Yajima, Y.; Kinoshita, M.; Ohnishi, M. Difference of Rhizopus Oryzae Strains in Organic Acid Synthesis and Fatty Acid Composition. Food Microbiology 2003, 20, 371–375.
- Sparringa, R.A.; Owen, J.D. Causes of Alkalinization in Tempe Solid Substrate Fermentation. Enzyme Microbial Technology 1999, 25, 677–681.
- Visessanguan, W.; Benjakul, S.; Potachareon, W.; Panya, A.; Riebroy, S. Accelerated Proteolysis of Soy Proteins During Fermentation of Thua-Nao Inoculated with Bacillus Subtilis. Food Biochemistry 2005, 29, 349–366.
- Sarrette, M.; Nout, M.J.R.; Gervais, P.; Rombouts, F.M. Effect of Water Activity on Production and Activity of Rhizopus Oligosporus Polysaccharidases. Journal of Applied Microbiological Biotechnology 1992, 37, 420–425.
- Prinyawiwatkul, W.; Beuchat, L.R.; McWatters, K.H.; Phillips, R.D. Changes in Fatty Acid, Simple Sugar, and Oligosaccharide Content of Cowpea (Vigna Unguiculata) Flour As a Result of Soaking, Boiling, and Fermentation with Rhizopus Microporus Var Oligosporus. Food Chemistry 1995, 57, 405–413.
- Charcosset, J.Y.; Chauvet, E. Effect of Culture Conditions on Ergosterol As An Indicator of Biomass in the Aquatic Hyphomycetes. Applied and Environmental Microbiology 2001, 67, 2051–2055.
- Feng, X.M.; Eriksson, A.R.B.; Schürer, J. Growth of Lactic Acid Bacteria and Rhizopus Oligosporus During Barley Tempeh Fermentation. International Journal of Food Microbiology 2005, 104, 249–256.
- Nout, M.J.R.; Rombouts, F.M. Recent Development in Tempe Research. Journal of Applied Bacteriology 1990, 69, 609–633.
- Je, J.Y.; Park, P.J.; Jung, W.K.; Kim, S.K. Amino Acid Changes in Fermented Oyster (Crassostrea Gigas) Sauce with Different Fermentation Periods. Food Chemistry 2005, 91, 15–18.
- Kim, S.H.; Lee, K.A. Evaluation of Taste Compounds in Water-Soluble Extract of a Doenjang (Soybean Paste). Food Chemistry 2003, 83, 339–342.
- Lioe, H.N.; Wada, K.; Aoki, T.; Yasuda, M. Chemical and Sensory Characteristics of Low Molecular Weight Fractions Obtained from Three Types of Japanese Soy Sauce (Shoyu)-Koikuchi, Tamari, and Shiro Shoyu. Food Chemistry 2007, 100, 1669–1677.
- Ogasawara, M.; Yamada, Y.; Egi, M. Taste Enhancer from Long-Term Ripening of Miso (Soybean Paste). Food Chemistry 2006, 99, 736–741.
- Kawai, M.; Uneyama, H.; Miyano, H. Taste-Active Components in Foods with Concentration on Umami Compounds. Journal of Health Science 2009, 55, 667–673.
- Arai, S.; Yamashita, M.; Noguchi, M.; Fujimaki, M. Tastes of L-Glutamyl Oligopeptides in Relation to Their Chromatographic Properties. Journal of Agriculture Biological Chemistry 1973, 36, 151–156.
- Noguchi, M.; Yamashita, M.; Arai, S.; Fujimaki, M. Isolation and Identification of Acidic Oligopeptides Occuring in a Flavor Potentiating Fraction from a Fish Protein Hydrolysate. Journal of Agricultural and Food Chemistry 1975, 23, 49–53.
- Manani, T.A.N.; Mwangwela, A.M.; Schüller, R.B.; Østlie, H.M.; Wicklund, T. Sensory Evaluation and Consumer Acceptance of Naturally and Lactic Acid Bacteria-Fermented Pastes of Soybeans and Soybean-Maize Blends. International Journal of Food Science and Nutrition 2014, 2, 114–131.
- Tseng, Y.H.; Lee, Y.L.; Li, R.C.; Mau, J.L. Non-Volatile Flavour Components of Ganoderma Tsugae. Food Chemistry 2005, 90, 409–415.
- Yimdee, T.; Wang, X.C. Comparison of Odor and Taste of Commercial Brand Fish Sauces from East and South East Asian Countries. International Journal of Food Properties 2015, accepted manuscript, DOI:10.1080/10942912.2015.1045517
- Ruiz, C.J.; Wray, K.; Delay, E.R.; Margolskee, R.F.; Kinnamon, S.C. Behavioral Evidence for a Role of α-Gustducin in Glutamate Taste. Chemical Sense 2003, 28, 573–579.