Abstract
Phenolic compound profiles were investigated by high-performance liquid chromatography-diode array detector in three vintages to assess genotypic variation in berry skins of six resistant grapevine varieties: “Cabernet Cortis,” “Chancellor,” “Léon Millot,” “Maréchal Foch,” “Monarch,” and “Regent.” The research has revealed a significant difference among the varieties in the total phenolic content, anthocyanins, flavanols, and flavan-3-ols. A clear differentiation of all varieties by means of the polyphenol profile using discriminant analyses has been achieved. “Regent” reached the significantly highest total polyphenol content, as well as total anthocyanin and total flavonol content. The polyphenol profile of “Chancellor” was quite different from all of the other studied varieties. Although being closely related, “Maréchal Foch” and “Léon Millot” showed qualitatively and quantitatively quite different polyphenol contents. No clear connection between the genetic origin of the varieties and their polyphenol profiles has been found.
INTRODUCTION
Viticulture worldwide is based mainly on the growth of numerous varieties of the species Vitis vinifera L. Due to their quality, these varieties are considered the only ones suitable for wine production. Aside from Vitis vinifera L. many other species are classified in the genus Vitis. Due to their evolution on different continents and under different environmental conditions they significantly differ in some important biological characteristics.[Citation1,Citation2]
After the root louse, phylloxera (Daktulosphaira vitifoliae Fitch), powdery mildew (Uncinula necator Burr), and downy mildew (Plasmopara viticola Berl.) were accidentally imported into the European vineyards in the mid-19th century, where they caused substantial losses on the highly susceptible V. vinifera vines planted there, resistant American varieties aroused the interest of breeders. They were first used as rootstocks and later in breeding programs with the aim to develop varieties resistant to phylloxera as well as fungal pathogens.[Citation3]
Despite the obstacles in achieving both good resistance to pathogens and high quality, by the end of the 20th century a certain number of varieties has been recognized and put into production.[Citation4–Citation8] Although the varieties arising from interspecies crossings long had a bad reputation, from the first hybrids that demonstrated good resistance but low quality, lately the interest for growing a new generation of resistant varieties increased.[Citation3,Citation9] One of the main reasons is the fact that grape production requires a lot of plant protection products, especially fungicides,[Citation10] and that, aside from increasing the production costs, raises concerns about their adverse influence on the environment and human health.[Citation11,Citation12]
A prerequisite for spreading new grape varieties is the awareness of their important biological and production characteristics. The chemical composition of grapes is essential for the quality of future wine. Among the numerous chemical constituents of grapes, phenolics have a special bearing on the sensory properties and stability of wine. In recent years, primarily due to the antioxidant activity of a variety of phenolic compounds, considerable attention has been focused on the health benefits of grapes and grape products.[Citation13,Citation14] Wine is recommended as part of a healthy diet and grapes are recognized as a valuable source of polyphenols for pharmaceuticals, cosmetic products, and food.[Citation15]
The polyphenolic composition of grapes varies significantly among grapevine varieties, as well as among different species of the genus Vitis and their interspecies hybrids.[Citation16–Citation18] Numerous researches have been done and a large amount of phenolic compounds have been identified and quantified in the grape berries and wines from V. Vinifera.[Citation19–Citation23] However, few reports have reported phenolic content and composition in different grape species and their hybrids.[Citation24,Citation25] Anthocyanins are the most studied group of phenols. There is a distinct separation among the different species with regard to monoglucoside and diglucoside anthocyanins. The V. vinifera grapes only contained the monoglucoside anthocyanins, but V. rotundifolia grapes only had diglucoside anthocyanins, while both existed in other species and hybrids. The presence of diglucoside anthocyanins characterized the first-generation of hybrids grown from American vines (V. labrusca, V. riparia, V. rupestris, V. berlandieri) and distinguished them from the Vitis vinifera varieties.[Citation26] However, this characteristic may disappear after further hybridizations.[Citation27,Citation28]
Among the hybrid varieties, some are characterized by a high percentage of diglucoside compounds and the others are characterized by fewer or almost no diglucosides.[Citation17] In order to obtain a better assessment of the full range of variation of polyphenolic compounds in grapes, a comprehensive and systematic evaluation of grape germplasm is needed. This is a prerequisite for its better utilization for different purposes.[Citation24]
The aim of this study was to determine the polyphenolic composition of six red resistant grapevine varieties with different but very complex pedigrees, involving several species of the genus Vitis. Through several cycles of backcrossing, the pedigrees of some of them were left only a small proportion of the genome originated from non-vinifera ancestors, while the others present true interspecies hybrids, meaning that one of their parents belongs to some other Vitis species. Research results would enable their mutual differentiation and be useful for their selection as suitable plant material for the extraction of phytochemicals as ingredients of functional foods, as well as for their use in wine production.
MATERIALS AND METHODS
Grape Samples
Six resistant red grapevine varieties with complex pedigrees, involving several species of the genus Vitis, encompassing Vitis riparia, Vitis aestivalis, Vitis amurensis, Vitis berlandieri, Vitis cinerea, Vitis labrusca, Vitis lincecumii, Vitis riparia, and some others, have been selected for the experiment. “Cabernet Cortis” (“Cabernet Sauvignon” × “Solaris;” VIVC variety numberFootnote1 20005), “Monarch” (“Solaris” × “Dornfelder,” VIVC variety number 19995), and “Regent” (“Diana” × “Chambourcin;” VIVC variety number 4572) belong to a new generation of varieties bred in “Germany,” where only a small proportion of the genome originated from non-vinifera ancestors was left. These varieties are formally recognized as Vitis vinifera and classified for wine production in European Union (EU) countries. “Chancellor” (“Seibel 5163” × “Seibel 880;” VIVC variety number 2446), Léon Millot (“Millardet et Grasset 101-14 OP” (V.riparia × V.rupestris) × “Goldriesling;” VIVC variety number 6806) and “Maréchal Foch” (“Millardet et Grasset 101-14 OP” (V.riparia × V.rupestris) × “Goldriesling;” VIVC variety number 7388) are Franco-American interspecies hybrids bred in France at the beginning of the 20th century.[Citation29]
The experiment was conducted in three vintages—2009, 2011, and 2012—in an experimental vineyard planted in the Zelina winegrowing region (near the Croatian capital, Zagreb). The area has continental climate with warm or hot summer and cold to very cold winter. The mean annual temperature (2009, 2011, 2012) was 12.3oC, with a mean vegetation temperature of 19.4oC. The annual precipitation was 696 mm, of where of 424 mm has fallen within the vegetation season (April to September).
The experimental vines were grafted on SO4 rootstocks. The vineyard has a planting density of 5681 vines per ha with vines spaced 0.8 m within and 2.2 m between rows. The vines were trained on a Guyot system. All vines were pruned to 10–12 nodes per vine. The cultural practices according to the EU regulation on organic farming were applied.
Grapes were harvested in the moment of technological ripeness of the grape. All varieties are early ripening and they were harvested during the last decade of August and first decade of September. Four random samples of about 1 kg of grapes were taken from each variety and stored frozen, pending analysis. This means that every variety was represented by four samples in all the three years of study, except for the variety “Chancellor” in the year 2009 which was represented by three samples.
Chemicals
All reagents and solvents of analytical or high-performance liquid chromatography (HPLC) grade were purchased from J.T. Baker (Deventer, The Netherlands). The standards used for identification and quantification purposes were as follows: delphinidin 3-O-glucoside, cyanidin 3-O-glucoside, peonidin 3-O-glucoside, malvidin 3-O-glucoside, delphinidin 3,5-O-diglucoside, cyanidin 3,5-O-diglucoside, malvidin 3,5-O-diglucoside, epigallocatechin, procyanidin B1, procyanidin B2, rutin (quercetin 3-O-rutinoside), quercetin 3-O-galactoside, and myricetin (Extrasynthese, Genay Cedex, France); (-)-epicatechin, (+)-catechin, and epicatechin gallate (Sigma-Aldrich, St. Louis, MO, USA); kaempferol, quercetin, and isorhamnetin (Fluka, Steinheim, Germany); quercetin 3-O-glucoside (Sigma, St. Louis, MO, USA).
Sample Preparation
The berry skins were manually removed from the pulp and air dried. Dry skins were ground and the obtained powder (500 mg) was extracted by a 10 mL of 70% aqueous ethanol containing 1% formic acid per 1 day in the dark. The extract was centrifuged at 5000 rpm in an LC-321 centrifuge (Tehtnica, Železnik, Slovenia) for 20 min at room temperature. The supernatant was collected, concentrated under vacuum to remove ethanol (40°C) on a Hei–Vap Adventage G3 rotary evaporator (Heidolph, Schwabach, Germany), and brought to the final volume of 10 mL with eluent A (water/phosphoric acid; 99.5:0.5, v/v). The extract was filtered with Phenex-PTFE 0.20 μm syringe filter (Phenomenex, Torrance, USA) and analyzed by HPLC. The extraction was performed in triplicate.
HPLC Analysis
Separation, identification, and quantification of phenolic compounds from grape skin extracts were performed on an Agilent 1100 Series system (Agilent, Germany), equipped with diode array detector (DAD; G1315B), fluorescence detector (FLD) (G1321 A) and coupled to an Agilent Chem Station (version B.01.03) data-processing station. The separation was performed with a reversed-phase column Luna Phenyl-Hexyl (4.6 × 250 mm; 5 μm particle (Phenomenex, Torrance, USA), with Phenyl guard column (4.0 × 3.0 mm) thermostated at 50°C. The solvents were water/phosphoric acid (99.5:0.5, v/v, eluent A) and acetonitrile/water/phosphoric acid; 50:49.5:0.5, v/v/v, eluent B). The flow rate was 0.9 mL/min. The linear gradient for eluent B was: 0 min, 0%; 7 min, 20%; 35 min, 40%; 40 min, 40%; 45 min, 80%; 50 min, 100%; 60 min 0%. The injection volume for all samples was 20 µL. The DAD was set to an acquisition range of 200–700 nm. Flavonols were detected at 360 nm and anthocyanins at 518 nm. Flavan-3-ols were detected at λex = 225 nm and λem = 320 nm.
Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis
For peak assignment berry skin extracts were analyzed with the Agilent 1200 Series system (Agilent, Germany) coupled online to an Agilent model 6410 mass spectrometer fitted with an electrospray ionization (ESI) source. The separation was performed with the column described in the previous section. The mass spectra of flavan-3-ols and flavonols were recorded in the negative mode and those of anthocyanins in the positive mode. Negative and positive ion mass spectra of the column eluate were recorded in the range m/z 100–1000. Mass spectrometric conditions were applied as previously reported.[Citation30]
Quantification of Individual Compounds
Individual phenolic compounds in berry skin extracts were identified by matching the retention time of each chromatographic peak with the external standard and DAD spectrum (). The quantification of individual phenolic peaks was done using a calibration curve of the corresponding standard compound. When reference compounds were not available, the calibration of a structurally related compound was used. The results are expressed in mg/kg of dry weight (dw) grape skin. The total content of a particular class of flavonoids was expressed as a sum of the contents of individual compounds determined by HPLC.
TABLE 1 HPLC-MS and HPLC-DAD-FLD data of flavonoids from grape berry skins extracts
Statistical Analysis
All variables were examined separately by the analysis of variance (ANOVA) for the 3 years of the study. Means separation by Duncan’s multiple range tests at P ≤ 0.05 was used to establish whether there were significant differences among the varieties using the SAS System Software, v. 9.0 (SAS Institute Inc., Cary, NC, USA, 2004).
Discriminant analyses using the PROC DISCRIM, and PROC CANDISC (SAS, 2004) were performed to evaluate the utility of polyphenol profiles for discrimination among the varieties. The polyphenol profile was evaluated for its performance as a discriminant criterion (PROC DISCRIM) to correctly classify samples into their respective varieties by estimating error rates (probabilities of misclassification) when using standard methods. A canonical discriminant analysis (PROC CANDISC) was performed based on the same traits and the first two canonical variables (CV) were plotted.
RESULTS AND DISCUSSION
Anthocyanins
shows, the content of individual anthocyanins detected in the skin of studied varieties in the three year research period, along with the total anthocyanins (TA) content, which were calculated as the sum of the contents of delphinidin 3,5-O-diglucoside, cyanidin 3,5-O-diglucoside, delphinidin 3-O-glucoside, peonidin 3,5-O-diglucoside, malvidin 3,5-O-diglucoside, cyanidin 3-O-glucoside, peonidin 3-O-glucoside, and malvidin 3-O-glucoside.
TABLE 2 Content of individual and total anthocyanin compounds in the skin of six grapevine varieties studied (mg/kg dw), years 2009, 2011, and 2012
A significant difference among the varieties has been observed in the TA content. As expected, monoglucosylated and diglucosylated anthocyanins have been detected in all varieties, but in differing proportions. According to earlier findings, the presence of monoglucosides only is characteristic for the Vitis vinifera varieties, while the presence of diglucosylated anthocyanins indicates non-vinifera ancestors in the pedigree of such varieties. Diglucosides are typical for grapes of other members of the Vitis genus (V. riparia, V. labrusca, V. rupestris, V. rotundifolia, etc.) and their hybrids.[Citation31,Citation32]
The highest TA content has been observed in Regent (9810 mg/kg dw of grape skin), which included a very high content of both monoglucosides (5945 mg/kg) and diglucosides (3865 mg/kg). “Maréchal Foch” had the second highest TA content but the highest monoglucosylated anthocyanin content of all the varieties. A very low TA has been detected in the grapes of “Cabernet Cortis” and “Léon Millot,” 2769 and 2062 mg/kg, respectively, both characterized by the lowest content of monoglucosides.
Our research results support the finding that a relative abundance of individual anthocyanins is genotype specific.[Citation33–Citation35]
A significant difference among the varieties in the content of eight detected anthocyanins (delphinidin 3-O-glucoside; delphinidin 3,5-O-diglucoside; cyanidin 3-O-glucoside; cyanidin 3,5-O-diglucoside; malvidin 3-O-glucoside, malvidin 3,5-O-diglucoside; peonidin 3-O-glucoside; peonidin 3,5-O-diglucoside) has been observed (). The most abundant anthocyanin in “Léon Millot,” “Cabernet Cortis,” and “Monarch” grapes is malvidin 3,5-O-diglucoside, accounting for 65.40, 59.00, and 55.30% of the total anthocyanin content, respectively. In “Regent” the proportion of malvidin 3-O-glucoside and malvidin 3,5-O-diglucoside in TA was almost equal (37.40 and 35.70%), which is in accordance with the findings of Balík et al.[Citation35] Based on the analysis of the anthocyanin composition in Euvitis (section of genus Vitis) grape varieties, Liang et al.[Citation18] concluded that malvidin derivatives were the most abundant anthocyanins in the majority of germplasm. But for Muscadinia grapes, 3,5-O-diglucosides of delphinidin, cyaniding, and petunidin accounted for approximately 90% of TAs.[Citation36]
According to its anthocyanin profile, the “Chancellor” was quite distant from other varieties having a high proportion of delphinidin, in the monoglucoside form, but especially as diglucoside (more than 40 times higher content than in any other variety studied) and cyanidin 3,5-O-diglucoside. Compared to the other varieties, a very low content of malvidin 3-glucoside was found in “Chancellor” and similar had previously been reported for some other interspecies hybrids as well.[Citation35] Beside the “Chancellor,” the highest proportion of delphinidin 3-O-glucoside has also been detected in “Maréchal Foch” grapes. Despite the fact that “Maréchal Foch” and “Léon Millot” have resulted from the same crossing, they differ significantly both in the TA value as well as in the content of individual anthocyanins.
In the study of Balík et al.[Citation35] the average levels of individual pigments in V. vinifera grapes were as follows: 49% of malvidin 3-O-glucoside, 10% of peonidin 3-O-glucoside, 6% of petunidin 3-O-glucoside, and 6% of delphinidin 3-O-glucoside. Anthocyanins with cyanidin occurred in lower amounts in the grapes of V. vinifera L. varieties. This is in accordance to the research results on 49 red Vitis vinifera varieties [Citation37] where the main anthocyanin was malvidin 3-O-glucoside (mean 42.28%; range 26.08–65.00% of TA), while delphinidin 3-O-glucoside accounted for only 10.72% of TA (range 1.90–26.24% of TA. Although a significant difference in the content of most individual anthocyanins has been observed over the research years, their relative abundance of any given variety remains broadly the same. The stability of the anthocyanin “fingerprint” has been confirmed in sundry researches involving other grapevine varieties.[Citation33,Citation38,Citation39]
Flavonols
Flavonols are only present in the outer epidermis of grape skin and their primary biological function is ultraviolet (UV) protection. Along with anthocyanins, they are considered to be the most important flavonoids that accumulate in this tissue. Flavonol patterns are considered to be an important chemo-taxonomic parameter as well, while varying across genotypes. In recent years, the flavonoid compounds attracted much interest due to their antioxidant proprieties and their potential benefit to human health.[Citation40]
In the present study the average total flavonols (TFO) of selected varieties have ranged between 111 and 840 mg/kg dw grape skin (). A significant difference has been observed among all the varieties. Quercetin 3-O-glucoside was dominant in all samples accounting for 56.44% (“Léon Millot”) to 82.44% (“Chancellor”) of TFOs on average. In the research by Zui et al.[Citation41] on the phenolic characteristics of red grapes, the domination of quercetin 3-O-glucoside in all samples (irrespective of their genetic background) has been observed as well. For all varieties except for “Cabernet Cortis,” the second most abundant flavonol was myricetin 3-O-glucoside. The two other quercetin derivatives, namely quercetin 3-O-glucuronide and rutin, have also been detected.
TABLE 3 Content of indivitual and total flavonol compounds in the skin of six grapevine varieties studied (mg/kg dw), years 2009, 2011, and 2012
The total content of flavonols, as well as of each flavonol detected, was highest in “Regent.” The wines of this variety are known for their strong dark color (rare in wines from cooler climates) which is probably due not only to a high anthocyanin content, but also to high content of flavonols and their mutual reaction producing more stable pigments in red wines.[Citation21]
The high content of TFOs was also observed in “Chancellor” grapes. In this variety the proportion of quercetin 3-O-glucuronide was highest, accounting for 82% of TFOs. The significantly lowest content of all flavonols was detected in “Léon Millot” and this is the only variety in which there was no quercetin 3-O-glucuronide detected in the grape skin. Although having the same parents, “Maréchal Foch” has twice as much TFOs as its sibling “Léon Millot,” and the amount is comparable to the results of Zhu et al.[Citation25]
In contrast to the differences found in the anthocyanin profiles, the flavonols present in grapes of resistant varieties were the same as those in V. vinifera varieties. Quercetin-type and myricetin-type flavonols dominated the flavonol profiles of Vitis vinifera red grape varieties as well. Their proportion and the proportion of their glycosides (glucoside and glucuronide) varied between the different varieties. According to the pattern of the flavonols in 49 red Vitis vinifera varieties obtained by Mattivi et al.,[Citation37] the main compound was quercetin (mean 43.99%; range, 12.34–87.76%), followed by myricetin, which is present in a similar percentage (mean 36.81%; range, 2.35–81.61%).
The “Pinot Noir” grapes had the highest proportion of quercetin-type flavonols and the lowest proportion of myricetin-type flavonols; “Cabernet Sauvignon” and “Merlot” had medium proportions of quercetin-type and myricetin-type flavonols, and varieties “Tempranillo,” “Syrah,” and “Petit Verdot” showed the lowest proportions of quercetin-type flavonols and the highest proportions of myricetin-type flavonols.[Citation42] The main quercetin glycoside detected in “Cencibel” and “Syrah” grape skin was glucoside, and glucuronide in case of Cabernet “Sauvignon” and “Merlot.”[Citation21] Therefore, the principal component (PC) analysis applied to red grape flavonol profiles allowed a high degree of grape variety differentiation.[Citation42]
In some articles only small amounts of rutin have been found in grape skin of Vitis vinifera varieties,[Citation19,Citation43] while in others[Citation21,Citation37,Citation44] rutin was not reported. As opposite to that, rutin is one of the dominant flavonols in muscadine grapes and in non-V. amurensis East Asian species.[Citation41]
Flavan-3-ols
In regard to the family of flavanols (), epicatechin, catechin, epigallocatechin, gallocatechin, as well as procyanidins B1, B2, and B4, have been detected in all skin extracts. Procyanidin B3 has also been determined in all samples except for “Regent” and “Monarch.” The average total flavan-3-ols content varies between the varieties but the differences are not so large as in other classes of phenolics. “Chancellor” had the highest content (187 mg/kg dw grape skin) and “Léon Millot,” as in the other phenolic class, the lowest (107 mg/kg dw grape skin). The most abundant monomer differs among the varieties: for “Regent” and “Maréchal Foch” this is epicatechin, for “Monarch” and “Cabernet Cortis” epigallocatechin and for “Chancellor” the contents of epigallocatechin and catechin are almost equal. Procyanidin B1 is the dominant dimer in all the varieties and, except for “Monarch” (having the lowest content), varies on average in a rather narrow range. The contents of flavonols and flavan-3-ols were also affected by environmental conditions. The differences between the years were also significant.
TABLE 4 Content of individual and total flavan-3-ol compounds in the skin of six grapevine varieties studied (mg/kg dw), years 2009, 2011, and 2012
Discriminant Analysis
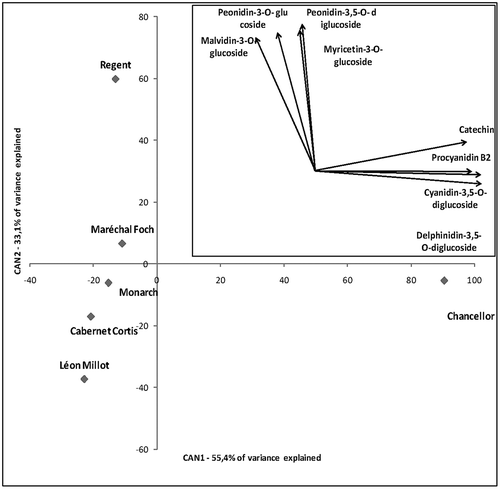
The discriminant function, based on the polyphenol profile 3-year study, correctly classified all the samples into their respective varieties. This means that the varieties used in this research can be clearly distinguished based on their polyphenol profile. The same classification success was attained using just the anthocyanin profile of the six varieties. Significant squared Mahalanobis distances were determined among the class means of all the varieties. The canonical discriminant analysis based on the polyphenol profile over the three years of the study showed that the first two CV explained 88.50% of the variation among varieties (CV1–55.40% and CV2–33.10%). The vector diagram () revealed a clear differentiation among all the varieties which can be explained based on the correlation of polyphenol content with CV1 and CV2.
FIGURE 1 Canonical discriminant analysis of Cabernet Cortis, Chancellor, Léon Millot, Maréchal Foch, Monarch, and Regent based on polyphenol profile in the three years of study. The inserted vector diagram indicates the direction of eight phenolic compounds with highest correlation (>0.9) with first two canonical variables in the space defined by CAN1 and CAN2.
CONCLUSION
This research has revealed a significant difference among the varieties in the total content of all chemical groups of studied polyphenols, anthocyanins, flavanols, and flavan-3-ols. A clear differentiation of all the varieties by means of the polyphenol profile has been attained. Since grapes are highly heterozygous, it was difficult to find a connection between the genetic origin of the varieties and their polyphenol profiles and for that reason it is necessary to characterize each variety before qualifying them as raw material for different purposes. A good example was set by “Maréchal Foch” and “Léon Millot,” having the same parents, but a qualitatively and quantitatively quite different polyphenol content. Nevertheless, the polyphenol (and especially anthocyanin) profile of “Chancellor,” the variety with a very complex pedigree, was quite distant from all the other varieties. Although it was supposed that true interspecies hybrids, due to a greater genetic distance of their ancestors, would have a higher content of polyphenols in comparison with the resistant varieties of the new generation, it was often not the case in this study. Among the tested varieties, the grapes of “Regent” had the highest total polyphenol content, as well as the total anthocyanin and total flavonol content. Moreover, the abundance of anthocyanins, both mono- and diglucosides, qualifies this variety as very suitable for wine production, as well as for the extraction of phytochemicals for foods and pharmaceuticals.
Notes
1 Vitis International Variety Catalogue (VIVC) www.vivc.de
REFERENCES
- Keller, M. Botany and Anatomy. In The Science of Grapevines: Anatomy and Physiology; Academic Press: San Diego, CA, 2010; 1–43.
- Mullins, M.; Bouquet, A.; Williams, L. The Grapevine and Its Relatives. In Biology of the Grapevine; Cambridge University Press: Cambridge, Great Britain, 1996; 17–36.
- Reisch, B.I.; Owens, C.L.; Cousins, P.S. Handbook of Plant Breeding: Fruit Breeding; Badenes, M.L.; Byrne, D.H.; Eds.; Springer: New York, NY, 2012; 225–263.
- Bouquet, A.; Pauquet, J.; Adam-Blondon, A.F.; Torregosa, L.; Merdinoglu, D.; Wiedemann-Merdinoglu, S. Towards the Obtention of Grapevine Varieties Resistant to Powdery and Downy Mildews by Conventional Breeding and Biotechnology. Bulletin OIV 2000, 833/834, 445–452.
- Cindric, P.; Korac, N.; Kovac, V. Grape Breeding for Resistance. Acta Horticulturae 2003, 603, 385–392
- Basler, P. Trends in Cultivation of Fungus Resistant Varietis. Swiss Journal of Horticulture and Viticulture (Engl. Transl.) 2003, 7, 14–18.
- Hajdu, E. Breeding of Table Grape Varieties in Hungary and Beyond Our National Borders. Hungarian Agricultural Research 2007, 4, 4–9.
- Morandell, W. Presentation of varietis from South Tirol, Obstaub-Weinbau (Engl. Transl.) 2008, 3, 89–91.
- Eibach, R.T.R. Success in Resistance Breeding “Regent” and Its Steps into the Market. Acta Horticulturae 2003, 603, 687–691.
- EU, C., Final Report Study of the Use of the Varieties of Interspecific Vines. http://ec.europa.eu/agriculture/markets/wine/studies/vine_en.pdf (accessed March 26, 2015).
- Weisenburger, D.D. Human Health-Effects of Agrichemical Use. Human Pathology 1993, 24, 571–576.
- Margni, M.; Rossier, D.; Crettaz, P.; Jolliet, O. Life Cycle Impact Assessment of Pesticides on Human Health and Ecosystems. Agriculture, Ecosystems & Environment 2002, 93, 379–392.
- Pezzuto, J.M. Grapes and Human Health: A Perspective. Journal of Agricultural and Food Chemistry 2008, 56, 6777–6784.
- Guilford, J.M.; Pezzuto, J.M. Wine and Health: A Review. American Journal of Enology and Viticulture 2011, 62, 471–486.
- Gollücke, A.P.B. Recent Applications of Grape Polyphenols in Food, Beverages, and Supplements. Recent Patents on Food, Nutrition & Agriculture 2010, 2, 105–109.
- Jin, Z.M.; He, J.J.; Bi, H.Q.; Cui, X.Y.; Duan, C.Q. Phenolic Compound Profiles in Berry Skins from Nine Red Wine Grape Cultivars in Northwest China. Molecules 2009, 14, 4922–4935.
- De Rosso, M.; Panighel, A.; Carraro, R.; Padoan, E.; Favaro, A.; Dalla Vedova, A.; Flamini, R. Chemical Characterization and Enological Potential of Raboso Varieties by Study of Secondary Grape Metabolites. Journal of Agricultural and Food Chemistry 2010, 58, 11364–11371.
- Liang, Z.C.; Wu, B.H.; Fan, P.G.; Yang, C.X.; Duan, W.; Zheng, X.B.; Liu, C.Y.; Li, S.H. Anthocyanin Composition and Content in Grape Berry Skin in Vitis Germplasm. Food Chemistry 2008, 111, 837–844.
- Gomez-Alonso, S.; Fernandez-Gonzalez, M.; Martinez, A.M.J.; Garcia-Romero, E. Anthocyanin Profile of Spanish Vitis Vinifera L. Red Grape Varieties in Danger of Extinction. Australian Journal of Grape and Wine Research 2007, 13, 150–156.
- Dimitrovska, M.; Bocevska, M.; Dimitrovski, D.; Murkovic, M. Anthocyanin Composition of Vranec, Cabernet Sauvignon, Merlot, and Pinot Noir Grapes As Indicator of Their Varietal Differentiation. European Food Research and Technology 2011, 232, 591–600.
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascuena, J.M.; Romero, E.G. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis Vinifera Varieties Grown in a Warm Climate. Journal of Food Composition and Analysis 2006, 19, 687–693.
- Guerrero, R.F.; Liazid, A.; Palma, M.; Puertas, B.; Gonzalez-Barrio, R.; Gil-Izquierdo, A.; Garcia-Barroso, C.; Cantos-Villar, E. Phenolic Characterisation of Red Grapes Autochthonous to Andalusia. Food Chemistry 2009, 112, 949–955.
- Vacca, V.; Del Caro, A.; Milella, G.G.; Nieddu, G. Preliminary Characterisation of Sardinian Red Grape Cultivars (Vitis Vinifera L.) According to Their Phenolic Potential. South African Journal of Enology and Viticulture 2009, 30, 93–100.
- Liang, Z.C.; Owens, C.L.; Zhong, G.Y.; Cheng, L.L. Polyphenolic Profiles Detected in the Ripe Berries of Vitis Vinifera Germplasm. Food Chemistry 2011, 129, 940–950.
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars Among Various Genetic Backgrounds and Originations. International Journal of Molecular Sciences 2012, 13, 3492–3510.
- Ribéreau-Gayon, P. Research of Plant Anthocyanins (Engl. Transl.), Librarie Générale de l’Enseignement: Paris, 1959.
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology Volume 2. The Chemistry of Wine Stabilization and Treatments, 2nd Ed; John Wiley and Sons, Ltd: Chichester, UK, 2006.
- Janvary, L.; Hoffmann, T.; Pfeiffer, J.; Hausmann, L.; Topfer, R.; Fischer, T.C.; Schwab, W. A Double Mutation in the Anthocyanin 5-O-Glucosyltransferase Gene Disrupts Enzymatic Activity in Vitis vinifera L. Journal of Agricultural and Food Chemistry 2009, 57, 3512–3518.
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes; Allen Lane: London, UK, 2012.
- Tomaz, I.; Maslov, L.; Stupić, D.; Preiner, D.; Ašperger, D.; Karoglan Kontić, J. Multi-Response Optimization of Ultrasound-Assisted Extraction for Recovery of Flavonoids from Red Grape Skins Using Response Surface Methodology. Phytochemical Analysis 2016, 27, 13–22.
- Mazzuca, P.; Ferranti, P.; Picariello, G.; Chianese, L.; Addeo, F. Mass Spectrometry in the Study of Anthocyanins and Their Derivatives: Differentiation of Vitis Vinifera and Hybrid Grapes by Liquid Chromatography Electrospray Ionization Mass Spectrometry and Tandem Mass Spectrometry. Journal of Mass Spectrometry 2005, 40, 83–90.
- Favretto, D.; Flamini, R. Application of Electrospray Ionization Mass Spectrometry to the Study of Grape Anthocyanins. American Journal of Enology and Viticulture 2000, 51, 55–64.
- Gonzalez-Neves, G.; Franco, J.; Barreiro, L.; Gil, G.; Moutounet, M.; Carbonneau, A. Varietal Differentiation of Tannat, Cabernet-Sauvignon and Merlot Grapes and Wines According to Their Anthocyanic Composition. European Food Research and Technology 2007, 225, 111–117.
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. American Journal of Enology and Viticulture 2006, 57, 257–268.
- Balik, J.; Kumsta, M.; Rop, O. Comparison of Anthocyanins Present in Grapes of Vitis Vinifera L. Varieties and Interspecific Hybrids Grown in the Czech Republic. Chemical Papers 2013, 67, 1285–1292.
- Huang, Z.L.; Wang, B.W.; Williams, P.; Pace, R.D. Identification of Anthocyanins in Muscadine Grapes with HPLC-ESI-MS. LWT–Food and Science Technology 2009, 42, 819–824.
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. Journal of Agricultural and Food Chemistry 2006, 54, 7692–7702.
- Ryan, J.M.; Revilla, E. Anthocyanin Composition of Cabernet Sauvignon and Tempranillo Grapes at Different Stages of Ripening. Journal of Agricultural and Food Chemistry 2003, 51, 3372–3378.
- Revilla, E.; Garcia-Beneytez, E.; Cabello, F. Anthocyanin Fingerprint of Clones of Tempranillo Grapes and Wines Made with Them. Australian Journal of Grape and Wine Research 2009, 15, 70–78.
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. Journal of Agricultural and Food Chemistry 2004, 52, 255–260.
- Zhu, L.; Zhang, Y.L.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars Among Various Genetic Backgrounds and Originations. International Journal of Molecular Sciences 2012, 13, 3492–3510.
- Hermosín-Gutiérrez, I.; Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E. Flavonol Profiles for Grape and Wine Authentication. In Progress in Authentication of Food and Wine. In ACS Symposium Series, vol. 1081; Ebeler, S.; Takeoka, G.R.; Winterhalter, P.; Eds.; American Chemical Society: Washington, DC, 2011; 113–129.
- Careri, M.; Corradini, C.; Elviri, L.; Nicoletti, I.; Zagnoni, I. Direct HPLC Analysis of Quercetin and Trans-Resveratrol in Red Wine, Grape, and Winemaking by Products. Journal of Agricultural and Food Chemistry 2003, 51, 5226–5231.
- Cheynier, V.; Rigaud, J. Hplc Separation and Characterization of Flavonols in the Skins of Vitis-Vinifera Var Cinsault. American Journal of Enology and Viticulture 1986, 37, 248–252.
