Abstract
An identification method of lard in chocolates using real-time polymerase chain reaction was developed to address Halal authentication. However, polymerase chain reaction detection of lard in chocolate has been in vain. In order to investigate the inhibitory effect exerted by each of the chocolate components, four basic chocolate components, sugar, milk powder, cocoa butter, and cocoa powder, were adulterated with lard and examined using porcine-specific real-time polymerase chain reaction assay. The results discovered cocoa powder, as the only component that prevents DNA extraction of lard in chocolate. No substantial polymerase chain reaction inhibition was detected, and thus confirms the cocoa powder’s inhibition on DNA extraction of lard from lard-adulterated chocolate. This finding will expedite new research to develop a method to dissociate the lard from the lard-cocoa powder complex, which will have high potential to be applied as a pre-treatment of the chocolate prior to the DNA extraction and polymerase chain reaction.
Keywords:
INTRODUCTION
Halal food authentication has been given the same concern as authentication of genetically modified foods and allergen-containing foods. Due to the high demand from consumers, Halal food has become a lucrative business conducted by both Muslims and non-Muslims. However, illegal fraud and adulteration of Halal food with non-Halal ingredients is not new, and mainly done due to economic reasons.[Citation1] Potential adulteration of Halal foods are commonly derived from porcine either from meat or its by-product such as lard and gelatin, which are totally banned by the religions of Islam and also Judaism.[Citation2]
Unexceptionally, chocolate, which has been a very popular commodity among consumers, is also potentially being exposed to fraud and adulteration. According to the Guidance on the Cocoa and Chocolate Products Regulations 2003, milk chocolate is basically composed of cocoa butter, sugar, milk powder, and cocoa powder. Cocoa butter is considered as the most essential chocolate component that determines its texture and melting behavior.[Citation3] It is produced from the cocoa nib through hydraulic presses at high pressure and is the most expensive chocolate component.[Citation4] Due to that, there have been long-term efforts to substitute it, fully or in part, with other vegetable fats and possibly, animal fats such as lard.[Citation5] Lard has been extracted from the adipose tissues of the swine by a rendering process and has found many applications in food preparations, mainly for its flavors and functional properties.[Citation6] The inclusion of lard in food products has been a major concern to certain consumers due to religious obligations[Citation7] and health complications such as hypercholesterolemia and coronary heart disease.[Citation8]
Detection of lard in food products has been demonstrated by several researchers. However, there were some limitations on the methods used. For instance, Che Man et al.[Citation4] used Fourier transform infrared spectroscopy to detect the lard in chocolate and chocolate products. However, the sensitivity of the method has not been determined and complicated data analysis is required to rule out the presence of lard.[Citation9] On the other hand, an attempt to use polymerase chain reaction (PCR) for lard detection in biscuit formulation had been failed to be demonstrated, which is probably due to the insufficient amount of degraded DNA being extracted from the lard-adulterated biscuit.[Citation10] Although lard has been known to unexceptionally contain a large amount of mtDNA in the adipose tissue,[Citation11] extensive processing of the product (high temperature and pressure) and composition of complex food matrices, such as chocolate, may cause DNA degradation and further contamination on the extracted DNA with other food components. Nevertheless, to date, there is no vigorous research being carried out to determine the key factor of the failure. Food products with complex food matrices, such as chocolate, require a detailed investigation of the inhibitory effect exerted by each of its components. This needs to be done in order to ensure none of the components halt DNA extraction from the lard contained in the chocolate and insignificant amount of PCR inhibitor, such as polyphenol,[Citation12,Citation13] is being co-extracted with the DNA. Polyphenols are compounds that consist of 1.3 and 4.5% of soluble polyphenols and condensed tannins, respectively, which gives cocoa its fiber material with intrinsic antioxidant capacity.[Citation14] However, the oxidized form of polyphenol is able to bind covalently to proteins and nucleic acids which may cause failure in DNA detection using PCR.[Citation15]
In this work, we hypothesized that there is one or more chocolate components; cocoa butter, cocoa powder, sugar, and milk protein that inhibit DNA extraction of lard from lard-adulterated chocolate. The effect of each chocolate component toward DNA extraction of lard from lard-adulterated chocolate was examined using highly porcine-specific and sensitive real-time PCR.
MATERIALS AND METHODS
Preparation of Chocolate
In this study, chocolate that contained milk was examined. The preparation of chocolate was done according to the double-boiling method being described by Gryson et al.[Citation15] Four basic components of chocolate, which consist of cocoa butter 44%, cocoa powder 11%, milk powder 1%, and sugar 44%, were obtained from several local baking suppliers in Selangor, Malaysia. Briefly, cocoa butter was incubated on warm water, followed by the mixing of cocoa powder, sugar, and milk powder. The mixture was then further homogenized for 30 min in warm water before being transferred into a mold and cooled at room temperature. The chocolate was stored at 4°C until further use.
Preparation of Lard-Adulterated Samples
Pure lard was purchased from local shopping mall in Seri Kembangan, Selangor. Prior to DNA extraction, lard was added to the previously prepared chocolate at 1, 10, 20, and 50% wt/wt spiking level. Additionally, lard was separately added to cocoa powder, cocoa butter, sugar, and milk powder at the spiking level of 50% wt/wt.
Preparation of Binary DNA Admixture
There was 10 ng/uL of DNA extracted from each pure lard, cocoa powder was mixed in 50% v/v ratio. The resulting DNA admixtures were mixed to ensure homogeneous mixture of DNA derived from both lard and cocoa powder. The DNA admixtures were either used directly or can be stored at 4°C until further use.
DNA Extraction and Quantification
Total DNA extraction was carried out using Cetyl trimethylammonium bromide (CTAB) method according to the method described by He et al.[Citation16] Briefly, 0.2 g of samples were mixed with 1 mL CTAB extraction buffer (1.4 M NaCl, 2% CTAB, 100 mM Tris, 20 mM EDTA, pH 8.0) and 100 µg/mL of proteinase K for 1 h at 65°C with shaking. The samples were extracted with 1 mL chloroform, centrifuged at 12,000 × g, precipitated with 2 volumes of CTAB precipitation butter (40 mM NaCl, 0.5% CTAB) at room temperature for 1 h, and centrifuged again for 20 min at 15,000 × g. The pellet was dissolved in 350 µL of 1.2 M NaCl and extracted with an equal volume of chloroform. DNA in the aqueous phase was precipitated with 350 µL 2-propanol. The pellet was washed with 70% ethanol and eluted in 50 µL of nuclease free water. DNA was quantified using Quant-itTM PicoGreen® dsDNA Assay Kit (Invitorgen, Paisley, U.K.) on microplate reader (TECAN Infinite M200) according to manufacturer’s protocol. The values of the emitted fluorescence were extrapolated on the standard curve of fluorescence versus DNA standards of known concentration. Lambda phage DNA was served as DNA standards and 1 × Tris-EDTA buffer was served as negative control.
Universal and Porcine-Specific Real-Time PCR
Real-time PCR was performed in a 20 µL reaction mixture containing 1× SsoFast EvaGreen Supermix (Bio-Rad), 10 µM of each forward and reverse primer and 5 ng of DNA samples were used. Universal real time PCR employed UF1 and UR1 primers, while porcine-specific real time PCR employed SUS_FWD and SUS_RVS primers (). The PCR program was initiated at a pre-denaturation step at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 s, and annealing for 1 min at 65 or 51.5°C, for porcine-specific and universal real time PCR, respectively. Melting curve analysis which composes of denaturation at 95°C for 5 s, annealing temperature for 1 min, and slow heating to 95°C for 5 s, with a transition rate of 0.2°C/s was done following the amplification. Each sample was run in triplicate. Sterile water was used to replace DNA in no-template control (NTC) tube. The assay was carried out using Mastercycler ep-realplex (Eppendorf, Germany).
TABLE 1 List of primers used in this work
Specificity and Sensitivity Determination of Universal and Porcine-Specific Real Time PCR
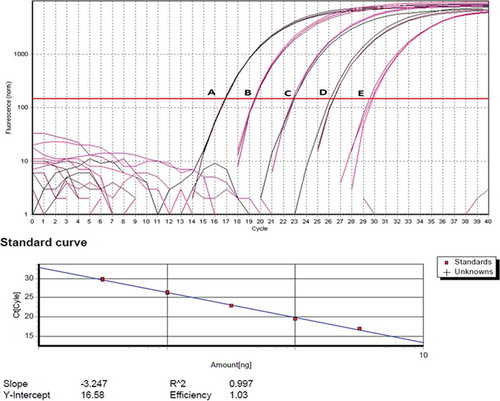
Specificity of the assay was determined by assessing 5 ng of DNA extracted from fresh muscle tissues of major meat-providing land animals (cow, chicken, sheep, buffalo, deer, and pig) in triplicate. The sensitivity of the porcine-specific assay was conducted by assessing 10-fold serial dilution (0.0001, 0.001, 0.01, 0.1, and 1 ng/µL) of pure porcine DNA in sterile water. The Cq values obtained from each dilution were used to construct a standard curve.
Statistical Analysis
The mean and the standard deviation of the Cq values obtained from the real time PCR assay were automatically calculated by the Mastercycler epRealplex Software (Eppendorf, Hilden, Germany).
RESULTS AND DISCUSSION
DNA Quality and Porcine Detection in Lard-Adulterated Chocolate
High DNA yield of approximately 200 to 500 ng/g were obtained from lard-adulterated chocolate samples (). This is in agreement with the findings from previous research[Citation13] that shows suitability of CTAB method for DNA extraction from chocolate. Initial attempt to measure the DNA concentration spectrophotometrically has failed, which is possibly due to the high amount of interference in the extracted DNA. Thus, DNA yield of lard-adulterated chocolate samples was evaluated using fluorimetric procedures that work based on double stranded DNA intercalation with the DNA-specific dye PicoGreenTM. Interferences such as free nucleotides, proteins, and aromatic compounds that are usually present in the complex food samples are unable to interfere with the reading due to specific binding of the dye to double stranded DNA, thus, promising a more reliable measurement.[Citation17]
TABLE 2 DNA yield and mean number of quantification cycles obtained in porcine-specific and universal real time PCR using DNA extracted from lard-adulterated samples
Specificity and Sensitivity Determination of Porcine-Specific and Universal Real Time PCR
The specificity of the porcine-specific PCR system was confirmed by specific amplification of porcine DNA at Cq of 16.90 ± 0.04. No amplification was detected using DNA from other animal species being tested, demonstrating the specificity of the assay to porcine ND5 gene. On the other hand, universal real time PCR successfully amplified DNA from all animal species being tested. Sensitivity of the porcine-specific real time PCR was determined by assaying 10-fold serial dilution of porcine DNA. The assay was found to be able to detect as low as 0.0001 ng porcine DNA, proving the sensitivity of the method to detect minute amount of porcine DNA ().
Quality Evaluation of DNA Extracted from Lard-Adulterated Chocolate
The universal primers were designed to amplify 16S rRNA sequence that is conserved in all mammals. Universal PCR system shows successful amplification of universal 16S rRNA sequences from all lard-adulterated chocolate samples (). This proves that the DNA of a good quality has been extracted from chocolate. Although the eluted DNA was observed to be light brown in color, which is the possible indicator of co-precipitation of polyphenol from cocoa with the DNA, successful universal PCR amplification shows that the extracted DNA is free or contain insignificant amount of PCR inhibitors, thus applicable for PCR.
Detection of Lard in Lard-Adulterated Chocolate Samples
However, the detection of lard in all lard-adulterated chocolate samples has been in vain. Successful amplification of porcine-specific sequence in the positive control, which utilizes DNA extracted from pure lard (100% w/w) shows that PCR reagents and equipment are in good working condition. Besides, successful amplification of universal 16S rRNA sequence proves that the extracted DNA has insignificant amount of PCR inhibitor that may interrupt the PCR process. Thus, we hypothesize that there is one or more chocolate component that inhibit DNA extraction of the lard when it is present in a homogenous chocolate mixture. In order to further investigate the actual chocolate component that is responsible for the unsuccessful detection of porcine in lard-adulterated chocolate, lard was used to separately adulterate cocoa butter, sugar, cocoa powder, and milk powder at 50% w/w concentration.
Evaluation of DNA Yield from Lard Adulterated-Cocoa Powder, Cocoa Butter, Sugar, and Milk Powder
Low DNA yield was obtained in lard-adulterated cocoa butter and sugar, 1.51 ± 1.50 and 1.98 ± 1.50 ng/g, respectively. On the other hand, a high DNA yield of 521.18 ± 3.83 ng/g was obtained in lard-adulterated cocoa powder. Besides, a DNA yield of 47.90 ± 1.60 ng/g was obtained from lard-adulterated milk powder (). From this observation, we can conclude that the high DNA yield of lard-adulterated chocolate is mostly contributed by the cocoa powder. Despite that, cocoa butter which has undergone many food technology processing techniques such as alkalization of the cocoa mass that involves high pH may cause DNA hydrolysis and contain a limited amount of undegraded DNA.[Citation13] In milk powder, significantly higher DNA amount as compared to cocoa butter and sugar was obtained due to the presence of somatic cells in milk.[Citation18]
Detection of Lard in Lard-Adulterated Cocoa Powder, Cocoa Butter, Sugar, and Milk Powder
The DNA extracted from lard-adulterated cocoa butter, sugar, cocoa powder, and milk powder were assessed using universal real time PCR to determine the purity of the extracted DNA from PCR inhibitor and to determine the presence of animal DNA. Detection of lard using porcine-specific real-time PCR was then carried out to determine the presence of lard-derived DNA in the extracted DNA.
Successful detection of both mammalian and porcine-specific sequence was obtained when assaying DNA extracted from lard-adulterated cocoa butter, sugar and milk powder (). Successful detection of mammalian-specific gene in lard-adulterated milk powder shows the availability of bovine or porcine DNA being derived from the bovine milk and lard, respectively. In spite of that, successful detection of mammalian-specific gene in lard-adulterated sugar and cocoa butter shows that the DNA being derived from lard was used as a template for amplification of universal 16S rRNA sequence (). He et al.[Citation16] failed to amplify plant-specific DNA from cocoa butter due to the large amplicon (600 bp) being targeted for amplification. Nonetheless, in this work, the ability to amplify plant-specific sequence from lard-adulterated cocoa butter was not tested. Overall, the ability of the extracted DNA to be amplified in universal PCR system suggests sufficient DNA purity from PCR inhibitors and the availability of animal DNA in lard-adulterated cocoa butter, sugar, and milk powder.
FIGURE 2 Amplification plot of lard-adulterated cocoa powder, milk powder, cocoa butter and icing sugar using porcine-specific real-time PCR assay. Only lard-adulterated cocoa powder failed to give positive amplification.
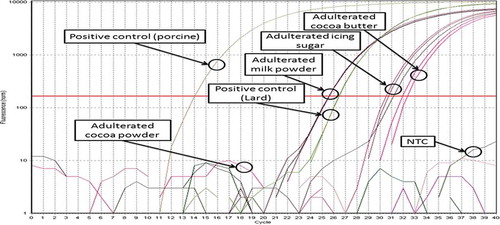
In contrast, both universal and porcine-specific sequence failed to be amplified in DNA extracted from lard-adulterated cocoa powder (). Failure in the detection of universal mammalian sequence in the DNA extracted from lard-cocoa powder suggests two possibilities. First, a significant amount of PCR inhibitor derived from cocoa powder may be present in the extracted DNA which inhibits PCR amplification of the gene. Second, there is no animal DNA contained in the extracted DNA.
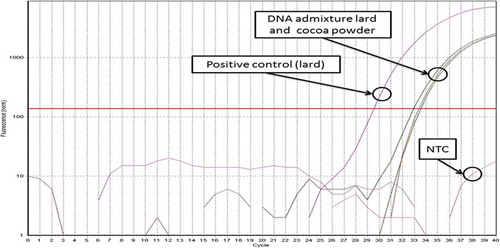
Thus, in order to further examine the purity of DNA being extracted from cocoa powder against PCR inhibitor, 10 ng/uL of binary DNA admixtures containing 50% v/v of DNA extracted from pure lard and pure cocoa powder were assessed in both universal and porcine-specific real time PCR. The presence of PCR inhibitor in DNA extracted from cocoa powder will inhibit PCR amplification of both universal and porcine-specific sequence in the binary DNA admixtures. As a result, both endogenous and porcine-specific sequences were successfully amplified, showing that the DNA extracted from both lard and cocoa powder is free from a significant amount of PCR inhibitor and in sufficiently good quality to allow it to be amplified in PCR assay ().
FIGURE 3 Amplification plot of binary DNA admixture containing mixture of DNA from pure lard and DNA from cocoa powder. Positive amplification being obtained proves that the extracted DNA is applicable for PCR (free or insignificant amount of PCR inhibitor).
Hence, the absence of animal DNA in the DNA extracted from lard-adulterated cocoa powder is confirmed. However, this hypothesis was questionable since a significantly high amount of lard (50% w/w) was introduced into the cocoa powder during the preparation of lard-adulterated cocoa powder. This condition can be explained through an inference that lard DNA from the lard-adulterated cocoa powder failed to be extracted in the presence of cocoa powder due to some inhibitory activity exerted by cocoa powder.
Possible explanation on the failure of DNA extraction from lard in lard-cocoa powder admixture may rely on the fact that a high amount of polyphenol is contained in the cocoa powder. Shishikura et al.[Citation19] proposed the ability of the hydroxyl moieties of some polyphenols to interact and form crosslinks with the polar phosphatidylcholine of the fatty acid. In our case, polyphenol from the cocoa powder may interact with the fatty acids from lard, which in turn, interrupts the DNA extraction process from lard. In addition, Do et al.[Citation20] has successfully observed the behavior of fat derived from rapeseed oil in the presence of cocoa powder using confocal laser scanning microscope. They proved that the fat globules were trapped in the porous network of the cocoa powder. In relation to our case, entrapped lard in cocoa powder network may limit the contact of DNA extraction reagent with the lard, thus prevents DNA extraction from lard. Although the previous study used plant-derived fat, the same occurrence of the behavior with animal fat in cocoa powder is hypothesized.
In order to prove these, more in depth research must be done to investigate the physical distribution of lard in cocoa powder and in chocolate. Future research should focus on investigating specific interactions that occur between these two components in order to resolve the inhibitory activity of the cocoa powder in DNA extraction of lard from lard-adulterated cocoa powder and lard-adulterated chocolate.
CONCLUSION
The CTAB method was found to be sufficient in extracting a high yield of DNA with limited co-precipitation of PCR inhibitors. The failure in detection of DNA extracted from lard-adulterated chocolate using PCR has showed cocoa powder inhibitory activity against DNA extraction of lard from lard-adulterated cocoa powder and lard-adulterated chocolate. This finding should expedite future research on investigating the inhibitory mechanism of cocoa powder and the physical distribution of lard in the presence of cocoa powder in order to find a mechanism to solve the emerging problem.
REFERENCES
- Rohman, A.; Che Man, Y.B. Fourier Transform Infrared (FTIR) Spectroscopy for Analysis of Extra Virgin Olive Oil Adulterated with Palm Oil. Food Research International 2010, 43, 886–892.
- Aida, A.A.; Che Man, Y.B.; Wong, C.M.V.L.; Raha, A.R.; Son, R. Analysis of Raw Meats and Fats of Pigs Using Polymerase Chain Reaction for Halal Authentication. Meat Science 2005, 69, 47–52.
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.M.; Omar, A.K.M. Cocoa Butter Fats and Possibilities of Substitution in Food Products Concerning Cocoa Varieties, Alternative Sources, Extraction Methods, Composition, and Characteristics. Journal of Food Engineering 2013, 117, 467–476.
- Bohačenko, I.; Kopicová, Z.; Pinkrová, J. Chocolate Authenticity Control Concerning Compliance with the Conditions for Adding Cocoa Butter Equivalents as Laid down by Directive 2000/36 EC. Czech Journal of Food Science 2005, 23, 27–35.
- Che Man, Y.B.; Syahariza, A.Z.; Mirghani, M.E.S.; Jinap, S.; Bakar, J. Analysis of Potential Lard Adulteration in Chocolate and Chocolate Products Using Fourier Transform Infrared Spectroscopy. Food Chemistry 2005, 90, 815–819.
- Marikkar, J.M.N.; Yanty, N.A.M. Effect of Chemical and Enzymatic Modifications on the Identity Characteristics of Lard: A Review. International Journal of Food Properties 2014, 17, 321–330.
- Marikkar, J.M.N.; Ghazali, H.M.; Che Man, Y.B.; Lai, O.M. The Use of Cooling and Heating Thermograms for Monitoring of Tallow, Lard, and Chicken Fat Adulterations in Canola Oil. Food Research International 2002, 35, 1007–1014.
- Ospina, E.J.C.; Cruz, S.A.; Pérez-Alvarez, J.A.; Fernández-López, J.; Development of Combinations of Chemically Modified Vegetable Oils as Pork Backfat Substitutes in Sausages Formulation. Meat Science 2010, 84, 491–497.
- Syahariza, Z.; Che Man, Y.B.; Jinap, S.; Bakar, J. Detection of Lard Adulteration in Cake Formulation by Fourier Transform Infrared (FTIR) Spectroscopy. Food Chemistry 2005, 92, 365–371.
- Che Man, Y.B.; Aida, A.A.; Raha, A.R.; Son, R. Identification of Pork Derivatives in Food Products by Species-Specific Polymerase Chain Reaction (PCR) for Halal Verification. Food Control 2007, 18, 885–889.
- Shikuma, C.M.; Hu, N.; Milne, C.; Yost, F.; Waslien, C.; Shimizu, S.; Shiramizu, B. Mitochondrial DNA Decrease in Subcutaneous Adipose Tissue of HIV-Infected Individuals with Peripheral Lipoatrophy. AIDS 2001, 15, 1801–1809.
- Gryson, N.; Messens, K.; Dewettinck, K. Evaluation and Optimisation of Five Different Extraction Methods for Soy DNA in Chocolate and Biscuits. Extraction of DNA as a First Step in GMO Analysis. Journal of the Science of Food and Agriculture 2004, 84, 1357–1363.
- Ha, L.T.V.; Vanlerberghe, L.; Toan, H.T.; Dewettinck, K.; Messens, K. Comparative Evaluation of Six Extraction Methods for DNA Quantification and PCR Detection in Cocoa and Cocoa-Derived Products. Food Biotechnology 2015, 29, 1–19.
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; L. Bravo, Dietary Fibre Composition, Antioxidant Capacity, and Physico-chemical Properties of a Fibre-Rich Product from Cocoa (Theobroma Cacao L.). Food Chemistry 2007, 104, 948–954.
- Gryson, N.; Dewettinck, K.; Messens, K. Influence of Cocoa Components on the PCR Detection of Soy Lecithin DNA. European Food Research and Technology 2007, 226, 247–254.
- He, X.; Carter, J.M.; Brandon, D.L.; Cheng, L.W.; McKeon, T.A. Application of a Real-Time Polymerase Chain Reaction Method to Detect Castor Toxin Contamination in Fluid Milk and Eggs. Journal of Agricultural and Food Chemistry 2007, 55, 6897–902.
- Pirondini, A.; Bonas, U.; Maestri, E.; Visioli, G.; Marmiroli, M.; Marmiroli, N. Yield and Amplificability of Different DNA Extraction Procedures for Traceability in the Dairy Food Chain. Food Control 2010, 21, 663–668.
- Li, N.; Richoux, R.; Boutinaud, M.; Martin, P.; Gagnaire, V. Role of Somatic Cells on Dairy Processes and Products: A Review. Dairy Science and Technology 2014, 94, 517–538.
- Shishikura, Y.; Khokhar, S.; Murray, B.S. Effects of Tea Polyphenols on Emulsification of Olive Oil in a Small Intestine Model System. Journal of Agricultural and Food Chemistry 2006, 54, 1906–1913.
- Do, T.A.L.; Vieira, J.; Hargreaves, J.M.; Mitchell, J.R.; Wolf, B. Structural Characteristics of Cocoa Particles and their Effect on the Viscosity of Reduced Fat Chocolate. LWT–Food Science and Technology 2011, 44, 1207–1211.