Abstract
Chicken breast muscle was treated with rosemary extract combined with ɛ-polylysine, and its quality was assessed throughout 10 days during refrigerated storage. Characteristics of peroxide value, thiobarbituric acid, total volatile basic nitrogen, K value, pH, color, sensory, and bacteriological were all periodically analyzed. Rosemary extract plus ɛ-polylysine treatment effectively improved physicochemical and sensory quality parameters, and reduced microbial growth as compared with rosemary extract or ɛ-polylysine alone or the control. Therefore, the treatment of rosemary extract combined with ɛ-polylysine could be a promising method of extending shelf life and maintaining storage quality of chicken breast muscle during refrigerated storage.
Introduction
With the outbreak of bird flu, sales of live chicken were banned in many markets in China, and fresh chicken meat has become the main trend in chicken consumption. Chilling is a common preservation method to control the quality of chicken meat during storage. However, due to the high polyunsaturated fatty acid and protein content in chicken meat, it does not completely inhibit biochemical reactions and bacteriological activity that affect the color, flavor, odor, and nutritional values of chicken meat. Synthetic preservatives, such as antioxidants, antimicrobial compounds, and chelating agents, have been used as food additives to maintain the storage quality and extend the shelf life of foods. As their use is restricted due to toxicological concerns and health risks, the development of effective and non-toxic measures to delay spoilage and to extend the shelf life of chicken meat has become an increasingly attractive alternative.[Citation1,Citation2]
Rosemary (Rosmarinus officinalis) is a plant species of the Labiatae family. Its major and most active extract components (e.g., carnosol, rosmarinic acid, carnosic acid, etc.) have proven to be useful in preventing cancer and inflammatory diseases in experimental animals and humans.[Citation3,Citation4] Rosemary extracts (REs) exhibit very strong antioxidant activity and effectively to protect the oxidative stability of natural sunflower oil, when compared with the synthetic antioxidants tert-butyl hydroquinone (TBHQ), butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA).[Citation5,Citation6] Moreover, REs also have strong antibacterial activity against Gram-negative and -positive bacteria (e.g., Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus, and Escherichia coli).[Citation7–Citation9] In 2010, the European Union authorized RE as a new food additive to use in foodstuffs, including meats.[Citation2]
ɛ-Polylysine (ɛ-PL), produced by fermentation of non-toxigenic and non-pathogenic microorganism Streptomyces albulus, is a homopolymer consisting of 25 to 35 L-lysine residues linked by isopeptide bonds between the α-carboxyl and ɛ-amino groups.[Citation10] Based on absorption, distribution, metabolism, and excretion (ADME) and toxicity studies, ɛ-PL has been tested to be safe for human consumption, and it has been used as a food preservative approved by the Food and Drug Administration (FDA) and Japan. ɛ-PL also exhibits antimicrobial activity against a broad spectrum of microorganisms including yeast, bacteria, and fungi,[Citation11,Citation12] and it has been widely used to inhibit microbial growth in poultry, beef, salad, cake, etc.[Citation11,Citation13]
Previous studies have been proofed that a food additive used alone can not provide sufficient protection against spoilage. Shelf life was improved but still limited in the presence of either RE or ɛ-PL.[Citation14,Citation15] At present, there are no published reports about using a natural antioxidant (RE) combined with a natural antimicrobial (ɛ-PL) to maintain the storage quality and enhance the shelf life of chicken meat. So, this study was to investigate the combined effects of RE and ɛ-PL treatments on the physicochemical, sensory, and bacteriological characteristics of chicken meat, and their ability to maintain the storage quality and extend the shelf life of chicken meat during refrigerated storage.
Material and methods
Preparation of Materials
Sanhuang chickens (120 days old, weighing approximately 1100 g) were slaughtered and processed within 2 h according to the Chinese National Standard: First, chicken legs were hanged on hooks; second, chicken was stunned by electricity (30–50 V); third, chicken neck was cut, and then it was exsanguinated for 3–5 min; fourth, chicken was scalded in hot water (60–62°C) for 60–90 s, and then the feather was depilated; fifth, chicken crop was pull off after its surface skin cutting off; finally, chicken was eviscerated.[Citation16,Citation17] Then the fresh breast muscles were cut into fillets (3 × 4 × 1 cm), weighing approximately 25 g. Two fillets of breast muscle were obtained from each chicken and immediately chilled at 4°C. They were then transported to the laboratory of Zhejiang Gongshang University within 2 h and kept at 4°C until use. ɛ-PL (50% w/w) was purchased from the Zhejiang Silver Elephant Bioengineering Co., Ltd. (Zhejiang Province, China), and RE was used as previously described.[Citation14]
Treatment of Chicken Samples
Samples of chicken breast were randomly divided into four treatment groups: (1) RE, (2) ɛ-PL, (3) RE combined with ɛ-PL (RE plus ɛ-PL), and (4) the control. For RE or ɛ-PL treatment, samples were given a dip treatment in 0.2% RE solution (w/v)[Citation14] or 0.5% ɛ-PL solution (w/v)[Citation18] at 4 ± 3°C for 30 min. For the RE plus ɛ-PL treatment, samples of RE treatment were drained at 4 ± 3°C about 1 h, and then they were treated with ɛ-PL solution (0.2% w/v) at 4 ± 3°C for 30 min. Samples of the control were immersed in distilled water at 4 ± 3°C for 30 min. After treatment, all samples were drained at 4 ± 3°C for approximately 1 h, and then individually packed into air-tight polyethylene packs and stored at 4 ± 1°C. Meat quality analyses were performed at 2-day intervals. Each analysis was repeated three times using 3 to 5 fillet samples.
Total Volatile Basic Nitrogen (TVB-N), Peroxide Value (PV), Thiobarbituric Acid (TBA), pH, and K-Value Analyses
The values of PV (peroxide meq/kg lipid), TBA (mg malonaldehyde equivalents/kg tissue), TVB-N (mg of TVB-N/100 g tissue), and pH were determined as in our previously described methods.[Citation19] Adenosine triphosphate (ATP) and its breakdown products adenosine diphosphate (ADP) adenosine monophosphate (AMP), inosine monophosphate (IMP) inosine, HxR; and hypoxanthine, Hx) in samples were extracted using the acid extraction method as described,[Citation20] and then measured using reverse phase high-performance liquid chromatography.[Citation21] All compounds were identified by their retention time and comparison with standard samples. The K-value was defined as the percentage of the sum of Hx and HxR divided by summed contents of ATP and its degradation products:
Color Analyses
The surface color of raw chicken fillets was measured using a Minolta Chroma Meter CR400 (Minolta, Japan), and parameters of L*, a*, and b* were reported as CIELab coordinates. The parameter L* indicates the luminosity (0–100 point scale from black to white); a* indicates reddish (+) or greenish (-) color; and b* indicates yellowish (+) or bluish (-) color. The whiteness indices (WI) are commonly measured to yield numbers that correlate closely with the choice and preferences of consumers for white colors. Therefore, the WI represents the overall whiteness of food, and is calculated as follows:[Citation22]
Color was measured within 30 min after the package opened, and all measurements were repeated at four randomly selected points of each sample.
Sensory Evaluation
The fillets of breast muscle were wrapped in an aluminum foil, and then they were placed in a 100°C water bath for about 15 min. After cooking, the muscles were placed in warm, insulated containers. Sensory evaluation of both raw and cooked chicken breast fillets was performed by serving them to an experienced, 10-person tasting panel (five men and five women, 19–53 years of age). The overall acceptability of the chicken breast muscle was calculated using the fuzzy reasoning as previously described.[Citation19]
Bacteriological Analyses
In a vertical laminar-flow cabinet, a sample of 25 g was taken aseptically and then transferred into a stomacher bag. Next, 225 mL of tryptone salt solution (0.85% tryptone and 0.85 NaCl) was added and the mixture was homogenized for 90 s using a Stomacher 400 (Seward, UK).[Citation23] Serial decimal dilutions were prepared, and 1 mL of each dilution was pipetted into an empty sterile Petri dish. Subsequently, about 20 mL of melted plate count agar (Qingdao Hope Bio-Technology Co., Ltd., China), which had been cooled in a water bath at 45°C, was poured into the dish, and then mixed thoroughly by gentle tilting and swirling. Twenty minutes later, the dishes were upside down and incubated at 30°C for 72 ± 4 h. Finally, the total colony forming units were recognized as the total viable counts (TVC).[Citation24]
Statistical Analyses
All analyses were carried out in triplicate experiments, and mean values with standard deviations (SDs) were reported for the each case. All data were interpreted using the one-way analysis of variance (ANOVA), and mean separations were performed using the Tukey’s multiple range test (SPSS 19.0). Differences were considered significant at the level of p < 0.05.
Results and discussion
PV and TBA Analyses
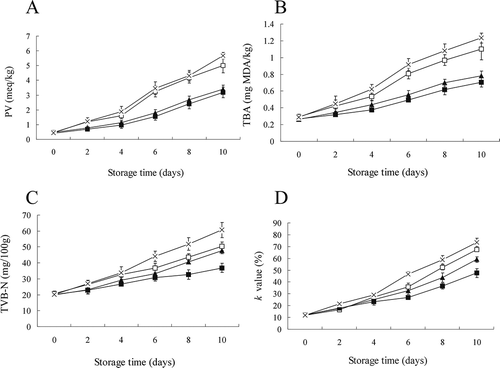
Lipid oxidation in all chicken breast muscle treatment groups was monitored as both PV and TBA throughout the 10-day storage period. All chicken meat started with low PV and TBA values of 0.39 to 0.57 meq/kg and 0.24 to 0.33 mg MDA/kg samples, respectively ( and ). Antioxidant (RE) changed the level of oxidation slightly in samples during the subsequent chilled storage (day 2). On the 6th day, the levels of both PV and TBA in the chicken breast samples without antioxidant (ɛ-PL and control) were significantly higher than in the others (RE and RE plus ɛ-PL; p < 0.05). The PV and TBA values of chicken fillets treated with ɛ-PL were close to those of the control treatment throughout the storage, indicating that ɛ-PL has almost no effect to reduce the lipid oxidation of chicken. RE, an excellent antioxidant, inhibits the lipid oxidation of foods by scavenging free radicals to terminate the free radical chain reaction, and it has been widely used as a preservative (e.g., for meat and poultry products, vegetable oils, and shortenings).[Citation2,Citation25,Citation26] In our study, the lipid oxidation of chicken breast muscle was strongly inhibited by RE. The PV and TBA values of samples (RE and RE plus ɛ-PL) treated with RE were significantly lower than those of control and ɛ-PL-treated samples from day 6 to day 10 (p < 0.05). Moreover, when combined with citric acid or ascorbyl palmitate, RE showed an additive antioxidative effect, but it had a negative synergism if mixed with α-tocopherol.[Citation27] However, there was no significant difference in PV and TBA values between the RE and RE plus ɛ-PL treatment groups during the whole storage period (p ≥ 0.05), suggesting that ɛ-PL has neither an additive nor a negative synergistic effect in reducing lipid oxidation of chicken breast muscle when combined with RE.
FIGURE 1 Changes in peroxide value (PV); A: thiobarbituric acid (TBA); B: total volatile basic nitrogen (TVB-N); C: and K value; D: of chicken breast muscle treated with RE (ϴ), ɛ-PL (▲), RE plus ɛ-PL (■), and control (×) stored at 4°C for 10 days. Each data point is the mean of three replicates. Vertical bars represent standard deviation of means.
TVB-N Analyses
Protein and non-protein nitrogenous compounds of meat could be degraded through several spoilage microbial activities and endogenous enzymatic processes, which produce TVB-N. The TVB-N content has been widely used to indicate meat freshness.[Citation28,Citation29] Changes in the TVB-N content of chicken breast muscle over the storage time are shown in . The initial TVB-N value was about 20 mg/100 g, and then it increased progressively with time of chilled storage for all treatment samples. At day 6, the TVB-N level in the control (44.35 ± 2.91 mg of TVB-N/100 g) exceeded the upper limit values of 40 mg of TVN-N/100 g for spoilage initiation of fresh chicken meat stored aerobically,[Citation30] while TVB-N levels in both the RE and ɛ-PL treatment groups reached the upper limit after 8 days. The final TVB-N values of the RE plus ɛ-PL treatment group did not exceed the upper acceptability limit after 10 days of chilled storage. TVB-N increased significantly faster in the control than in the RE and ɛ-PL treatment groups, in which it was significantly faster than in the RE plus ɛ-PL treatment group. This result indicates that the combination of RE and ɛ-PL inhibited endogenous enzymes and spoilage microbial activity more effectively than either treatment alone.
K-Value Analyses
Generally, after death, ATP in chicken meat decomposes gradually to produce ADP, AMP, IMP, HxR, and Hx, which are important components of the flavor-precursor complex in chicken muscle. The higher the IMP content in the muscle, the better the meat will taste, while HxR and Hx are bitter. Hence, the K-value is an excellent indicator of the freshness of fish and chicken.[Citation31] A fish product with a K-value less than 20% is very fresh, and less than 50% is moderately fresh, while greater than 70% is not fresh.[Citation32] Changes in the K-value of chicken breast muscle over storage time are shown in . The K-value of the control increased markedly from the 2nd day (21.34%) and throughout the storage period. The addition of RE, alone or in combination with ɛ-PL, reduced the degradation of ATP and yielded scores below 20% on the 2nd day. On day 6, large significant differences (p < 0.05) were evident between the treated and control groups, and the RE plus ɛ-PL treatment group had the lowest score (26.85%), followed by the RE and ɛ-PL treatment groups (32.47 and 35.86%, respectively). At the end of storage, the mean K-values were 73.45, 67.57, 59.1, and 47.55% for the control, ɛ-PL, RE, and RE plus ɛ-PL treatment groups, respectively. Thus, RE plus ɛ-PL treatment can effectively reduce the degradation of ATP and maintain the storage quality and extend the shelf life of chicken breast muscle, suggesting a synergistic effect of RE combined with ɛ-PL treatment might effectively inhibit the microbial activity that degrades ATP into its breakdown products.
pH Value Analyses
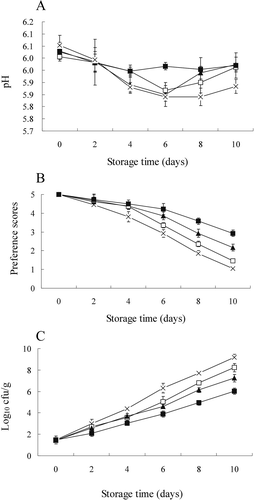
Due to the accumulation of H+ ions, the pH value will decline because of post-mortem muscle energy metabolic activity. During the rigor mortis period, an occurrence of abnormal high temperature accompanied by a rapid decline of pH will probably result in protein denaturation and consequent adverse effects on the quality of the meat (it becomes pale, soft, and exudative) that have been observed in muscles of pork, beef, sheep, poultry, and turkey.[Citation33,Citation34] In this study, the pH value of all samples ranged from 5.84 to 6.05 during post-mortem storage (), and no PSE-like meat was observed in any sample, suggesting that low temperature inhibited post-mortem muscle energy metabolism, reducing glycolysis of the residual glycogen to pyruvic acid and then to lactic acid during storage. However, no significant difference (p ≥ 0.05) was observed in pH values between treated groups and the control during the post-mortem storage. Similar results have been found in beef[Citation35] and pompano (Trachinotus ovatus) fish.[Citation14] The main reason for this might be that only a small amount of lactic acid was produced in the chicken breast muscle tissue during the storage.
FIGURE 2 Changes in pH A: overall acceptability of sensory; B: and total viable counts (TVC); C: of chicken breast muscle treated with RE (ϴ), ɛ-PL (▲), RE plus ɛ-PL (■), and control (×) stored at 4°C for 10 days. Each data point is the mean of three replicates. Vertical bars represent standard deviation of means.
Color Analyses
Color has a major influence on the consumer’s food choice and preferences. Food color is determined by the chemical, physical, biochemical, and microbial changes that exist during growth, maturation, post-mortem processing, and storage. Surface color parameters of the chicken breast muscle during the entire storage are shown in . As storage time increased, values of lightness (L*) and whiteness markedly decreased (p < 0.05), while blueness (b*) increased (p < 0.05). At the end of storage, values of L* and whiteness in the RE plus ɛ-PL treatment group were higher than those in the RE, ɛ-PL, or control, while the value of b* was lower. Regarding redness (a*), no significant differences were observed between all treatments, nor were significant correlations observed over the period of storage. Previous studies reported that muscle color was influenced by both muscle structure characteristics and pigment concentrations,[Citation36,Citation37] and it was also attributed to the oxidation of protein during storage.[Citation38] Our results suggest that RE combined with ɛ-PL protects the lightness (L*), blueness (b*), and whiteness of chicken breast muscle during chilled storage.
TABLE 1 Color measurements within each treatment group of the chicken breast muscle during the storage period
Sensory Analyses
Fuzzy reasoning was used to analyze the chicken breast sensory evaluation data. Preference degrees for sensory attitudes of appearance, odor, and taste of raw or cooked meat were represented by fuzzy sets. In the defuzzification process, the element values for preference levels of “neutral,” “slight smell,” “smell,” “stale,” and “spoiled” were assigned scores of 5, 4, 3, 2, and 1, respectively, based on the fact that the higher preference level, the higher element scores (). The evaluation of sensory attributes of both raw and cooked chicken breast muscle is shown in . All samples were started with a score of 5. No significant differences were observed among the different samples within 2 days of storage (p ≥ 0.05). Four days later, large significant differences were evident, and the control had scores significantly lower than any other treated samples at that time (p < 0.05). Previous studies suggested that values greater than 3 are acceptable.[Citation39,Citation40] Samples treated with RE plus ɛ-PL maintained their good quality up to day 8 and were of acceptable quality up to day 10. When RE or ɛ-PL alone was used, the acceptable quality lasted up to day 6. However, it should be emphasized that the value for RE was significantly higher than the value for ɛ-PL on day 6 (p < 0.05). Thus, the use of RE alone produced a significant increase in maintaining good quality and the shelf life of chicken breast muscle compared to the control, and this trend was strengthened when ɛ-PL was added.
TABLE 2 Sensory scheme for evaluating the chicken breast muscle during the storage period
Bacteriological Analyses
Chicken breast muscle contains a variety of microbes that are either naturally occurring on the meat or are introduced in processing. The bacteria present can present a public health hazard as well as lead to spoilage. Changes in the TVC on chicken breast muscle during the entire storage are shown in . All samples were of good starting quality, with low microbial counts of around 1.4 log10 cfu/g. Up to day 4, there were no significant differences among all the samples (p ≥ 0.05). On the 6th day of storage, counts grew rapidly in control samples, and reached 6.67 ± 0.22 log10 cfu/g, which was significantly higher than those of the treated samples (p < 0.05). On day 8, both RE- and ɛ-PL-treated samples had significantly higher values (7.09 ± 0.17 log10 cfu/g and 5.94 ± 0.22 log10 cfu/g, respectively) than the RE plus ɛ-PL group (4.94 ± 0.21 log10 cfu/g; p < 0.05), while the control was 7.69 ± 0.23 log10 cfu/g, more than the maximal recommended limit (7 log10 cfu/g) in meat.[Citation41] Treated samples of RE plus ɛ-PL had the lowest counts during the entire storage, and they did not reach the maximal recommended limit even at the end of storage (day 10). These results obviously demonstrate that the RE had as strong an inhibitory effect on microbial growth in stored chicken breast muscle as ɛ-PL, and that the two treatments were synergistic in inhibiting microorganism growth and extending the shelf life of chicken breast muscle.
Conclusions
This study showed that the RE in combination with ɛ-PL treatment could effectively inhibit lipid oxidation, protein decomposition, nucleotide breakdown, and microbial growth, and they could also improve color and sensory attributes within acceptable limits during the refrigerated storage period, and maintain the storage quality and extend the shelf life of chicken breast muscle compared with the control. So, natural antioxidant (RE) combined with natural antimicrobial (ɛ-PL) may be a promising method of maintaining the storage quality of chicken breast muscle and extending its shelf life.
FUNDING
This work was supported by the National Natural Science Foundation of China (No. 31301566), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20133326120008), International Science & Technology Cooperation Program of China (No. 2013DFG32390), Food Science and Engineering—the Most Important Discipline of Zhejiang Province (No. JYTSP20142071), Non-Profit Technology Research Projects of Analysis and Testing of Zhejiang Province (No. 2015C37067).
Additional information
Funding
References
- Hygreeva, D.; Pandey, M.C.; Radhakrishna, K. Potential Applications of Plant Based Derivatives As Fat Replacers, Antioxidants, and Antimicrobials in Fresh and Processed Meat Products. Meat Science 2014, 98, 47–57.
- Karre, L.; Lopez, K.; Getty, K.J. Natural Antioxidants in Meat and Poultry Products. Meat Science 2013, 94, 220–227.
- Johnson, J.J. Carnosol: A Promising Anti-Cancer and Anti-Inflammatory Agent. Cancer Letter 2011, 305, 1–7.
- Ngo, S.N.; Williams, D.B.; Head, R.J. Rosemary and Cancer Prevention: Preclinical Perspectives. Critical Reviews in Food Science and Nutrition 2011, 51, 946–954.
- Chen, X.Q.; Zhang, Y.; Zu, Y.G.; Yang, L.; Lu, Q.; Wang, W. Antioxidant Effects of Rosemary Extracts on Sunflower Oil Compared with Synthetic Antioxidants. International Journal of Food Science & Technology 2014, 49, 385–391.
- Zhang, Y.; Yang, L.; Zu, Y.G.; Chen, X.Q.; Wang, F.J.; Liu, F. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants During Accelerated Storage. Food Chemistry 2010, 118, 656–662.
- Dussault, D.; Vu, K.D.; Lacroix, M. In Vitro Evaluation of Antimicrobial Activities of Various Commercial Essential Oils, Oleoresin, and Pure Compounds Against Food Pathogens and Application in Ham. Meat Science 2014, 96, 514–520.
- Weerakkody, N.S.; Caffin, N.; Lambert, L.K.; Turner, M.S.; Dykes, G.A. Synergistic Antimicrobial Activity of Galangal (Alpinia Galanga), Rosemary (Rosmarinus Officinalis), and Lemon Iron Bark (Eucalyptus Staigerana) Extracts. Journal of the Science of Food and Agriculture 2011, 91, 461–468.
- Han, J.H.; Patel, D.; Kim, J.E.; Min, S.C. Retardation of Listeria Monocytogenes Growth in Mozzarella Cheese Using Antimicrobial Sachets Containing Rosemary Oil and Thyme Oil. Journal of Food Science 2014, 79, E2272–2278.
- Yamanaka, K.; Kito, N.; Imokawa, Y.; Maruyama, C.; Utagawa, T.; Hamano, Y. Mechanism of Epsilon-Poly-L-Lysine Production and Accumulation Revealed by Identification and Analysis of An Epsilon-Poly-L-Lysine-Degrading Enzyme. Applied and Environmental Microbiology 2010, 76, 5669–5675.
- Chang, Y.H.; McLandsborough, L.; McClements, D.J. Antimicrobial Delivery Systems Based on Electrostatic Complexes of Cationic ɛ-Polylysine and Anionic Gum Arabic. Food Hydrocolloids 2014, 35, 137–143.
- Moschonas, G.; Geornaras, I.; Stopforth, J.D.; Wach, D.; Woerner, D.R.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Activity of Caprylic Acid, Carvacrol, Epsilon-Polylysine, and Their Combinations Against Salmonella in Not-Ready-to-Eat Surface-Browned, Frozen, Breaded Chicken Products. Journal of Food Science 2012, 77, M405–411.
- Chang, S.S.; Lu, W.Y.; Park, S.H.; Kang, D.H. Control of Foodborne Pathogens on Ready-to-Eat Roast Beef Slurry by Epsilon-Polylysine. International Journal of Food Science & Technology 2010, 141, 236–241.
- Gao, M.S.; Feng, L.F.; Jiang, T.J.; Zhu, J.L.; Fu, L.L.; Yuan, D.X.; Li, J.R. The Use of Rosemary Extract in Combination with Nisin to Extend the Shelf Life of Pompano (Trachinotus Ovatus) Fillet During Chilled Storage. Food Control 2014, 37, 1–8.
- Juneja, V.K.; Dwivedi, H.P.; Yan, X. Novel Natural Food Antimicrobials. Annual Review of Food Science and Technology 2012, 3, 381–403.
- GBT 19478 -2004. Operating Procedure of Chicken Slaughtering; The Chinese National Hygiene Ministry: Beijing, 2004.
- Saleem, R.; Hasnain, A.; Ahmad, R. Changes in Some Biochemical Indices of Stability of Broiler Chicken Actomyosin at Different Levels of Sodium Bicarbonate in Presence and Absence of Sodium Chloride. International Journal of Food Properties 2015, 18, 1373–1384.
- Hou, W.F.; Xie, J. Preservation Effects of ε-Polylysine, Chitosan, and Phytic Acid on Penaeus Vannamei. Food Science 2012, 33, 308–312.
- Feng, L.F.; Jiang, T.J.; Wang, Y.B.; Li, J.R. Effects of Tea Polyphenol Coating Combined With Ozone Water Washing on the Storage Quality of Black Sea Bream (Sparus Macrocephalus). Food Chemistry 2012, 135, 2915–2921.
- Aliani, M.; Farmer, L.J.; Kennedy, J.T.; Moss, B.W.; Gordon, A. Post-Slaughter Changes in ATP Metabolites, Reducing, and Phosphorylated Sugars in Chicken Meat. Meat Science 2013, 94, 55–62.
- Ryder, J.M. Determination of Adenosine Triphosphate and Its Breakdown Products in Fish Muscle by High-Performance Liquid Chromatography. Journal of Agricultural and Food Chemistry 1985, 33, 678–680.
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food and Bioprocess Technology 2013, 6, 36–60.
- Vasconcelos, H.; Saraiva, C.; de Almeida, J.M.M.M. Evaluation of the Spoilage of Raw Chicken Breast Fillets Using Fourier Transform Infrared Spectroscopy in Tandem with Chemometrics. Food and Bioprocess Technology 2014, 7, 2330–2341.
- GBT 4789.2-2010. Food Microbiological Examination: Aerobic Plate Count; The Chinese National Hygiene Ministry: Beijing, 2010.
- Karpinska-Tymoszczyk, M. The Effect of Antioxidants, Packaging Type and Frozen Storage Time on the Quality of Cooked Turkey Meatballs. Food Chemistry 2014, 148, 276–283.
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Comprehensive Reviews in Food Science and Food Safety 2009, 8, 345–358.
- Hraš, A.R.; Hadolin, M.; Knez, Ž.; Bauman, D. Comparison of Antioxidative and Synergistic Effects of Rosemary Extract with α-Tocopherol, Ascorbyl Palmitate, and Citric Acid in Sunflower Oil. Food Chemistry 2000, 71, 229–233.
- Cai, J.R.; Chen, Q.S.; Wan, X.M.; Zhao, J.W. Determination of Total Volatile Basic Nitrogen (TVB-N) Content and Warner–Bratzler Shear Force (WBSF) in Pork Using Fourier Transform Near Infrared (FT-NIR) Spectroscopy. Food Chemistry 2011, 3, 1354–1360.
- Özyurt, G.A.; Özkütük, A.S.; Şimşek, A.; Yeşilsu, A.F.; Ergüven, M. Quality and Shelf Life of Cold and Frozen Rainbow Trout (Oncorhynchus Mykiss) Fillets: Effects of Fish Protein-Based Biodegradable Coatings. International Journal of Food Properties 2015, 18, 1876–1887.
- Economou, T.; Pournis, N.; Ntzimani, A.; Savvaidis, I.N. Nisin–EDTA Treatments and Modified Atmosphere Packaging to Increase Fresh Chicken Meat Shelf-Life. Food Chemistry 2009, 114, 1470–1476.
- Lin, C.Y.; Lin, L.C.; Hsu, J.C. Effect of Caponization on Muscle Composition, Shear Value, ATP Related Compounds and Taste Appraisal in Taiwan Country Chicken Cockerels. Asian-Australasian Journal of Animal Sciences 2011, 24, 1026–1030.
- Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Marquez-Ríos, E.; Canizales-Rodríguez, D.F.; Castillo-Yáñez, F.J.; Ruíz-Bustos, E.; Graciano-Verdugo, A.Z.; Plascencia-Jatomea, M. Freshness Assessment of Ray Fish Stored in Ice by Biochemical, Chemical, and Physical Methods. Food Chemistry 2011, 125, 49–54.
- Kim, Y.H.B.; Warner, R.D.; Rosenvold, K. Influence of High Pre-Rigor Temperature and Fast pH Fall on Muscle Proteins and Meat Quality: A Review. Animal Production Science 2014, 54, 375–395.
- Swatland, H.J. How pH Causes Paleness Or Darkness In Chicken Breast Meat. Meat Science 2008, 80, 396–400.
- Reihani, S.F.; Tan, T.C.; Huda, N.; Easa, A.M. Frozen Storage Stability of Beef Patties Incorporated with Extracts from Ulam Raja Leaves (Cosmos Caudatus). Food Chemistry 2014, 155, 17–23.
- Mørkøre, T.; Rødbotten, M.; Vogt, G.; Fjæra, S.O.; Kristiansen, I.Ø.; Manseth, E. Relevance of Season and Nucleotide Catabolism on Changes in Fillet Quality During Chilled Storage of Raw Atlantic Salmon (Salmo Salar L.). Food Chemistry 2010, 119, 1417–1425.
- Özcan, A.U.; Bozkurt, H. Physical and Chemical Attributes of a Ready-to-Eat Meat Product During the Processing: Effects of Different Cooking Methods. International Journal of Food Properties 2015, 18, 2422–2432.
- Özyurt, G.; Kuley, E.; Balikçi, E.; Kaçar, Ç.; Gökdogan, S.; Etyemez, M.; Özogul, F. Effect of the Icing with Rosemary Extract on the Oxidative Stability and Biogenic Amine Formation in Sardine (Sardinella Aurita) During Chilled Storage. Food and Bioprocess Technology 2012, 5, 2777–2786.
- Seung, J.L.; Young, A.K. Study on Fuzzy Reasoning Application for Sensory Evaluation of Sausages. Food Control 2007, 18, 811–816.
- Martínez, L.; Djenane, D.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of Different Concentrations of Carbon Dioxide and Low Concentration of Carbon Monoxide on the Shelf-Life of Fresh Pork Sausages Packaged in Modified Atmosphere. Meat Science 2005, 71, 563–570.
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of Chitosan Coatings Enriched with Cinnamonoil on the Quality of Refrigerated Rainbow Trout. Food Chemistry 2010, 120, 193–198.
