Abstract
The effects of collagen and collagen peptide from goose bone on osteoporosis were studied. Fourier transform infrared spectrometry, ultraviolet spectrum, differential scanning calorimetry, and sodium dodecyl sulfate polyacrylamide gel electrophoresis identified the goose collagen as typical type I collagen. Results showed that osteopontin, bone morphogenetic protein 2, runt-related transcription factor 2, and type I collagen levels in bone marrow mesenchymal stem cells supernatant were dramatically higher in the experimental groups than in the control group (p < 0.05), and was highest in the experimental group composed of low molecular weight collagen peptide linked with calcium. Then the osterix, runt-related transcription factor 2, transforming growth factor-β1, and bone morphogenetic protein 2 expression levels related to osteogenetic differentiation were detected by real-time fluorescent quantitative polymerase chain reaction. The findings showed that goose collagen and collagen peptide can improve the gene expression related to osteogenetic differentiation which reflected the level of bone marrow mesenchymal stem cells to osteoblasts.
Introduction
Osteoporosis (OP) is one of the top 10 most common health conditions globally, especially in the elderly. More than 200 million individuals suffer from OP worldwide, and China is expected to have more than 200 million cases by the middle of the 21st century. OP is a serious threat to an individual’s health and presents a worldwide challenge in the quest to prevent and treat the condition.
No researcher has unequivocally determined the pathogenesis of OP, which may include an interaction between multiple factors including habits,[Citation1] hormone regulation,[Citation2] and genetics.[Citation3] Abnormal bone metabolism appears to be a main factor in OP. Osteoblasts (OB) and osteoclasts (OC) are involved in a dynamic process of bone formation and bone resorption to reconstruct bones.[Citation4] As the most critical cells in bone formation, OB not only secrete bone matrices, which are essential in bone rebuilding, but can generate collagen and new bone tissue via matrix calcification. OB are mainly created by bone marrow mesenchymal stem cells (BMSCs), which are closely linked to OP. The number and function of BMSCs directly affect OB activity. BMSCs help maintain the balance between bone formation and resorption. A key research issue in relation to developing OP treatments is to determine ways to improve the proliferation of BMSCs and promote differentiation toward osteogenesis.
Collagen is the most common protein in an animal’s body, representing about 30% of the total proteins.[Citation5] It is distributed extensively in connective tissues, such as in ligaments and cartilage. Collagen has been widely used in biomedical materials and in clinical applications due to its toughness, low toxicity, weak antigenicity, and excellent biocompatibility. There are more than 27 different types of collagen, such as type I collagen (Col I), type II collagen and type III collagen; among these, Col I is the most common and has been the most thoroughly researched. Col I plays a significant role in the maturation and mineralization of bone cells, and in supporting organic bone growth.[Citation6] Studies confirm that eating collagen can promote the absorption of minerals, increase bone density, and decrease the risk of OP.[Citation7,Citation8]
Collagen peptide can be hydrolyzed from collagen with acid, alkali, heat, and enzyme. It is safe and causes no allergic reactions in humans. It can also promote the absorption of other proteins.[Citation9] China has rich resources of animal bones. Traditionally, collagen has been extracted from the skins of land animals, such as cows and pigs. For technical reasons, animal bones are usually processed into bone meal or bone paste for animal feed, with a very low utility value. Goose bones contain abundant collagen, calcium, phosphorus, and other minerals. In this study, we extracted goose collagen and collagen peptide, analyzed their effect on OP, and sought to increase their effectiveness in staving off and treating OP.
Materials and methods
Chemicals
Goose bones were extracted from Zhedong white geese, a famous type of goose in China. BMSCs were obtained from the Saiye Co., China. High molecular weight dialysis bags (14,000 Da), pepsin, trypsin, and Tris were purchased from the Beijing Solarbio Co., China. A bicinchoninic acid kit was purchased from Beyotime Biotechnology Co., China. N, N, N’, N’-tetramethyl ethylene diamine (TEMED) and sodium dodecyl sulphate (SDS) were obtained from Bio-Rad Laboratories, USA. All chemicals were of analytical grade. Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Qiao Du Biotechnology Co., Shanghai, China. An EasyPure RNA Kit and TransStart Tip Green qPCR SuperMix were purchased from TransGen Biotech Co., Beijing, China. An iScript cDNA Synthesis Kit was obtained from Bio-Rad Laboratories, USA. GelRed was purchased from Biotium, USA.
Preparation of Goose Skeleton
One-year-old goose carcasses were purchased from a local market and brought to the lab on ice within 30 min. All procedures were carried out in 4°C conditions.[Citation10] The skins were removed manually using a knife. The bones were extracted and washed with cold distilled water, then cut into small pieces (0.5 cm each in length)[Citation11] and stored at –20°C. The bone pieces were blended with 0.1 M NaOH at a 1:10 ratio (bone/alkali solution, w/v) for 18 h, and washed with cold distilled water until the pH value reached 7.0. The bone pieces were suspended in 2.5% (w/v) NaCl for 6 h, washed several times with cold distilled water, and immersed in 0.5 M EDTA-2Na (pH 7.0) solution for 5 days to remove any calcium. The solution was replaced every 24 h. The bone pieces were then soaked in 10% (v/v) butanol for 24 h to remove the fat. The butanol solution was changed every 4 h and the bone pieces were washed with cold distilled water.
Extraction of Macromolecular Collagen
The obtained bone pieces were soaked and continuously stirred in 0.5 M acetic acid containing 4% pepsin at a 1:10 ratio of solid/solvent (w/v) for 24 h at a pH of 2.0.[Citation12] The pieces were placed in boiling water for 5 min. The mixture was centrifuged at 4500 × g for 30 min at 4°C. The supernatant was collected, salted-out by the addition of 2 M NaCl, stirred for 20 min, and placed in a refrigerator overnight. The precipitate was collected by centrifuging the samples at 4500 × g for 10 min and dissolved in 0.5 M acetic acid. It was then dialyzed against 0.5 M acetic acid in a dialysis tube with the molecular weight cut off set at 14,000 Da for 1 day, 0.1 M acetic acid for 1 day, and then distilled water for 3 days with a change of solvent every 6 h.[Citation13] Finally, dialysate was collected by freeze drying and stored at –20°C.
Extraction of Collagen Peptide
The bone pieces obtained by the above steps were soaked and continuously stirred in 0.5 M acetic acid containing 5% trypsin[Citation14] (10,000 u/g) with a 1:5 ratio of solid/solvent (w/v) at 50°C for 5 h at pH 7.5. The pieces were placed in boiling water for 5 min.[Citation15] By centrifuging at 4500 × g for 30 min, the supernatant was collected and then freeze dried. The freeze dried content was used as collagen peptide.
Scanning Electron Microscopic (SEM) Analysis
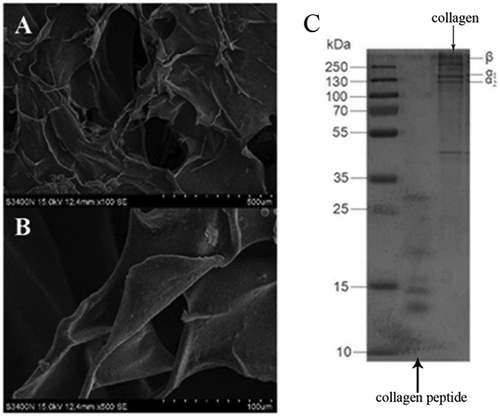
The morphological characteristics of the isolated goose collagen and collagen peptide were observed using a Hitachi S-3400N VP-SEM (Tokyo, Japan). The collagen and collagen peptide samples were introduced to the SEM’s specimen chamber and the surface morphology examined. The SEM observations were made at a setting of 15 KV accelerating voltage.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE of the collagen and collagen peptide was performed by the method described by Laemmli.[Citation16] Samples were mixed with 2 × loading buffer (every 10 mL 2 × loading buffer included 2 mL glycerinum, 0.0202 g bromophenol blue, 1 mL 1 M Tris-HCl, 0.14 mL mercaptoethanol, and 4 mL 10% SDS at pH 6.8) at a 1:1 ratio. The mixtures were heated at 10°C for 5 min. Ten microliters of each sample was loaded onto a polycrylamide gel composed of 12% separating and 5% stacking gel. While samples were electrophoresed, a constant 80 V of separating gel and 120 V of stacking gel were applied. The gels were then stained using 0.125% (w/v) Coomassie Brilliant Blue R-250 in 8% (v/v) acetic acid and 25% (v/v) methanol for 30 min. Finally, the gels were destained using an 8% (v/v) acetic acid and 25% (v/v) methanol mixture to visualize the bands.
Ultraviolet (UV) Spectrum
Acetic acid (0.5 M) was used to dissolve the collagen and collagen peptide samples. With a UV-spectrophotometer (UV 3300, Zhi Xin Co., Shanghai, China), UV absorption spectra were measured between 200 to 400 nm at a scan speed of 2 nm/s with a 1 nm interval.[Citation17]
Fourier Transform Infrared Spectrometry (FT-IR)
The Fourier infrared spectra of the collagen and collagen peptide samples were recorded on potassium bromide (KBr) disks using a Fourier transform IR spectrophotometer (Tensor 27, Bruker Corporation, Germany). One milligram of dry collagen and 1 mg of collagen peptide were separately mixed with the dry KBr (100 mg) and then pelleted for the spectrum recording. Spectra of the samples were obtained in the 400 to 4000 cm–1 range.
Differential Scanning Calorimetry (DSC)
DSC was performed by DSC20F3 (Maia Corp., Germany). Indium was used as a standard to calibrate the instrument. Three milligrams of dry collagen and collagen peptide were placed into separate aluminum pans and sealed. An empty pan was used as a reference. The samples remained at 20°C for 1 min and were scanned from 20 to 120°C with a heating rate of 5°C/min. The samples then remained at 120°C for 1 min. The flow rate of nitrogen was 20 mL/min.[Citation18] Thermal contraction temperatures were estimated using a DSC thermogram.
ELISA Analysis
The solutions for the control and experimental groups with added collagen and collagen peptide were used to cultivate BMSCs to a density of 1 × 105/mL in 6-well cell plates. The solution was changed every 3 days. Cell-culture supernatant was collected by centrifuging at 250 × g for 15 min on days 3, 6, 9, 12, and 14. Finally, the production of osteopontin (OPN), bone morphogenetic protein 2 (BMP-2), runt-related transcription factor 2 (Runx2), and Col I from the cell culture supernatant related to osteogenic differentiation were determined by ELISA. All procedures were carried out according to the kits.
Quantification of Gene Expression by Real-Time Fluorescent Quantitative Polymerase Chain Reaction (RT-PCR)
For reverse transcription RNA, an iScript cDNA Synthesis kit (Bio-Rad, USA) was used. RT-PCR was performed using the SYBR Green real time PCR method with a TransStart Tip Green qPCR SuperMix kit. PCR reactions were performed and quantitated using the following parameters: 30 s at 94°C, 45 cycles of 5 s each at 94°C and 45 cycles of 30 s each at 60°C. To analyze the relative gene expression of messenger ribonucleic acid (mRNA), a 2−△△CT method was used (see ).
TABLE 1 List of primer sequences used in this study
Results and Discussion
Molecular Weight and Structure
Enzymolysis of the collagen with pepsin did not alter its unique triple helical structure or its biological characteristics.[Citation19] In this study, the yield of goose collagen and collagen peptide were 11.4 and 13.2%, respectively. As shown in , β, α1, and α2 chains were observed. The molecular weight of the collagen peptide was small, showing that the collagen had already decomposed. The molecular weight was under 30 KDa in a discontinuous state. Electrophoresis of the collagen protein showed three clear bands: a β chain with a molecular weight of about 200 KDa; and α1 and α2 chains with molecular weights of about 130 KDa. In , the total free amino acid of collagen peptide higher than collagen, protein was formed by amino acid and then absorbed in intestinal, but it does not had the biology active of proteins or peptides. Meanwhile, as high molecular substance, there will be a certain space steric effect to collagen when it entered human bodies. On the contrary, people didn’t need to digest collagen peptide after they entering the human bodies due to the low molecular, so the utilization rate of collagen peptide was high. They were absorbed directly and had a faster absorption rate than amino acid.[Citation20,Citation21] Research showed that the only less than five peptide can be transported, among them, dipeptide had a fastest transport rate, so the molecular weight was a key factor in collagen and collagen peptide absorption rate.[Citation22]
UV of Collagen and Collagen Peptide
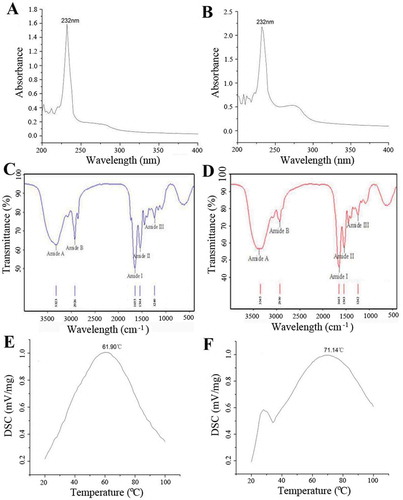
Peptide chains of collagen contain some UV chromophores, such as COOH, C=O, and CONH2.[Citation23] As a result, a solution of collagen and collagen peptide can absorb some UV wavelengths to form specific absorption spectra. As shown in and , collagen and collagen peptide had the highest absorption peaks at 232 nm because of the transition of the C=O in the peptide chains from n to π. Both had no absorption peaks around 280 or 250 nm.
FT-IR Spectra
The infrared spectra of collagen and collagen peptide from goose bones are shown in and . The amide-A peak was detected at 3323 cm–1. The peptide chains displayed the absorption characteristics commonly associated with N-H stretching frequency and the existence of hydrogen bonds. In general, in the 3400 to 3440 cm–1 range, free N-H stretching vibration occurs. The position of the NH group of a peptide will shift to a lower frequency to about 3300 cm–1 when it is involved in a hydrogen bond.[Citation11] The amide-B peak was at 2926 cm–1, which resulted from the asymmetric stretching vibration of CH2.
The amide I peak observed at 1655 cm–1 was caused by the stretching vibration of C=O. The amide II band was found at 1544 cm–1, which originated from the bending vibration of N-H.[Citation24] The characteristic peak of the amide III was observed at 1240 cm–1, which was caused by the bending vibration of N-H coupled to the stretching vibration of C=N, the bending vibration of C=O in a plane and the stretching vibration of C=C.[Citation25] The peaks of amide B, I, II and III of the collagen peptide were observed at 3345, 2930, 1654, 1543, and 1242 cm–1, respectively.
Thermal Stability of Collagen and Peptide
As shown in and , the shrinkage temperature of collagen and collagen peptide were 61.90 and 71.14°C, respectively. The amount of imide acid (proline and hydroxyproline, especially the later) in collagen played a key role in thermal stability.[Citation26–Citation28] Proline and hydroxyproline contain tetrapyrrole rings which can limit changes to the secondary structure of collagen peptide chains. Therefore, it appears that the presence of more tetrapyrrole rings caused a higher thermal shrinkage temperature.
Effect on Secretion of BMP-2 and BMP-2 mRNA Expression
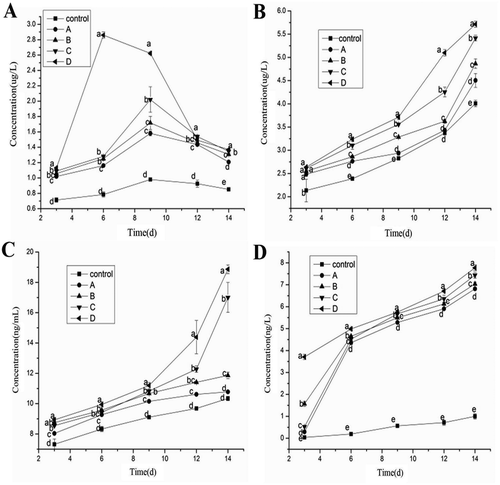
BMP-2 is a specific factor for bone growth, and is commonly found in bone matrices. BMP-2 is an acidic peptide and is mainly produced by OB. As shown in , the control group’s BMP-2 expression level was significantly lower than that of groups A, B, C, or D (p < 0.05). BMP-2 secretion increased and then decreased. Group D’s secretion level was higher than any other experimental group (p < 0.05). The peak for the control group was on day 9. However, the increasing secretion levels over the entire process were far from obvious. The maximum secretion of BMP-2 for groups A, B, and C was on day 9; for group D it was on day 6. On days 3 and 6, the experimental groups displayed significantly different BMP-2 secretion levels, except for groups B and C. On day 14, there were significant differences (p < 0.05) between the control and experimental groups, as well as among the experimental groups.
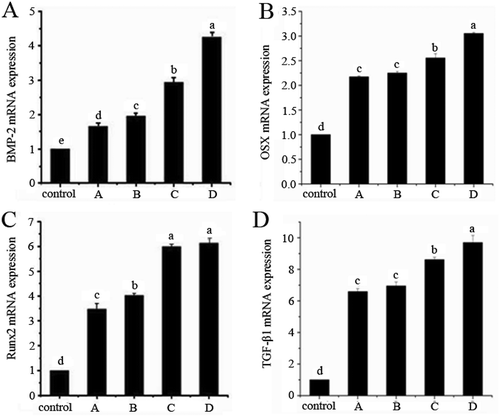
BMP is the original signal caused by ectomesenchymal cell differentiation. The expression of BMP-2 mRNA increases during the early period of fracture healing. However, the ability of BMP-2 mRNA to synthesize decreases as cells gradually differentiate and mature. Each groups’ BMP-2 expression after 14 days of cultivation is shown in . Compared with the control group, BMP-2 mRNA expression by groups A, B, C, and D increased 1.6599, 1.6599, 2.9432, and 4.2588 times, respectively, with significant differences between them (p < 0.05). Small molecule collagen peptide with calcium exhibited the greatest effect.
Effect on Runx2 Secretion and Runx2 mRNA Expression
Runx2 contains 596 amino acids, and a Runt structure domain. Runx2 is an early sign of differentiation related to osteogenesis and is a transcription protein with multi-functionality and specificity, which plays an important role in promoting bone differentiation and formation. As shown in , over time all of the control and experimental groups’ Runx2 secretion gradually increased. The control group’s increase was far from obvious, but the experimental groups’ increases were apparent especially from day 3 to 6. There were significant differences (p < 0.05) between the control and experimental groups, as well as between each experimental group from day 3 to 14.
As a key factor for differentiation during osteogenesis, Runx2 expression indicates the differentiation of BMSCs during osteogenesis.[Citation29] Runx2 plays a vital role in metabolic regulation and bone formation. The results of RT-PCR were correspond to the ELISA analysis. As shown in , on day 14, the Runx2 mRNA expression of groups A, B, C, and D compared to the control group increased 3.4791, 4.0283, 5.9935, and 4.0283 times, respectively. In addition to groups C and D, the Runx2 gene expression among all the experimental groups showed significant differences.
Effect on Col I and OPN Secretion
Col I can reflect the degree of osteogenetic differentiation. The production of Col I is closely associated with salt deposition in bones, as well as bone mineralization and formation. As shown in , the control and experimental groups’ Col I secretion increased with the induction time. On day 3, the four experimental groups were significantly different from the control group (p < 0.05), although there were no notable differences among the experimental groups. On days 6, 9, and 14, the control group’s Col I secretion was lower than that of the experimental groups. On day 12, the experimental groups’ Col I secretion significantly differed from the control group, and there were significant differences between the other experimental groups and group A. There were also statistically significant differences between each of the experimental groups (p < 0.05).
OPN is a phosphoric acid protein that is expressed by bone. In recent years, research has shown that OPN plays important biological functions in cell regeneration, tissue repair, immunity, and bone mineralization. As shown in , the control and experimental groups’ OPN secretion increased gradually over time. By day 3, there was no noticeable difference between groups B and D, or between B and C, while all of the other experimental groups displayed significant differences (p < 0.05). By day 6, the experimental groups showed significant differences, as well as differences between group A and groups B and C. Group C and D’s OPN secretion increased quickly from day 9. The experimental groups were significantly different than the control group (p < 0.05) except on day 14 when group A showed no difference compared with the control group.
mRNA Expression of Osterix (OSX) and Transforming Growth Factor-β1 (TGF-β1)
OSX is an indispensable transcription factor in regulating differentiation in osteogenesis, which can promote directional differentiation of stems cells from different sources into OB and lead to the formation of new bone.[Citation30,Citation31] As shown in , after 14 days of cultivation, the OSX mRNA expression of BMSCs in the experimental groups dramatically increased. Compared with the control group, the OSX expression of groups A, B, C and D increased 2.2503, 2.1735, 2.5560, and 2.1735 times, respectively. There were also significant differences between the experimental groups, except between groups A and B.
TGF-β1 can promote the production of Col I and bone matrix proteins, as well as the formation of osteoid and the mineralization of bones, thereby increasing the flexibility and hardness of bone. TGF-β1, which plays a crucial role in the synthesis of bone matrices and bone reconstruction, inhibits stem cell differentiation into OC.[Citation32] As shown in , on day 14, the TGF-β1 expression of groups A, B, C, and D compared to the control group increased 6.5904, 6.9512, 8.6148, and 9.6983 times, respectively. Except for between groups A and B, all of the other experimental groups displayed significant differences.
Conclusion
Collagen and collagen peptide were successfully extracted from goose bone with pepsin and trypsin under different conditions. FT-IR and UV investigations showed that the extracted substances were typical Col I and collagen peptide. SDS-PAGE and SEM showed that the molecular weight of the collagen and collagen peptide differed, although the triple-helical structure was intact. There was also a difference in thermal stability. ELISA investigations indicated that OPN, BMP-2, Col I, and Runx2 expression all increased to varying degrees when collagen and collagen peptides were administered. The results of RT-PCR on day 14 showed that BMP-2, OSX, Runx2, and TGF-β1 secretion all increased significantly. Therefore, the effect of collagen and collagen peptide on OP is well supported.
Table_S1.doc
Download MS Word (47.5 KB)FUNDING
This work were supported by the Science and Technology Department of China (2013GB2C200191 & 2014GB2C220153), grants from the Modern Agricultural Technical System Foundation of China (CARS-43-17), Major Scientific and Technological Special Emphasis on Agriculture Project of Zhejiang (2014C02020), the Science and Technology Department of Ningbo (2012B82017), and the K. C. Wong Magna Fund at Ningbo University.
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Marini, F.; Brandi, M.L. Pharmacogenetics of Osteoporosis. Best Practice & Research Clinical Endocrinology & Metabolism 2014, 28 (6), 783–793.
- Montalcini, T.; Romeo, S.; Ferro, Y.; Migliaccio, V.; Gazzaruso, C.; Pujia, A. Osteoporosis in Chronic Inflammatory Disease: The Role of Malnutrition. Endocrine 2013, 43 (1), 59–64.
- Beurskens, L.W.J.E.; Tibboel, D.; Steegers-Theunissen, R.P. Role of Nutrition, Lifestyle Factors, and Genes in the Pathogenesis of Congenital Diaphragmatic Hernia: Human and Animal Studies. Nutrition Reviews 2009, 67 (12), 719–730.
- Perrone, S.; Tataranno, M.L.; Buonocore, G. Oxidative Stress and Bronchopulmonary Dysplasia. Journal of Clinical Neonatology 2012, 1 (3), 109–114.
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and Characterisation of Pepsin-Solubilised Collagen from Fins, Scales, Skins, Bones, and Swim Bladders of Bighead Carp (Hypophthalmichthys Nobilis). Food Chemistry 2012, 133 (4), 1441–1448.
- Cheng, F.Y.; Hsu, F.W.; Chang, H.S.; Lin, L.C.; Sakata, R. Effect of Different Acids on the Extraction of Pepsin-Solubilised Collagen Containing Melanin from Silky Fowl Feet. Food Chemistry 2009, 113 (2), 563–567.
- Glimcher, M.J. Composition, Structure, and Organization of Bone and Other Mineralized Tissues and the Mechanism of Calcification. Handbook of Physiology 1976, 7, 25–116.
- Piez, K.A.; Eigner, E.A.; Lewis, M. The Chromatographic Separation and Amino Acid Composition of the Subunits of Several Collagens. Biochemistry 1963, 2 (1), 58–66.
- Nagata, H.; Kojima, T.; Kubota, N. Mitogenic Activity of Gelatin to Murine Spleen Cells. Cancer Biotherapy and Radiopharmaceuticals 2000, 15 (3), 279–283.
- Lin, Y.K.; Lin, T.Y.; Su, H.P. Extraction and Characterisation of Telopeptide-Poor Collagen from Porcine Lung. Food Chemistry 2011, 124, 1583–1588.
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, J.F. Isolation and Characterization of Acid Soluble Collagens and Pepsin Soluble Collagens from the Skin and Bone of Spanish Mackerel (Scomberomorous Niphonius). Food Hydrocolloids 2013, 31, 13–103.
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Use of Pepsin for Collagen Extraction from the Skin of Bigeye Snapper (Priacanthus Tayenus). Food Chemistry 2007, 104, 593–601.
- Jeevithan, E.; Wu, W.H.; Wang, N.P.; He, L.; Bin, B. Isolation, Purification, and Characterization of Pepsin Soluble Collagen Isolated from Silvertip Shark (Carcharhinus Albimarginatus) Skeletal and Head Bone. Process Biochemistry 2014, 49, 1767–1777.
- Jiaojiao, L.; Cheng, L.; Gang, F.; Yong, Y.; Zhao, S.; Li, H.; Bin, H. Optimization of Enzymatic Hydrolysis of Goose Bone Protein for Production of Antioxidant Peptides. Science and Technology of Food Industry 2013, 20, 194–198.
- Duan, R.; Zhang, J.J.; Du, X.Q.; Yao, X.C.; Konno, K. Properties of Collagen from Skin, Scale and Bone of Carp (Cyprinus Carpio). Food Chemistry 2009, 112, 702–706.
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685.
- Lin, L.C.; Chen, W.T. The Physical and Chemical Properties of Melanin Extracted from Silky Fowl. Taiwanese Journal of Agricultural Chemistry and Food Science 2004, 45, 335–342.
- Rochdi, A.; Foucat, L.; Renou, J.P. NMR and DSC Studies During Thermal Denaturation of Collagen. Food Chemistry 2000, 69, 9–295.
- Feng, W.; Zhao, T.; Zhou, Y.; Fang, L.; Ye, Z.; Shiqi, B.; Wei, W.; Liuqing, Y.; Xiangyang, W. Optimization of Enzyme-Assisted Extraction and Characterization of Collagen from Chinese Sturgeon (Acipenser Sturio Linnaeus) Skin. Pharmacognosy Magazine 2013, 9 (Suppl 1): S32.
- Ning, Z.; Jiyao, K.; Jianping, G.; Haiyan, W.; Yingjun, K.; Guifeng, G.; Zhiguo, S. The Influence of Molecular Weight on Absorption of Collagen Peptides. Journal of Biology 2013, 30 (2), 10–13.
- Wenhong, C.; Chaohua, Z. The Absorption Mechanism of Bioactive Peptide. Pharmaecutical Biotechnology 2006, 13 (5), 384–388.
- Ziv, E.; Bendayan, M. Intestinal Absorption of Peptides Through the Enterocytes. Microscopy Research and Technique 2000, 49 (4), 346–352.
- Edwards, H.G.M.; Farwell, D.W.; Holder, J.M.; Lawson, E.E. Fourier Transform Raman Spectroscopy of Ivory: II. Spectroscopic Analysis and Assignments. Journal of Molecular Structure 1997, 435, 49–58.
- Barth, A.; Zscherp, C. What Vibrations Tell Us About Proteins. Quarterly Reviews of Biophysics 2002, 35 (4), 369–430.
- Plepis, A.M.D.G.; Goissis, G.; Das Gupta, D.K. Dielectric and Pyroelectric Characterization of Anionic and Native Collagen. Polymer Engineering and Science 1996, 36 (24), 2932–2938.
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of Acid Soluble Collagen from Scale of Spotted Golden Goatfish (Parupeneus Heptacanthus). Food Chemistry 2011, 129, 1179–1186.
- Regenstein, J.M.; Zhou, P. Collagen and Gelatin from Marine by-Product. In Maximising the Value of Marine by-Products; Shahidi, F.; Ed.; Woodhead Publishing: Cambridge, England, 2007; 279–303.
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and Characterisation of Collagen Extracted from the Skin of Striped Catfish (Pangasianodon Hypophthalmus). Food Chemistry 2011, 124, 97–105.
- Dong, S.W.; Ying, D.J.; Duan, X.J.; Zhu, C.H.; Liu, G.J.; Mi, J.H. Effect of Core Binding Factor Α1 on the Expression of Osteoblast Gene Marker Mesenehymal Stem Cells. Chinese Journal of Reparative and Reconstructive Surgery 2005, 9, 746–750.
- Nakashima, K.; de Crombrugghe, B. Transcriptional Mechanisms in Osteoblast Differentiation and Bone Formation. Trends Genet 2003, 19 (8), 458–466.
- Tu, Q.; Valverde, P.; Li, S. Osterix Overexpression in Mesenchymal Stem Cells Stimulates Healing of Critical-Sized Defects in Murine Calvarial Bone. Tissue Engineering 2007, 13 (10), 2431–2440.
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Biology Science 2012, 8 (2), 272–288.