Abstract
Phytochemicals content and antimicrobial activity of Artocarpus heterophyllus, Cyclosorus extensa, Oldendia corymbosa, and Alpinia malaccensis were investigated. Maximum alkaloids and terpenoids were found in A. heterophyllus; tannins and saponins in C. extensa; flavonoids, polyphenols, and phytosterols in O. corymbosa and anthraquinone, glycosides, and anthocyaninin A. malaccensis. Aqueous, methanolic, ethyl acetate, and hexane extracts were prepared from all the leaves. Attenuated total reflectance Fourier transform infrared spectrophotometric (ATR-FTIR) analysis revealed that alkanes and alkyl halides were prevalent in all the extracts and the ethyl acetate extracts contained comparatively higher number of functional groups, which were also more effective against Candida albicans, Escherichia coli, and Staphylococcus aureus. The minimum inhibitory concentration values of A. malaccensis against the tested pathogens were found to be lesser than the other species.
INTRODUCTION
The consumption of alcoholic beverage in the form of rice beer is a very common practice among the tribal people in Assam, India. Various processing technologies are followed for preparing rice beer and each of these correlates with the utilization of natural plants and herbs. There are also reports of rice beer being used as a drug and is believed to be effective against insomnia, headaches, body aches, inflammation of body parts, diarrhea and urinary problems, expelling worms, and as a treatment of cholera.[Citation1] The nutritional aspects of various types of rice beers have also been studied and a variety of organic acids, carbohydrates, amino acids, and aromatic compounds have been detected.[Citation2] The starters for rice beer fermentation are in the form of dry powder, hard balls, or cakes and various plant materials are used in the preparation of these starters.[Citation3] Out of these plants,[Citation1] leaves of Artocarpus heterophyllus, Cyclosorus extensa, Oldenlandia corymbosa, and Alpinia malaccensis were found to be with the highest content of total phenolic compounds and antioxidant activity (2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity) based on an initial screening test by us.[Citation4] All these four plants are native to Southeast Asia and grow abundantly in Assam as wild plants. In our previous study,[Citation5] it was also seen that all these four species contained various phenolic compounds and these phenolics were found to have fairly good antioxidative property.
Cyclosorus is a genus of ferns of the family Thelypteridaceae. Antibacterial activity of the closely related fern C. interruptus against Staphylococcus aureus has already been reported.[Citation6] Oldenlandia is a genus of flowering plant belonging to the family Rubiaceae. It is a weedy annual herb with ascending or erect 4-angled stems.[Citation7] Its extracts are said to be active against appendicitis, hepatitis, pneumonia, cholecystitis, urinary infection, cellulites and snake bite, skin sores, ulcers, sore throat, bronchitis, gynecologic infections, and pelvic inflammatory diseases.[Citation8] Ten new compounds have also been isolated from the whole plants of O. corymbosa of Thai origin.[Citation9] A. heterophyllus, also known as jakfruit, is a species of tree in the Moraceae family. Reports are also available on the wound healing activity of flavonoid rich fraction of ethyl acetate (EA) extract of the leaves of A. heterophyllus using porcine skin wound healing model.[Citation10] The methanolic extract of A. heterophyllus leaves exhibited significant analgesic and immunomodulator effect on Swiss albino mice.[Citation11] The hypoglycemic activity of the leaves of A. heterophyllus in normal and streptozocin induced diabetic rats has also been reported.[Citation12] A. malaccensis is a plant in the Zingiberaceae family and is a native of Indonesia and Malaysia. It has a wide distribution, extending from north-east India to Indochina, southward to Peninsular Malaysia and Java.[Citation13] Many species of the genus Alpinia containing flavonoids, terpenoids,[Citation14] and kavalactones provide a variety of medicinal properties.[Citation15] Traditionally A. malaccensis is used to cure wounds and sores, to make the voice strong and clear, applied on gastralgia with tympanites, for bathing feverish people, and as an anti-vomiting agent.[Citation16,Citation17]
The naturally occurring phytochemicals in plants like alkaloids, tannins, flavonoids, steroid, terpenoid, phenol-saccharide conjugate, and other phenolic compounds have various bioactive properties[Citation18] and have various antimicrobial and antioxidant activities, both in in vitro and in vivo conditions. In recent years, there has been renewed interest in plant derived natural bioactive compounds which might confer health benefits and help in preventing the infections caused by pathogenic microorganisms. In this study, phytochemical evaluation of the leaves of these four plant species has been evaluated and the antimicrobial activities exhibited by extracts of these species have also been assessed.
MATERIALS AND METHODS
Materials
The leaves which are used for preparing starters have already been reported in our previous study.[Citation1] The rice beer starter cakes are prepared by mixing various proportions of ground leaves with powdered rice. The leaves are not directly added to the fermentation mixture. The focus of this study is to primarily evaluate the phytochemical composition and antimicrobial activity of the extracts of these leaves, which might significantly affect the quality of the alcoholic beverage. Leaves of the four plant species viz. A. heterophyllus Lam., C. extensa (Blume) Ching, O. corymbosa L., and A. malaccensis (Burm.f.) Roscoe were collected from the botanical gardens of Tezpur University Campus, Assam. Taxonomical identification of the collected plant species were done in the Department of Botany, Darrang College, Tezpur, Assam. The young and tender leaves were selected for analysis based on the traditional uses of the plants for starter cake making. The chemicals and solvents used for analysis were of high purity analytical grade and obtained from Sigma-Aldrich Corporation (USA) and HiMedia (India). The mould Fusarium oxysporum MTCC 1755, yeast Candida albicans MTCC 183, and Saccharomyces cerevisae ATCC, bacteria Escherichia coli MTCC 40, Staphylococcus aureus MTCC 3160, Lactobacillus plantarum ATCC 8014, and Bacillus subtilis ATCC 11774 were kindly provided by the Department of Food Engineering and Technology, Tezpur University, Assam. The equipment which was used during the study were the tray dryer (BDI-51, Labotech, India), spectrophotometer (Spectrascan UV2600, Thermo Scientific, USA), incubator (LBI-150M, Labtech, South Korea), incubator shaker (Excella E24R, NBS, USA), water bath (BW-20G, Jeiotech, South Korea), rotary vacuum evaporator (N-1200AS, Eyela, Japan), ultrasonic bath (Sonorex RK510H, Bandelin, Germany), high-performance liquid chromatography (HPLC) system (Ultimate 3000, Dionex, Germany), and FTIR spectrophotometer (Spectrum 100, Perkin Elmer, USA).
Phytochemical Analysis of the Leaves
Total phenolic compounds in the plant extracts were determined as per method of Slinkard and Singleton[Citation19] by using Folin–Ciocalteu reagent and was expressed as gallic acid equivalent (GAE). The content of alkaloids was estimated on a % weight basis following the ammonium hydroxide method of Harborne.[Citation20] The tannin content was estimated by Folin-Denis method[Citation21] and a standard curve of tannic acid was used for quantification. Terpenoids was estimated on a % weight basis as per the method described by Ferguson.[Citation22] Total flavonoids content was determined following aluminium chloride method of Zhishen et al.[Citation23] and a standard curve of quercetin was used for quantification. Anthraquinone content was estimated according to the method of Soladoye and Chukwuma[Citation24] and a standard curve of alizarin was used for quantification. Determination of gylcosides was done by modification of the method of El-Olemy[Citation25] and a standard curve of digitoxin was used for quantification. Anthocyanin content in the plant materials was estimated according to Ozsoy et al.[Citation26] Determination of saponin content was done according to Brunner[Citation27] and quantification was done based on disogenin standard curve. Estimation of phytosterols was done according to the method of Sabir et al.[Citation28] by using Liberman-Burchard reagent and a standard curve of stigmasterol was used for quantification.
Preparation of Solvent Extracts (SEs)
Leaves were washed with deionized water, cut into small pieces, and dried in a tray dryer at 45ºC until complete dryness. The dried leaves were ground to a fine powder and sieved through 50 U.S. mesh size. The powders were extracted with distilled water (H2O), methanol (MeOH), EA and hexane (Hex) at room temperature in an incubator shaker at 150 rpm for 24 h. An ultrasonic bath was used to enhance the dissolution process at regular intervals. The extracts were filtered through four layers of muslin cloth and centrifuged at 3000 rpm for 10 min and filtered through Whatman No. 1 filter paper in a vacuum assisted filtration unit. The extracts were further dried in a rotary vacuum evaporator at room temperature under reduced pressure. The weight of the dried extracts was recorded and again dissolved in deionized water to a final concentration of 1000 µg/mL (stock solution). The dried extracts were dissolved in deionized water in order to nullify any effect which other synthetic solvents might have on the desired property. In order to facilitate dissolution of the non-polar extracts like methanol and Hex, an ultrasonic probe (UW2070, Bandelin, Germany) was used to bring uniform dispersion.
FTIR Analysis of the SEs
Attenuated total reflectance (ATR) FTIR spectrophotometric analysis was used to identify the functional group present in the SEs in liquid state. A horizontal ATR accessory which allows direct sample application of liquids was used for obtaining the spectra. A zinc selenide (ZnSe) crystal trough plate at 45º was used as a liquid sampling top-plate. The data were collected in absorbance (A) mode and the selected wavelength ranged from 4000 to 400 cm–1. A resolution of 4 cm–1 was used while the number of scans per sample was four. Detection was done based on the peak value in the region of infrared radiation and compared with previously reported data and other libraries.
Antimicrobial Activity of the SEs
The agar well diffusion method of Perez et al.[Citation29] was followed for carrying out the assays. Various media were used based on the microorganisms viz. potato dextrose agar for F. oxysporum and C. albicans, Baird Parker agar base supplemented with egg yolk emulsion and potassium tellurite for S. aureus, Eosin Methylene Blue (EMC) agar for E. coli, de Man, Rogosa and Sharpe (MRS) agar for Lactobacillus plantarum and modified Mannitol Yolk Polymyxin (MYP) agar for Bacillus subtilis. Cultures (100 µL, 24 h old) with the count adjusted to 108 CFU/mL were spread on the respective plates. Two wells were made in all the plates with a sterile cork borer. A volume of 100 µL of the SEs were added to one well and deionized water was used as a negative control. Diffusion of the extracts was allowed at room temperature for 1 h in a laminar air flow work chamber. In case of mould, 10 mm diameter circles of 72 h old culture of F. oxysporum were dug out using a cork borer and inoculated on the center of the respective plates and the extracts were allowed to diffuse in the same manner as in case of the other organisms. The plates were incubated for 96 h at 25ºC for F. oxysporum, 48 h at 30ºC for C. albicans, 48 h at 37ºC for E. coli, S. aureus, L. plantarum, and B. subtilis. The zones of inhibition were measured in terms of their diameter with an antibiotic zone scale (HiMedia, India).
Determination of Minimum Inhibitory Concentration (MIC)
MIC value of each of the plant extract showing antimicrobial activity against the tested microorganism was determined according to the broth microdilution method of Basri and Fan.[Citation30] The SEs were serial diluted on antibiotic assay broth (Medium No 3, HiMedia, India) in test tubes to make final concentrations ranging from 10 to 500 µg/mL. Inoculum (100 μL) of bacteria (108 CFU/mL) and yeast (107 CFU/mL) cultures were added to each tube. Assay broth without inoculation was used as negative control, while broth containing antibiotic was used as positive control. The tubes were incubated at 37ºC for 24 h for bacteria and 27ºC for 48 h for yeast. Each extract was assayed in triplicate and 100 µL of media from each tube was plated onto agar plates for observation of apparent growth. The MIC value was taken as the least concentration of extract showing no visible growth of the microbes on plating. In order to bring out a comparison, standard antimicrobial agents, viz. streptomycin and chloramphenicol were assessed for their MIC values against the bacteria and fungi, respectively.
Statistical Analysis
Statistical analysis was carried out using the software Origin Pro (Version 8.0) and values were taken as mean of replicates and the standard deviation (SD) was calculated. The Fisher’s least significant difference (LSD) was taken at p < 0.05.
Results AND DISCUSSIONS
Various Phytochemicals in the Leaves
The content of various phytochemicals in the leaf samples are presented in . The highest content of total polyphenols (2.06%) was found in O. corymbosa, followed by A. malaccensis and A. heterophyllus with no significant difference and lowest (0.62%) was found in C. extensa. The highest content of alkaloids content was recorded in A. heterophyllus (6.00 mg/g) and lowest in A. malaccensis (2.47 mg/g). No significant difference in content was recorded between C. extensa and O. corymbosa. The highest content of tannins (1.95 mg/g) was found in C. extensa and lowest in A. malaccensis (0.75 mg/g). No significant difference in content between A. heterophyllus and O. Corymbosa was observed. A. heterophyllus had the highest content of terpenoids (5.79 mg/g), followed by O. corymbosa (3.55 mg/g), A. malaccensis (2.50 mg/g) and C. extensa (1.71 mg/g). The highest content of flavonoids was recorded in O. corymbosa (6.82 mg/g) followed by C. extensa (5.47 mg/g), whereas in both A. heterophyllus and A. malaccensis the content was below 2.00 mg/g. Anthocyanins which belong to the family of flavonoids were also detected and A. heterophyllus and C. extensa revealed the same anthocyanin content of 0.23 mg/g, but significant difference between O. corymbosa (0.45 mg/g) and A. malaccensis (0.59 mg/g) was observed. No significant difference in glycosides content among all the species was observed and ranged from 1.45 to 1.52 mg/g. The anthraquinone content was low in all the plant species and ranged from 0.13 mg/g (C. extensa) to 0.18 mg/g (A. malaccensis). The highest content of saponins was recorded in C. extensa (1.78 mg/g), followed by A. heterophyllus (1.49 mg/g), A. malaccensis (1.26 mg/g), and O. corymbosa (1.12 mg/g). The content of phytosterols was relatively high in O. corymbosa (0.70 mg/g) and C. extensa (0.57 mg/g), while both A. heterophyllus and A. malaccensis were at par (0.14 mg/g).
TABLE 1 Content of different phytochemicals in the leaves of the four plant species
The methanolic extract of A. malaccensis leaves was also examined by Sahoo et al.[Citation31] and found the total phenolic content 76.25 mg GAE/g of the extract. In another study by Ahmed et al.,[Citation32] the methanolic extract of the closely related species A. nigra leaf showed the presence of medicinally active secondary metabolites such as alkaloids, glycosides, cardiac glycosides, flavonoids, steroids, tannins, anthraquinone glycosides, and saponins. The presence of alkaloids, tannins, glycosides, terpenoids, steroids, flavonoids, and saponins has already been reported in the extracts of O. corymbosa by Hussain and Kumaresan.[Citation33] The total phenolic and flavonoid contents of the aqueous extract of O. corymbosa were also found to be 11.6 mg/gm and 4.4 mg/gm respectively by Yadav and Agarwala.[Citation34] The presence of polyphenols in fairly high amount is interesting, as it has high potential to be used as anticancer agents, antioxidants, food preservatives, antifibrillogenic agents for the fight against neurodegenerative pathologies, and other functionalized materials.[Citation35] Tannins were found in minimal amount and intake of a small quantity of tannin may be beneficial to human health by affecting the metabolic enzymes, immunomodulation, or other functions.[Citation36] The presence of terpenoids and flavonoids also suggest that these plants may be effective against cancer, malaria, inflammation, coronary heart diseases, and a variety of infectious diseases.[Citation37,Citation38] Anthraquinones, glycosides, and saponins were significantly less in occurrence in all the species. The phytosterols were also present and have role in inhibition of the absorption of intestinal cholesterol, including recirculating endogenous biliary cholesterol.[Citation39]
Functional Groups Detected in the SEs
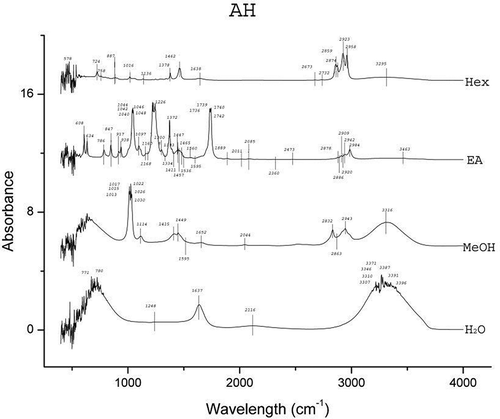
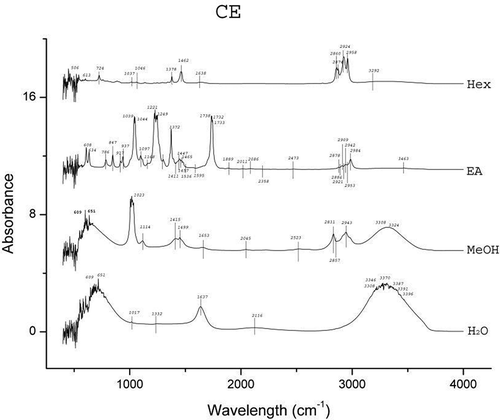
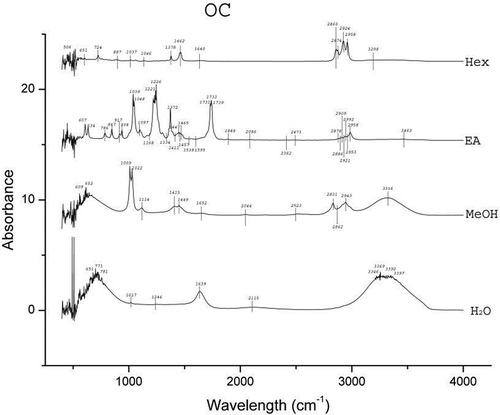
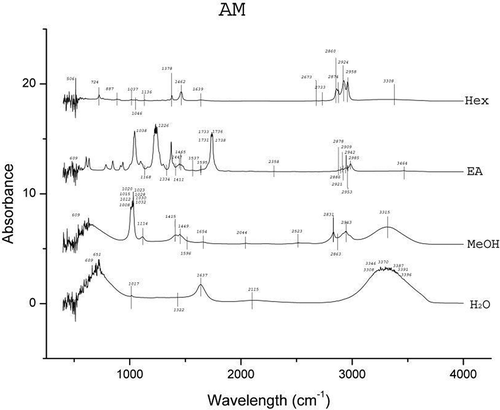
The results of FTIR peak values and the respective functional groups are presented in and the spectra are illustrated in , , , and . The FTIR spectra indicated the presence of various functional groups and compounds in the tested plant extracts and variations in the peaks of all the extracts were observed. Alkanes and alkyl halides were invariably present in all the extracts. Except for the Hex extracts, phenolic groups were present in all the extracts. Carboxylic acids were present in extracts of H2O, MeOH, and EA of A. heterophyllus, H2O, and Hex of C. extensa, H2O, MeOH, and Hex of O. corymbosa and A. malaccensis. The alcohols were present in EA extracts of all the species and the H2O extracts of A. heterophyllus, O. corymbosa, and A. malaccensis. Results revealed that the primary amines were present in the H2O, MeOH and EA extracts. The alkynes were totally absent from A. heterophyllus and were present in H2O, MeOH, and Hex extracts of C. extensa, O. corymbosa, and A. malaccensis. The carbonyl groups were present in the H2O and EA extracts of A. heterophyllus, C. extensa, and A. malaccensis, but in case of EA extract of O. corymbosa their presence was detected. Aliphatic amines were present in the MeOH and EA extracts of A. heterophyllus, MeOH, and Hex extracts of C. extensa and O. corymbosa and MeOH, EA, and Hex extracts of A. malaccensis. Esters or ethers were present in the EA extract of A. heterophyllus, H2O, and EA extract of C. extensa, EA extract of O. corymbosa, H2O, and EA extract of A. malaccensis. Aromatics were present in the EA and Hex extracts of all the species. The ketones, nitro compounds, and aromatic amines were present only in the EA extract of all the species. Aldehydes were found only in the H2O extract of A. heterophyllus and O. corymbosa. These functional groups suggest the presence of compounds such as flavonoids, saponins, alkaloids, phenols, glycosides, steroids, and naphthoquinone.[Citation40] The occurrence of similar pattern of functional groups has also been reported in the leaf extracts of some other plant species like Albizia lebbeck by Bobby et al.,[Citation41] Aerva lanata by Mariswamy et al.,[Citation42] and Hybanthus enneaspermus by Anand and Gokulakrishnan.[Citation43]
TABLE 2 The functional groups detected by ATR-FTIR analysis of the water, methanol, ethyl acetate, and hexane extracts of the four plant species
Antimicrobial Activity of the SEs
The antimicrobial activities of the SEs are presented in and wide variations in the activity of the extracts against different pathogens were observed. C albicans is a normal commensal of humans but can be pathogenic if a person’s immunity is lowered resulting in oral and genital infections and is the causative organism for candidaitis.[Citation44] The H2O extract of none of the species was found to be effective against C. albicans. The MeOH extracts of A. heterophyllus and C. extensa were also found to have no activity; however, the MeOH extracts of O. corymbosa and A. malaccensis showed zones of 5.00 and 11.25 mm, respectively. The EA extract of A. heterophyllus, C. extensa, and A. malaccensis revealed inhibition zones of 16, 10, and 14 mm, respectively, but EA extract of O. corymbosa did not show any activity. On the other hand, the Hex extract of only A. malaccensis showed a zone of 15.50 mm. However, it was observed that none of the SEs were effective against the mould F. oxysporum. E. coli is an important member of the coliform group and most strains of E. coli are commonly a part of the normal flora of the gut and are harmless, but some causes serious food poisoning, gastroenteritis, diarrhea, and urinary tract infections.[Citation45] The H2O extract of only A. malaccensis showed inhibition zone of 6.25 mm. In case of MeOH extracts largest zone was shown by A. malaccensis (19.50 mm), followed by C. extensa (12.75 mm) and O. corymbosa (11.50 mm). All the EA extracts showed activity and the highest was shown by A. heterophyllus (33.25 mm) and A. malaccensis (29.00 mm), while in case of both C. extensa and O. corymbosa the zones remained in the range of 16 mm. The Hex extract of A. heterophyllus showed a zone of 22.00 mm and less activity was shown by A. heterophyllus (3 mm) and there was no activity by C. extensa and O. corymbosa. Some strains of S. aureus are a common cause of skin infections, respiratory diseases, and food poisoning.[Citation40] The H2O extract of none of the species was found to be effective against S. aureus. In case of MeOH extracts, A. heterophyllus showed a zone of 12.25 and 5.00 mm in case of O. corymbosa. All the EA extracts were found to be effective, with A. heterophyllus showing the largest zone of 23.50 mm followed by A. malaccensis (17.50 mm). Both the EA extracts of C. extensa and O. corymbosa showed zones in the range of 12 mm. The Hex extract of only O. corymbosa showed a zone of 5.00 mm. The Gram-positive bacterium B. subtilis can form endospore and can survive in extreme environmental conditions of temperature and desiccation. It is also responsible for the formation of biofilms.[Citation46] Even in this case the EA extracts were found to be effective and highest activity was that of A. heterophyllus and A. malaccensis (18.22 and 18.19 mm zones of inhibition, respectively).
TABLE 3 Antimicrobial activity of the four plant extracts
Substantial activity of the SEs against the tested water and foodborne pathogens was observed. The folklore or traditional belief stands that rice beer has the potential to treat various ailments of microbial origin. Hence, the inhibitory effects shown by the SEs justifies the usage of these plant leaves in the rice beer making process. Moreover, this effectiveness also implies their usage in food preservation in general and rice beer in particular. It was interesting to see that none of the SEs could inhibit the growth of the fermenting organisms viz. S. cerevisae and L. plantarum. S. cerevisiae, also known as brewer’s yeast is one of the most notable and well-known species of yeast in fermentation, health, and wellness. It is used as a protein supplement, energy booster, immune enhancer, or other vehicle for compound insertion.[Citation47] L. plantarum is found in a variety of fermented foods and is a natural inhabitant of the human gastrointestinal tract. It is a potentially probiotic lactic acid producing bacteria.[Citation48] The ineffectiveness of the SEs against these representative beneficial fermentative microorganisms signifies a positive implication of these plants’ use in the fermentation process of rice beer. The results also revealed that the EA extracts of all the species exhibited high antimicrobial activity. The FTIR analysis () also favorably supported the higher number of functional groups in EA extracts as compared to the other solvents. The major peaks shown in the EA extracts were of ketones, alcohols and carboxylic acids in case of A. heterophyllus (), carbonyl group, alcohols, and aliphatic amines in case of C. extensa (), carbonyl group and alcohols in case of O. corymbosa and carbonyl group, alcohols, and aliphatic amines in case of A. malaccensis. The MeOH extracts of C. extensa, O. corymbosa, and A. malaccensis also exhibited antimicrobial properties, especially against E. coli. In the MeOH extracts, the major peaks were shown by phenolics in case of C. extensa, O. corymbosa, and A. malaccensis; carboxylic acids in case of C. extensa and A. malaccensis; alkanes in case of C. extensa and aliphatic amines in case of A. malaccensis. In Hex extracts, substantial activity was exhibited by A. malaccensis and the major peaks were shown by the alkanes, alkynes, and aromatics.
In the work by Pauline et al.,[Citation6] with antibacterial effect of C. interruptus extracts against S. aureus, it was seen that the highest activity was of the crude acetone extracts at a minimum concentration 31.255 µg/100 µL. This was followed by the EA and chloroform fractions of the crude extract. In the work of Loizzo et al.[Citation49] the total water extract, EA, and aqueous fractions from the leaves of A. heterophyllus were evaluated for antibacterial activities by agar diffusion method against E. coli, B. cereus, and S. aureus. They reported the diameters of inhibition 12.2 mm for the total extract, 10.7 and 11.5 mm for EA and aqueous fractions, respectively. The antibacterial activity observed for the methanolic extract of the closely related species A. nigra leaf at 2 mg/disc was found to be mild as compared to tetracycline (50 mg/disc).[Citation32] The extract O. corymbosa were also found to have antimicrobial activity against both Gram (+) and Gram (–) bacteria by Hussain and Kumaresan.[Citation33]
MIC Values of the SEs
The MICs are defined as the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism. MICs are very helpful in determining the in vitro activity of new antimicrobials against a wide range of test organisms.[Citation50] The MIC values of the extracts which evinced positive antimicrobial activity are shown in . In case of C. albicans, the EA extract of A. heterophyllus was found to be the most effective with a MIC value of 75 µg/mL and was followed by the EA extract of A. malaccensis with a value of 100 µg/mL. Similar values were observed for the EA extract of C. extensa and Hex extract of A. malaccensis (120 µg/mL) and MeOH extract of O. corymbosa and MeOH extract of A. malaccensis (150 µg/mL). For E. coli the EA extract of A. malaccensis was found to be the most effective with a MIC value of 45 µg/mL. Both the EA extract of A. heterophyllus and the MeOH extract of A. malaccensis evinced a value of 50 µg/mL, which was followed by Hex extract of A. malaccensis (60 µg/mL), EA extract of O. corymbosa (70 µg/mL), EA extract of C. extensa (80 µg/mL), MeOH extract of O. corymbosa (120 µg/mL), and MeOH extract of C. extensa (170 µg/mL), respectively. The Hex extract of A. heterophyllus (270 µg/mL) and H2O extract of A. malaccensis (350 µg/mL) were found to be the least effective. For S. aureus the highest activities were shown by the EA extracts of A. heterophyllus (60 µg/mL) and A. malaccensis (100 µg/mL). The MIC value of EA extract of O. corymbosa and MeOH extract of A. malaccensis were similar (130 µg/mL), and followed by MeOH extract of O. corymbosa (190 µg/mL), EA extract of C. extensa (200 µg/mL), and Hex extract of O. corymbosa (280 µg/mL), respectively. In case of B. Subtilis also, the lowest MIC value was of the EA extracts of A. heterophyllus (130µg/mL) and was followed by the EA extracts of A. malaccensis (180 µg/mL), while the EA extracts of C. extensa and O. corymbosa had similar MIC values. In comparison, the effectivity of streptomycin against E. coli, S. arueus and B. subtilis in terms of its MIC value was found to be 20, 50, and 30 µg/mL, respectively. While, the MIC value of chloramphenicol against C. albicans was 25 µg/mL. In similar studies, Loizzo et al.[Citation49] also found the MICs of A. heterophyllus leaves to range from 221.9 µg/mL for EA fraction to 488.1 µg/mL for total extract against some common foodborne pathogens. Sahoo et al.[Citation31] also reported the MIC values of the methanolic extract of A. malaccensis to be 2.5, 10, 8.5, and 9.5 µL/mL against S. aureus, P. aeruginosa, C. albicans, and A. niger, respectively.
TABLE 4 Minimum inhibitory concentration (MIC) values of the four plant extracts
CONCLUSION
Results revealed the presence of diverse group of phytochemicals were observed in all the four plant species under investigation. All the four plant species exhibited antimicrobial properties and the EA fractions produced the most effective antimicrobial properties and also contained wide array of functional groups. Moreover, it was observed that the extracts were more effective against E. coli as compared to the other pathogens. The MIC values of some of the extracts particularly A. malaccensis was close to establish antimicrobial agents and justifies the importance of these plants for using as natural food preservers and also in the preparation of antimicrobial drugs. All the tested plants have high potentials for extraction of bioactive compounds and antimicrobial agents, which could be used in the brewing, food and pharmaceutical industries.
FUNDING
The financial support provided by the Ministry of Defence, GoI, New Delhi is duly acknowledged.
Additional information
Funding
REFERENCES
- Das, A.J.; Deka, S.C.; Miyaji, T. Methodology of Rice Beer Preparation and Various Plant Materials Used in Starter Culture Preparation by Some Tribal Communities of North-East India: A Survey. International Food Research Journal 2012, 1, 101–107.
- Das, A.J.; Khawas, P.; Miyaji, T.; Deka, S.C. HPLC and GC-MS Analyses of Organic Acids, Carbohydrates, Amino Acids, and Volatile Aromatic Compounds in Some Varieties of Rice Beer from Northeast India. Journal of the Institute of Brewing 2014, 120, 244–252.
- Das, A.J.; Deka, S.C. Mini Review: Fermented Foods and Beverages of the Northeast India. International Food Research Journal 2012, 19(2), 377–392.
- Final Report of the R&D project “Quality Improvement of Traditional Method of Rice Beer Production by the Tribal People of North-East India”. Submitted to: Ministry of Food Processing Industries, New Delhi, India by Tezpur University, Assam, India.
- Das, A.J.; Das, G.; Miyaji, T.; Deka, S.C. In Vitro Antioxidant Activity of Polyphenols Purified from Four Different Plant Species Used in the Preparation of Rice Beer in Assam, India. International Journal of Food Properties 2015, DOI:10.1080/10942912.2015.1038835
- Pauline, V.C.; Irudayaraj, V.; Johnson, M. Anti-Bacterial Efficacy of Macroscopic, Microscopic Parts of Sporophyte and in Vitro Cultured Gametophyte of a Fern Cyclosorus Interruptus (Willd.) H. Ito (Thelypteridaceae–Pteridophyta). Journal of Chemical and Pharmaceutical Research 2012, 4(2), 1167–1172.
- Stone, B.C. The Flora of Guam. Micronesica 1970, 6, 1–659.
- Patel, T.D.; Jain, V.; Dodia, R. Oldenlandia Corymbosa L.: A Phytopharmacological Review. International Journal of Phytopharmacy 2014, 4(3), 79–82.
- Noiarsa, P.; Ruchirawat, S.; Otsuka, H.; Kanchanapoom, T. Chemical Constituents from Oldenlandia Corymbosa L. of Thai Origin. Journal of Natural Medicines 2008, 62(2), 249–250.
- Periyanayagam, K.; Karthikeyan, V. Wound Healing Activity of the Leaves of Artocarpus Heterophyllus Lam. (Moraceae) on Ex-Vivo Porcine Skin Wound Healing Model. Innovare Journal of Life Sciences 2013, 1(1), 28–33.
- Prakash, O.; Jyoti, Kumar, A.; Kumar, P. Screening of Analgesic and Immunomodulator Activity of Artocarpus Heterophyllus Lam. Leaves (Jackfruit) in Mice. Journal of Pharmacognosy and Phytochemistry 2013, 1(6), 33–36.
- Shahin, N.; Sanjar Alam, S.; Ali, M. Pharmacognostical Standardisation and Antidiabetic Activity of Artocarpus Heterophyllus Leaves Lam. International Journal of Drug Development and Research 2012, 4(1), 346–352.
- Holttum, R.E. The Zingiberaceae of the Malay Peninsula. The Gardens’ Bulletin 1950, 13(1), 1–249.
- Bhuiyan, M.N.I.; Chowdhury, J.U.; Begum, J.; Nandi, N.C. Essential Oils Analysis of the Rhizomes of Alpinia Conchigera Griff. and Leaves of Alpinia Malaccensis (Burm. f.) Roscoe from Bangladesh. African Journal of Plant Science 2010, 4(6), 197–201.
- Victório, C.P. Therapeutic Value of the Genus Alpinia, Zingiberaceae. Brazilian Journal of Pharmacognosy 2011, 21(1), 194–201.
- Smith, R.M. Alpinia (Zingiberaceae): A Proposed New Infrageneric Classification. Edinburgh Journal of Botany 1990, 47(1), 1–75.
- Kress, W.J.; Ai-Zhong, L.; Mark, N.; Qing-Jun, L. The Molecular Phylogeny of Alpinia (Zingiberaceae): A Complex and Polyphyletic Genus of Gingers. American Journal of Botany 2005, 92, 167–178.
- Pascaline, J.; Charles, M.; Lukhoba, C.; George, O. Phytochemical Constituents of Some Medicinal Plants Used by the Nandis of South Nandi District Kenya. Journal of Animal and Plant Science 2011, 9(3), 1201–1210.
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. American Journal of Enology and Viticulture 1977, 28, 49–55.
- Harborne, J.B. Phytochemical Methods; Chapman and Hall: London, 1973; 49–188.
- Schanderi, S.H. Method in Food Analysis; Academic Press: New York, NY, 1970; 709.
- Ferguson, N.M. A Text Book of Pharmacognosy. Mac Millan Company: New Delhi, 1956; 191.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chemistry 1999, 64, 555–559.
- Soladoye, M.O.; Chukwuma, E.C. Quantitative Phytochemical Profile of the Leaves of Cissus Populnea Guill. & Perr. (Vitaceae)—An Important Medicinal Plant in Central Nigeria. Archives of Applied Science Research 2012, 4(1), 200–206.
- El-Olemy, M.M.; Al-Muhtadi, F.J.; Afifi, A.F.A. Experimental Phytochemistry: A Laboratory Manual; King Saud University Press: Saudi Arabia, 1994.
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant Activity of Smilax Excelsa L. Leaf Extracts. Food Chemistry 2008, 110, 571–583.
- Brunner, J.H. Direct Spectrophotometric Determination of Saponins. Analytical Chemistry 1984, 34, 1314–1326.
- Sabir, S.M.; Hayat, I.; Gardezi, S.D.A. Estimation of Sterols in Edible Fats and Oils. Pakistan Journal of Nutrition 2003, 2(3), 178–181.
- Perez, C.; Paul, M.; Bazerque, P. An Antibiotic Assay by the Agar Well Diffusion Method. Acta Biologiae et Medecine Experimentaalis 1990, 15, 113–115.
- Basri, D.F.; Fan, S.H. The Potential of Aqueous and Acetone Extracts of Galls of Quercusin Fectoria As Antibacterial Agents. Indian Journal of Pharmacology 2005, 7(1), 26–29.
- Sahoo, S.; Singh, S.; Nayak, S. Chemical Composition, Antioxidant, and Antimicrobial Activity of Essential Oil and Extract of Alpinia Malaccensis Roscoe (Zingiberaceae). International Journal of Pharmacy and Pharmaceutical Sciences 2014, 6(7), 183–188.
- Ahmed, A.M.A.; Sharmen, F.; Mannan, A.; Rahman, M.A. Phytochemical, Analgesic, Antibacterial, and Cytotoxic Effects of Alpinia Nigra (Gaertn.) Burtt Leaf Extract. Journal of Traditional and Complementary Medicine 2014, 1–5.
- Hussain, A.Z.; Kumaresan, S. GC-MS Analysis and Antimicrobial Evaluation of Oldenlandia Corymbosa. Journal of Environmental Nanotechnology 2014, 3(2), 161–167.
- Yadav, R.N.S.; Agarwala, M. Phytochemical Analysis of Some Medicinal Plants. Journal of Phytology 2011, 3(12), 10–14.
- Quideau, S.P.; Deffieux, D.; Douat-Casassus, C.L.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angewandte Chemie International Edition in English 2011, 50(3), 586–621.
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Critical Reviews in Food Science and Nutrition 1998, 38(6), 421–464.
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as Therapeutic Drugs and Pharmaceutical Agents. In Natural Products; Zhang, L.; Demain, A.L.; Eds.; Humana Press Inc.: Totowa, NJ, 2005; 197–227.
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods for Human Nutrition 2004, 59(3), 113–122.
- Ostlund Jr, R.E. Phytosterols in Human Nutrition. Annual Review of Nutrition 2002, 22, 533–549.
- Ahmad, I.; Aqil, F. In Vitro Efficacy of Bioactive Extracts of 15 Medicinal Plants Against ESβL-Producing Multidrug-Resistant Enteric Bacteria. Microbiological Research 2007, 162(3), 264–275.
- Bobby, M.N.; Gnanaraj, W.E.; Antonisamy, J.M. FT-IR Studies on the Leaves of Albizia Lebbeck Benth. International Journal of Pharmacy and Pharmaceutical Sciences 2012, 4(3), 293–296.
- Mariswamy, Y.; Gnanaraj, W.E.; Antonisamy, J.M. FTIR Spectroscopic Studies on Aerva Lanata (L.) Juss. Ex Schult. Asian Journal of Pharmaceutical and Clinical Research 2012, 5(2), 82–86.
- Anand, T.; Gokulakrishnan, K. Phytochemical Analysis of Hybanthus Enneaspermus Using UV, FTIR, and GC-MS. International Organization of Scientific Research Journal of Pharmacy 2012, 2(3), 520–524.
- Ryan, K.J. Candida, Aspergillus and Other Opportunistic Fungi. In Sherris Medical Microbiology, 4th ed. ; Ryan, K.J.; Ray, C.G.; Ahmad, N.; Drew, W.L.; Plorde, J.; Eds.; McGraw Hill: New York, USA, 2004; 659–668.
- Singleton, P. Bacteria in Biology, Biotechnology, and Medicine, 5th Ed.; Wiley: West Sussex, England, 1999; 444–454.
- Morikawa, M. Beneficial Biofilm Formation by Industrial Bacteria Bacillus Subtilis and Related Species. Journal of Bioscience and Bioengineering 2006, 101(1), 1–8.
- Moyad, M.A. Brewer’s/Baker’s Yeast (Saccharomyces Cerevisiae) and Preventive Medicine: Part II. Urologic Nursing 2008, 28(1), 73–75.
- Zago, M.; Fornasaria, M.E.; Carminatia, D.; Burnsb, P.; Suàrezb, V.; Vinderolab, G.; Reinheimerb, J.; Giraffa, G. Characterization and Probiotic Potential of Lactobacillus Plantarum Strains Isolated from Cheeses. Food Microbiology 2011, 28(5), 1033–1040.
- Loizzo, M.R.; Tundis, R.; Chandrika, U.G.; Abeysekera, A.M.; Menichini, F.; Frega, N.G. Antioxidant and Antibacterial Activities on Foodborne Pathogens of Artocarpus Heterophyllus Lam. (Moraceae) Leaves Extracts. Journal of Food Science 2010, 75(5), M291–M295.
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. Journal of Antimicrobial Chemotherapy 2001, 48, S15–S16.