Abstract
The current aim was to evaluate antidiabetic potential of Syzygium aromaticum and Cuminum cyminum essential oils and their emulsions by alpha amylase inhibition assay. Antidiabetic activity of C. cyminum and S. aromaticum was examined in dose dependent mode (1 to 100 µg/mL). The maximum antidiabetic activity for S. aromaticum and C. cyminum essential oils was noted at the highest dose (100 µg/mL). Five emulsions (essential oil + surfactant [tween 80] + co-surfactant [ethanol] + water) of different concentrations for S. aromaticum (A1 to A5) and C. cyminum (B1 to B5) essential oils were formulated. Among different emulsions, A5 of S. aromaticum and B5 of C. cyminum essential oil exhibited a maximum antidiabetic activity with 95.30 and 83.09% inhibition of α-amylase, respectively. Moreover, the analysis of essential oils showed that eugenol (18.7%) and α-pinene (18.8%) were the major components of S. aromaticum and C. cyminum essential oils, respectively.
INTRODUCTION
Syzygium aromaticum (family Myrtaceae, common name “clove”) is an evergreen plant with height of 8 to 12 m. Its traditional medicinal uses include treatment of indigestion, headache, insect bites, asthma, wounds, atherosclerosis, scabies, cough, and many other disorders.[Citation1,Citation2] Recent literature provided evidence of the antioxidant, antimicrobial, anticancer, and hepatoprotective potential of this plant.[Citation3–Citation5]
Cuminum cyminum (C. cyminum) of family Apiaceae, is a plant of vernacular name “Zeera.” It is widely used in the traditional healthcare system for the treatment of chronic diarrhea, hypertension, toothaches, scorpion bites, gastrointestinal disorders, etc.[Citation6–Citation8] Several studies have reported that C. cyminum too has broad of a range of biological activities, including antitussive, anticancer, antidiarrhoeal, and anti-inflammatory effects.[Citation9–Citation12]
Though the biological activities of S. aromaticum and C. cyminum were previously reported, there has not been any report regarding the antidiabetic potential of S. aromaticum and C. cyminum emulsions. Therefore, the aim of the current study was to evaluate the antidiabetic potential of S. aromaticum and C. cyminum essential oils and their emulsions. The physico-chemical analysis of essential oils was also conducted.
MATERIALS AND METHODS
Collection of Plant Material
The buds and seeds of Syzygium aromaticum and Cuminum cyminum collected from different areas of Province Khyber Pakhtunkhwa, Pakistan were identified by Dr. Mansoor Hameed (Taxonomist), Department of Botany, University of Agriculture, Faisalabad. A voucher specimen was also submitted to the same department.
Extraction of Essential Oil
The dried plant materials of S. aromaticum and C. cyminum buds were separately ground to a fine powder with the help of an electric grinder. Two hundred fifty grams of finely ground powder of each plant was suspended in distilled water and subjected to hydrodistillation for 3 h using a Clevenger type apparatus. The condensed vapors were collected and the oil layers were separated by using a separating funnel. The extracted essential oils were dried over anhydrous sodium sulfate and stored in airtight glass vials at 4°C, until analysis.
Preparation of Emulsions
Emulsion formulations were prepared as shown in and . Five emulsions of different concentrations of both essential oils were formulated and named as A1 to A5 for S. aromaticum and B1 to B5 for C. cyminum essential oil keeping amount of water and essential oil as two variables. The emulsions were prepared by mixing essential oils with the surfactant before adding to the required amount of water followed by sonication for 30 min.[Citation13]
TABLE 1 Emulsion formulations (V/V %) of S. aromaticum essential oil
TABLE 2 Emulsion formulations (V/V %) of C. cyminum essential oil
Physical Analysis of Essential Oil Emulsions
The different physical parameters of essential oil emulsions such as solubility, color, specific gravity, and refractive index were determined according to methods described previously.[Citation14] The refractive index of all the emulsions of S. aromaticum and C. cyminum essential oil was measured with a refractometer (ATAGO Digital Reractometer, R3261).
Antidiabetic Activity Assay
The antidiabetic activity of S. aromaticum and C. cyminum essential oils and emulsions was determined by α-amylase assay as described previously.[Citation15] Five hundred microliter quantities of different concentrations (1 to 100 µg/mL) of each essential oil and their emulsion formulations (A1 to A5 and B1 to B5) were mixed with 500 µL of a solution of alpha amylase enzymes and then incubated at 25°C for 10 min. Five hundred microliters of starch solution was added to the above solution and incubated at room temperature for 10 min. Then 1 mL of 3,5-Dinitrosalicylic acid (DNSA) solution was added to the mixture and placed in a boiling water bath for 5 min and cooled to room temperature. Ten milliliters of water was added into the reaction mixture for dilution and the absorbance was measured at 540 nm wavelength. The percentage inhibition was calculated using the following formula:
where Ablank and Asample are the absorbance of the blank and test compound, respectively.
Gas Chromatographic (GC) Analysis
Essential oils of S. aromaticum and C. cyminum were analyzed using a GC (Shimadzu GC-17A) equipped with a flame ionization detector (FID). Helium was used as carrier gas and at a flow rate of 1.4 mL/min. The column temperature was programmed from 70 to 210°C at the rate of 5°C/min. Injector and detector temperatures were set at 250 and 260°C, respectively. All quantifications were carried out using a built-in data-handling program provided by the manufacturer of the GC (Perkin Elmer, Norwalk, CT, USA).
Statistical Analysis
All experiments were carried out in triplicate. Data was represented as mean ± SD of three replications. Statistical analysis of data was conducted by one way analysis of variance using Minitab software version 16.
RESULTS AND DISCUSSION
Physical Analysis
contains yield, color, solubility, and stability of S. aromaticum and C. cyminum essential oils. Higher yield of essential oil (8.5%) was observed for S. aromaticum compared to C. cyminum (2.1%). These results are in agreement with previous findings, in which a yield of 3.4% was found for C. cyminum seed oil.[Citation16] However, in contrast to our findings, another research group noticed a comparatively higher yield (17.95%) for essential oil of S. aromaticum.[Citation17] Variations in yield of essential oil might be attributed to differences in phonological states and agroclimatic conditions of the plant.[Citation18] The color of S. aromaticum essential oil was pale yellow, while the color of C. cyminum essential oil was greenish yellow. Appearance of both S. aromaticum and C. cyminum essential oil emulsions were from clear liquid to milky solution. All the emulsions of S. aromaticum and C. cyminum passed the centrifugation stability test and were found to be thermodynamically stable. As far as the solubility concerns, S. aromaticum and C. cyminum essential oils were soluble in alcohol but insoluble in water.
TABLE 3 Yield, color, solubility, and refractive indices of S. aromaticum and C. cyminum essential oils
Antidiabetic Activity of S. Aromaticum and C. Cyminum Essential Oils and Their Emulsions
Plant-based products particularly the essential oils are reported for wide range of biological activities.[Citation18–Citation21] In the present study, essential oils, as well as emulsions, of S. aromaticum and C. cyminum were investigated for their antidiabetic activity using α-amylase enzyme assay. α-amylase is an enzyme in humans which breaks starch into simple sugars leading to diabetes. Thus by inhibiting this enzyme, the carbohydrate digestion can be delayed and ultimately the rate of glucose absorption is reduced.[Citation22] Therefore, postprandial rise can be decreased in blood glucose. In the current study the α-amylase inhibiting potential of S. aromaticum and C. cyminum essential oils was noted in dose dependent mode (1 to 100 µg/mL). From the results as shown in Fig. 1, it is evident that α-amylase inhibition increases in dose dependant manner for both S. aromaticum and C. cyminum essential oils. These results are in agreement with previous findings, where inhibition of α-amylase activity was found to be dose dependent.[Citation23] Compared to standard antidiabetic compound like ascorbase (IC50 = 14.79 µg/mL), the essential oil of S. aromaticum exhibited very weak antidiabetic activity. However, antidiabetic activity of S. aromaticum (IC50 = 74.53 µg/mL) essential oil was significantly higher than C. cyminum essential oil (IC50 = 80.01 µg/mL). Our results are superior to a previous study, who examined poor inhibition of α-amylase activity by free phenol (IC50 = 497.2 µg/mL) and bound phenol (IC50 = 553.7 µg/mL) extracts of S. aromaticum.[Citation24] Antidiabetic activity of S. aromaticum might be due to presence of insulin mimetic agents in it.[Citation25,Citation26] Another research group revealed the antihyperglycemic potential of S. aromaticum buds.[Citation27] Besides S. aromaticum, C. cyminum is also reported for its antidiabetic influence.[Citation28–Citation32] Some other research groups isolated antidiabetic compounds from extracts of S. aromaticum and C. cyminum.[Citation33–Citation35] Emulsions (mixture of essential oil, tween-80, ethanol, and water) of both (S. aromaticum and C. cyminum) essential oils exhibited significant antidiabetic activity. In the current study, five different emulsion formulations of S. aromaticum (A1 to A5) and C. cyminum (B1 to B5) were used to assess their activity against α-amylase enzyme. The results are shown in . Among all the emulsions (A1 to A5, B1 to B5), maximum α-amylase inhibition potency was observed for the emulsion formulations A5 and B5 which contained the highest amount of respective essential oil as shown in and . Minimum inhibition (%) of 68.14 and 14.32% was examined for A1 and B1 emulsion formulations of S. aromaticum and C. cyminum essential oils, respectively. A significant difference (p < 0.05) in α-amylase inhibition (%) was observed with respect to emulsion formulation and plant species. Antidiabetic activity of S. aromaticum and C. cyminum emulsions is rarely reported in literature.
Figure 1 Inhibition of α-amylase (%) by two (S. aromaticum and C. cyminum) essential oils and positive control (acarbose).
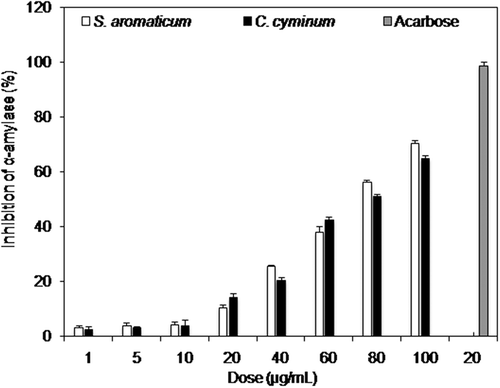
TABLE 4 Antidiabetic activity of emulsion formulations of S. aromaticum and C. cyminum essential oil
Chemical Analysis
GC is the best method due to its simplicity, rapidity, and efficiency for both the identification and quantification of essential oil components and composition variations. In the current study chemical components of S. aromaticum and C. cyminum essential oils were analyzed using GC. The results are given in . In S. aromaticum essential oil 14 compounds were detected which constituted 99% of the oil, while in C. cyminum essential oil 16 compounds were identified which were 98.5% of the oil. Eugenol (18.7%) was the main chemical constituent of S. aromaticum essential oil, followed by α-pinene (15.6%), eugenyl acetate (15.3%), acetaldehyde (8.84%), n-hexane (8.12%), geraniol (5.79%), methyl benzoate (4.51%), γ-terpinene (3.84%), citronellyl acetate (3.72%), eucalyptol (3.36%), and caryophyllene oxide (2.93%). The major component of C. cyminum essential oil was α-pinene (18.8%) followed by α-terpinene (15.1%), Cumin aldehyde (10.2%), octanal (7.57%), geranyl acetate (6.85%), p-cymene (6.71%), limonene (6.06%), α-thujene (5.89%), methyl benzoate (5.48%), hexane (5.02%), eugenol (4.01%), citronellyl acetate (3.09%), β-pinene (2.34%), and geraniol (2.21%). Besides above mentioned components, chemical contribution of all other components in both (S. aromaticum and C. cyminum) essential oils was less than 2%. The chemical composition of S. aromaticum essential oil in current study is different from previous finding,[Citation36] who reported eugenol (up to 82.36%) as major component of S. aromaticum essential oil from Malaysian, Indonesian, and Madagascar region. Similar to our findings, some other studies reported the presence of eugenol, α-pinene, limonene, α-terpinene, caryophyllene oxide, benzoic acid,3-(1-methyl ethyl), eugenyl acetate, and eucalyptol in S. aromaticum essential oil from different regions of the world.[Citation37–Citation40] Present findings for main components of C. cyminum essential oil are in accordance with previous study who also examined the presence of α-thujene, α-pinene, p-cymene, geraniol, and limonene in C. cyminum essential oil of Iranian region.[Citation41,Citation42] β-pinene found in current study was also detected in earlier studies from C. cyminum essential oil.[Citation43–Citation49] β-pinene and its enantiomers were also reported for biological activities.[Citation50,51]
TABLE 5 Components analysis of S. aromaticum oil and C. cyminumessential oil
CONCLUSION
In conclusion, our study for the first time reports the antidiabetic activity of S. aromaticum and C. cyminum emulsions. The results of the current study showed that S. aromaticum and C. cyminum essential oils and emulsions had potent antidiabetic activities. GC analysis evidenced that both essential oils are potential sources of bioactive compounds. However, further studies are required to investigate antidiabetic potential of S. aromaticum and C. cyminum emulsions for in vivo disease models.
References
- Shukri, M.A.M.; Alan, C.; Noorzuraini, A.R.S. Polyphenols and Antioxidant Activities of Selected Traditional Vegetables. Journal of Tropical Agriculture and Food Science 2005, 39, 1–15.
- Saeed, S.; Tariq, P. In Vitro Antibacterial Activity of Clove Against Gram Negative Bacteria. Pakistan Journal of Botany 2008, 40, 2157–2160.
- Pinto, E.; Vale-Silva, L.; Cavalerio, C.; Salgueiro, L. Antifungal Activity of the Clove Essential Oil from Syzygium Aromaticum on Candida, Aspergillus, and Dermatophyte Species. Journal of Medicinal Microbiology 2009, 58, 1454–1462.
- Dwivedi, V.; Shrivastava, R.; Hussain, S.; Ganguly, C.; Bharadwaj, M. Comparative Anticancer Potential of Clove (Syzygium Aromaticum)—An Indian Spice—Against Cancer Cell Lines of Various Anatomical Origin. Asian Pacific Journal of Cancer Prevention 2011, 12, 1989–1993.
- Prasad, R.; Ali, S.; Khan, L.A. Hepatoprotective Effect of Syzygium Aromaticum Extract on Acute Liver Injury Induced by Thioacetamide. International Journal of Pharmaceutical and Clinical Research 2010, 2, 68–71.
- Al–Yahya, M.; Collpharm, A. Phytochemical Studies of Plant Used in Traditional Medicine in Saudi Arabia. Fitoterapia 1986, 57, 179–182.
- Leporatti, M.L.; Ghedira, K. Comparative Analysis of Medicinal Plants Used in Traditional Medicine in Italy and Tunisia. Journal of Ethnobiology Ethnomedicine 2009, 26, 5–31.
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological Survey of Plants Used in the Traditional Treatment of Hypertension and Diabetes in South-Eastern Morocco (Errachidia Province). Journal of Ethnopharmacology 2007, 110, 105–117.
- Sahoo, H.B.; Sahoo, S.K.; Sarangi, S.P.; Sagar, R.; Kori, M.L. Anti-Diarrhoeal Investigation from Aqueous Extract of Cuminum Cyminum Linn. Seed in Albino Rats. Pharmacognosy Research 2014, 6, 204–209.
- Parkash, E.; Gupta, D.K. Cytotoxic Activity of Ethanolic Extract of Cuminum Cyminum Linn Against Seven Human Cancer Cell Line. Universal Journal of Agricultural Research 2014, 2, 27–30.
- Boskabady, M.H.; Kiani, S.; Azizi, H.; Khatami, T. Antitussive Effect of Cuminum Cyminum Linn. in Guinea Pigs. Natural Product Radiance 2006, 4, 266–269.
- Shivakumar, S.I.; Shaapurkar, A.A.; Kalmath, K.V.; Shivakumar, B. Anti-Inflammatory Activity of Fruits of Cuminum Cyminum Linn. 2010. Der Pharmacia Lettre 2010, 2, 22–24.
- Saranya, S.; Chandrasekaran, N.; Mukherjee, A. Antibacterial Activity of Eucalyptus Oil Nanoemulsion Against Proteus Mirabilis. International Journal of Pharmacy and Pharmaceutical Sciences 2012, 4, 668–671.
- Ahmad, M.M.; Rehman, S.; Iqbal, Z.; Anjum, F.M.; Sultan, J.I. Genetic Variability to Essential Oil Composition in Four Citrus Fruits Species. Pakistan Journal of Botany 2006, 38, 319–324.
- Ali, H.; Houghton, P.J.; Amala, S. Alpha-Amylase Inhibitory Activity of Some Malaysian Plants Used to Treat Diabetes; with Particular Reference to Phyllanthusamarus. Journal of Ethnopharmacolog 2006, 107, 449–451.
- Razafimamonjison, G.; Jahiel, M.; Duclos, T.; Ramanoelina, P.; Fawbush, F.; Danthu, P. Bud, Leaf, snd Stem Essential Oil Composition of Syzygium Aromaticum from Madagascar, Indonesia, and Zanzibar. International Journal of Basic and Applied Sciences 2014, 3, 224–233.
- Chuhan, N.K.; Singh, S.; Haider, S.Z.; Lohani, H. Influence of Phonological Stages on Yield and Quality of Oregano (Origanum Vulgare L.) Under the Agroclimatic Condition of Doon Valley (Uttarakand). Indian Journal of Pharmceutical Sciences 2013, 75, 489–493.
- Uysal, B.; Gencer, A.; Oksal, B.S. Comparative Antibacterial, Chemical and Morphological Study of Essential Oils of Thymbra Spicata Var. Spicata Leaves by Solvent-Free Microwave Extraction and Hydro-Distillation. International Journal of Food Properties 2015, 18, 2349–2359.
- Abderrahmane, H.; Hechachna, H.; Ozogul, F. In Vitro Determination of the Antifungal Activity of Artemisia campestris Essential Oil from Algeria. International Journal of Food Properties 2016, 19, 1749–1756.
- Pirbalouti, A.G.; Neshat, S.H.; Rahimi, E.; Hamedi, B.; Malekpoor, F. Chemical Composition and Antibacterial Activity of Essential Oils of Iranian Herbs Against Staphylococcus Aureus Isolated from Milk. International Journal of Food Properties 2014, 17, 2063–2071.
- Abdossi, V.; Kazemi, M. Bioactivties of Achillea millefolium Essential Oil and its Main Terpenes from Iran. International Journal of Food Properties, 2016, 19, 1798–1808.
- Betterworth, P.J.; Warren, F.J.; Ellis, P.R. Human α-Amylase and Starch Digestion: An Interesting Marriage. Starch 2011, 63, 395–406.
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-Amylase Inhibitory Activity of Indian Ayurvedic Medicinal Plants. BMC Complementary and Alternative Medicine 2011, 1, 5.
- Adefegha, S.A.; Oboh, G. In Vitro Inhibition Activity of Polyphenol-Rich Extracts from Syzygium Aromaticum (L.) Merr. & Perry (Clove) Buds Against Carbohydrate Hydrolyzing Enzymes Linked to Type 2 Diabetes And Fe2+- Induced Lipid Peroxidation in Rat Pancreas. Asian Pacific Journal of Tropical Biomedicine 2012, 10, 774–781.
- Kuroda, M.; Mimaki, Y.; Ohtomo, T.; Yamada, J.; Nishiyama, T.; Mae, T. Hypoglycemic Effects of Clove (Syzygium Aromaticum Flower Buds) on Genetically Diabetic KK-Ay Mice and Identification of the Active Ingredients. Journal of Natural Medicine 2012, 66, 394–399.
- Prasad, R.C.; Herzog, B.; Boonem, B.; Sims, L.; Waltner-Law, M. An Extract of Syzygium Aromaticum Represses Genes Encoding Hepatic Gluconeogenic Enzymes. Journal of Ethnopharmacology 2005, 96, 295–301.
- Adefegha, S.A.; Oboh, G.; Adefegha, O.M.; Boligon, A.A.; Athayde, M.L. Antihyperglycemic, Hypolipidemic, Hepatoprotective, and Antioxidative Effects of Dietary Clove (Szyzgium Aromaticum) Bud Powder in a High-Fat Diet/Streptozotocin-Induced Diabetes Rat Model. Journal of Science Food and Agriculture 2014, 94, 2726–2737.
- Jagtap, A.G.; Patil, P.B. Antihyperglycemic Activity and Inhibition of Advanced Glycation End Product Formation by Cuminum Cyminum in Streptozotocin Induced Diabetic Rats. Food and Chemical Toxicology 2010, 48, 2030–2036.
- Lee, H.S. Cuminaldehyde: Aldose Reductase and Alpha-Glucosidase Inhibitor Derived from Cuminum Cyminum L. Seeds. Journal of Agricultural and Food Chemistry 2005, 53, 2446–2450.
- Roman-Ramos, R.; Flores-Saenz, J.L.; Alarcon-Aguilar, F.J. Anti-Hyperglycemic Effect of Some Edible Plants. Journal of Ethnopharmacology 1995, 48, 25–32.
- Srinivasan, K. Plant Foods in the Management of Diabetes Mellitus: Spices As Beneficial Antidiabetic Food Adjuncts. International Journal of Food Science and Nutrition 2005, 56, 399–414.
- Willatgamuwa, S.A.; Platel, K.; Sarawathi, G.; Srinivasan, K. Anti-Diabetic Influence of Dietary Cumin Seeds (Cuminum Cyminum) in Streptozocin Induced Diabetic Rats. Nutrition Research 1998, 18, 131–142.
- Toda, M.; Kawabata, J.; Kasai, T. Alpha-Glucosidase Inhibitors from Clove (Syzgium Aromaticum). Bioscience, Biotechnology, and Biochemistry 2000, 64, 294–298.
- Patil, S.B.; Takalikar, S.S.; Joglekar, M.M; Haldavnekar, V.S.; Arvindekar, A.U. Insulinotropic and β-Cell Protective Action of Cuminaldehyde, Cuminol and An Inhibitor Isolated from Cuminum Cyminum in Streptozotocin-Induced Diabetic Rats. The British Journal of Nutrition 2013, 8, 1434–1443.
- Khathi, A.; Serumula, M.R.; Myburg, R.B.; Heerden, F.R.; Musabayane, C.T. Effects of Syzygium Aromaticum-Derived Triterpenes on Postprandial Blood Glucose in Streptozotocin-Induced Diabetic Rats Following Carbohydrate Challenge. PLoS ONE 2013, 8, e81632.
- Lee, S.; Najiha, M.; Wendy, W.; Nadirah, M. Chemical Composition and Antimicrobial Activity of the Essential Oil of Syzygium Aromaticum Flower Bud (Clove) Against Fish Systemic Bacteria Isolated from Aquaculture Sites. Frontiers of Agriculture in China 2009, 3, 332–336.
- Alma, M.H.; Ertas, M.; Nitz, S.; Kollmannsberger, H. Chemical Composition and Contents of Essential Oil from the Bud of Cultivated Turkish Clove (Syzygium Aromaticum L.). Bioresources 2007, 2, 265–269.
- Bhuiyan, M.N.I.; Begum, J.; Nandi, N.C.; Akter, F. Constituents of the Essential Oil from Leaves and Buds of Clove (Syzigium Caryophyllatum (L.) Alston). African Journal of Plant Science 2010, 11, 451–454.
- Fayemiwo, K.A.; Adeleke, M.A.; Okoro, O.P.; Awojide, S.H.; Awoniyi, I.O. Larvicidal Efficacies and Chemical Composition of Essential Oils of Pinus Sylvestris and Syzygium Aromaticum Against Mosquitoes. Asian Pacific Journal of Tropical Biomedicine 2014, 4, 30–34.
- Mohammadpour, H.; Moghimipour, E.; Rasooli, I.; Fakoor, M.H.; Astaneh, S.A.; Moosaie, S.S.; Jalili, Z. Chemical Composition and Antifungal Activity of Cuminum Cyminum L. Essential Oil from Alborz Mountain Against Aspergillus Species. Jundishapur Journal of Natural Pharmaceutical Products 2012, 2, 50–55.
- Naeini, A.; Shokri, H. Chemical Composition and in Vitro Antifungal Activity of the Essential Oil from Cuminum Cyminum Against Various Aspergillus Strains. Journal of Medicinal Plants Research 2012, 6, 1702–1706.
- Moghadam, A.R.L. Essential oil of the Seeds of Cuminum Cyminum L.(Apiaceae). Bulletin of Environment, Pharmacology, and Life Sciences 2015, 4, 161–163.
- Li, R.; Jiang, Z.T. Chemical Composition of the Essential Oil of Cuminum Cyminum L. from China. Flavour and Fragrance Journal 2004, 19, 311–313.
- Lacobellis, N.S.; Cantore, P.L.; Capasso, F.; Senatore, F. Antibacterial Activity of Cuminum Cyminum L. and Carum Carvi L. Essential Oils. Journal of Agricultural Food Chemistry 2005, 53, 57–61.
- Hajlaoui, H.; Mighri, H.; Noumi, E.; Snoussi, M.; Trabelsi, N.; Ksouri, R.; Bakhrouf, A. Chemical Composition and Biological Activities of Tunisian Cuminum Cyminum L. Essential Oil: A High Effectiveness Against Vibrio spp. Strains. Food and Chemical Toxicology 2010, 48, 2186–2192.
- Neda, H.; Ghasemi, P.A.; Masoud, H.; Ahmadreza, G.; Behzad, H. Diversity in Chemical Composition and Antibacterial Activity of Essential Oils of Cumin (Cuminum Cyminum L.) Diverse from Northeast of Iran. Australian Journal of Crop Science 2013, 11, 1752–1760.
- Tavakoli, H.R.; Mashak, Z.; Moradi, B.; Sodagari, H.R. Antimicrobial Activities of the Combined Use of Cuminum Cyminum L. Essential Oil, Nisin, and Storage Temperature Against Salmonella Typhimurium and Staphylococcus Aureus in Vitro. Jundishapur Journal of Microbiology 2015, 8, 248–338.
- Wanner, J.; Bail, S.; Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Gochev, V.; Girova, T.; Atansova, T.; Stoyanova, A. Chemical Composition and Antimicrobial Activity of Cumin Oil (Cuminum Cyminum, Apiaceae). Natural Product Communication 2010, 5, 1355–1358.
- Silva, R.D.A.C.; Lopes, P.M.; Azevedo, B.D.M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological Activities of α-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316.
- Leite, M.; Lima, E.D.O.; Souza, E.L.D.; Diniz, M.D.F.F.M.; Trajano, V.N.; Medeiros, I.A.D. Inhibitory Effect of β-Pinene, α-Pinene, and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria Aristides. Brazilian Journal of Pharmaceutical Sciences 2007, 43, 121–126.
