Abstract
The purpose of this study was to investigate the effect of packaging film on physicochemical properties, microbial profile, and biogenic amines content of barramundi (Lates calcarifer Bloch) fillets packed in polyamide, polypropylene, and low-density polyethylene films and kept at 8°C more than 20 days under modified atmosphere packaging. Putrescine and cadaverine were the most abundant amines, whereas the concentration of histamine ranged from less than 0.5 (not detected) to 198.0, 264.3, and 308.5 mg/kg for polyamide, polypropylene, and polyethylene (low-density polyethylene) films, respectively. Among the three, the psychrotrophic bacteria count was initially 4.26 log colony forming units/g and exceeded the acceptable limit of 7 log colony forming units/g on the 16th day of storage for polyamide and on 12th day of storage for polypropylene and polyethylene. However, the total plate count, among the three packaging films, was initially 3.54 log colony forming units/g and exceeded the acceptable limit of 6 log colony forming units/g on the 12th day of storage. The histamine-forming bacteria count was significantly (p < 0.05) lower in barramundi fillets packaged with polyamide compared to polypropylene and polyethylene. The significant difference (p < 0.05) was observed between the concentration of amines in polyamide as compared with polypropylene and polyethylene. Among the three packaging materials, polyamide was found to be the best for prolonging the storage of barramundi fillets.
INTRODUCTION
Packaging polymers, such as plastics, have become the material of choice in many food packaging applications.[Citation1] One of the functions of packaging materials is to preserve the packaged goods; however, the extension of the storage period is dependent on the properties of the packaging materials and foods.[Citation1] There are three major advantages of using packaging plastic: variety, versatility, and efficiency.[Citation2] The most commonly used polymers for food packaging are polyethylene (PE; low-density polyethylene [LDPE] and high-density polyethylene [HDPE]), polypropylene (PP), polystyrene (PS), polyvinylchloride (PVC), polyethylene terephthalate (PET), ethylene vinyl alcohol copolymer (EVOH), ethylene vinyl acetate (EVA), and polyamide (PA).[Citation3]
PE is the most frequently used polymer in food-packaging applications due to the low cost, easy availability, high-pressure polymer, easy heat sealing, toughness, high elasticity, and good mechanical properties.[Citation1,Citation2] PP is ranked third in bulk plastic production after PE and PVC and is one of the lightest thermoplastics having good water vapor barrier, and heat resistance properties, and low temperature resistance. Nylon is a generic name for a family of PA thermoplastic. It is the common name of PA used for food-packaging films, which have good gas barrier, puncture, and heat-resistance properties.[Citation1,Citation2]
The amount of gas transmission through a packaging material depends on various factors, such as the type, size, thickness, and gas permeability properties of the film and differences in the partial pressure on both sides of the film and storage temperature.[Citation4] Packaging film with different qualities and thicknesses affect gas composition and respiratory metabolism of the commodity inside the package.[Citation4,Citation5] Several investigations have documented the effects of packaging materials on microbiological, physiological, and chemical qualities of fruits such as strawberries,[Citation6] carrots,[Citation7] fresh-cut salad savoy,[Citation8] fresh ginseng,[Citation4] and meat,[Citation9,Citation10] octopus (Octopus vulgaris; PE/PA) barrier bags,[Citation11] salmon (placed in bio-oriented PA bags with a base of three layers of polyolefins) bags.[Citation12] In ideal condition, the usage of high-barrier packaging film, such as PA, might extend the storage period of foods but on cost considerations is overlooked (three to four times higher than other films such as PE and PP), at the cost of storage life. However, not much research has been done on the use of modified atmosphere packaging (MAP) to extend the storage life of fresh fish fillets such as barramundi on biogenic amine formation. Therefore, the objective of this study was to investigate the effects of MAP using different packaging films on the quality attribute of fresh barramundi fillets kept at 8°C.
MATERIALS AND METHODS
Experimental Design
A total of 54 barramundi fillets obtained from 27 fish (average weight of 0.5 kg) were purchased from local wet market, Selangor D.E. Malaysia and transported on ice to the laboratory. Upon arrival, they were immediately decapitated and filleted manually and randomly divided into three equal groups. All fish used in the study were within 5 h of landing. Two pieces of fillets were obtained from each fish. Three different packaging films, that is, PA, PP, and PE, were selected, and 18 fillets were packed individually in each packaging film, follow-up flushed with 75% carbon dioxide [CO2]/10% O2/15% N2 (food grade) inside the package (Multivac packaging, GOOD-And-WELL, Taiwan; Gas/Product 2:1) and sealed immediately. The gas composition (O2 and CO2) was analyzed using a CheckMate gas-analyzer (PBI Dansensor MAP Mix 9001 ME, USA) in triplicate. The fillets were kept at 8°C for 20 days and sampled periodically at 4-day intervals.
Properties of Packaging Film
The material was made of PA (thickness, 0.09 mm; dimension, 220 × 300 mm) and had an O2, N2, CO2 (at 25°C), and H2O (at 38°C, 90% relative humidity) transmission rate of 1.55, 0.465, 6.15 cm3/m2/day atm, and 15 g/m2/day atm; PP (thickness, 0.025 mm; dimension, 220 × 300 mm) 2000, 400, 8000 cm3/m2/day atm and 6–7 g/m2/day atm; and PE (thickness, 0.025 mm; dimension, 220 × 300 mm) 2600, 650, 7600 cm3/m2/day atm and 7–10 g/m2/day atm, respectively.
Biogenic Amine Determination
Biogenic amine analysis was done according to the method of Bakar et al.,[Citation13] and Yassoralipour et al.[Citation14] Samples were homogenized in a Waring blender (Model 32BL79, USA) and 5 g samples were transferred to 50 mL centrifuge tubes and homogenized with 20 mL 6% trichloroacetic acid (TCA) solution for 3 min. The homogenates were then centrifuged at 8000 × g for 10 min in a refrigerated high-speed centrifuge (KUBOTA7800) and the supernatant was filtered through Whatman No. 1 filter paper (Whatman, Maidstone, UK). The filtrates were then placed in volumetric flasks and 6% TCA was added to achieve a final volume of 50 mL. After which, an aliquot of each extract was derivatized with benzoyl chloride using the same procedure as the benzoylation of the standard amine solution. Amines were determined using a Perkin Elmer liquid chromatograph (Perkin Elmer Series 200, USA) system which was equipped with an ultraviolet-visible (UV-Vis) detector, liquid chromatography (LC) Chromato-integrator and Vacuum Degasser. A Lichrospher 100 RP-18 reserved-phase column (5 μm, 150 × 4.6 mm I.D., Merck) was used for the peak separation which was detected at 254 nm. The gradient elution program was set at 0.8 mL/min, starting with a methanol–water mixture (50:50, v/v) for 0.5 min and the program preceded linearly to methanol–water (85:15, v/v) at a flow rate of 0.8 mL/min over 6.5 min. This was followed by the same composition and flow rate for 5 min, then a decrease over 2 min to methanol–water (50:50, v/v) at a flow rate of 0.8 mL/min.[Citation13]
Microbiological Analysis
Total plate count (TPC) and psychrotrophic bacterial counts were determined according to the Association of Official Analytical Chemists (AOAC).[Citation15] Twenty-five grams of the samples were aseptically weighed and homogenized in stomacher bags (BagMixer® 400, Model P) with 225-mL sterile peptone water for 1 min. The homogenized samples were serially diluted using 9-mL peptone water. Further serial dilutions were made, and 0.1 mL of each dilution was pipetted onto the surface of plate count agar (Merck), in triplicate, after which, they were incubated for 2 days at 30°C for TPC and one week at 8°C for psychrotrophic bacteria. The enumeration of histamine-forming bacteria (HFB) was carried out according to the procedure of Niven’s,[Citation16] and anaerobic bacteria were enumerated on tryptose sulphite cycloserine (TSC) agar and incubated anaerobically in anaerobic jars at 30°C for 5 days.[Citation17,Citation18]
Total Volatile Basic-Nitrogen (TVB-N)
The TVB-N content of barramundi fillet was determined according to the method of Goulas and Kontominas[Citation19] and expressed as mg TVB-N per 100 g muscle. A 10 g sample of fish fillet was mixed with 50 mL of distilled water using a Moulinex mixer. The mixture was quantitatively transferred with 200 mL of distilled water into a 500 mL round bottom flask and was distilled after the addition of 2 g of MgO and one drop of silicone to prevent foaming. A 250 mL Erlenmeyer flask containing 25 mL of 3 % aqueous solution of boric acid, 0.04 mL of methyl red and methylene blue mixed indicators for the titration of ammonia was used as the distillate receiver. Distillation was continued until a final volume of 125 mL of distillate was obtained. The boric acid solution turned green when it was made alkaline by the distilled TVB-N. This was titrated with an aqueous 0.1 N hydrochloric acid solution. Complete neutralization was achieved when the color of the distillate turned pink upon addition of a further drop of hydrochloric acid. The quantity of TVB-N in mg N/100 g of fish fillet was calculated from the volume (V) of hydrochloric acid added and its concentration (C) as follows:
Statistical Analysis
All data collected were analyzed using analysis of variance (ANOVA) to determine the effect of packaging films and storage time on the parameters measured in the packaged barramundi fillets. Tukey’s test was used for mean comparison when a significant variation was found through the ANOVA test using SPSS software for Windows (SPSS Inc., 2008).
RESULTS AND DISCUSSION
Biogenic Amine Content
and summarize the content of biogenic amines in the fillet in different packaging materials stored at 8°C for 20 days. Histamine was not detected on day 0 but increased to 198.03, 264.34, and 308.50 mg/kg at the end of the period (20 days) in PA, PP, and PE, respectively (). After 16, 12, and 8 days of storage, the histamine content in PA, PP, and PE was greater than 50 mg/kg of histamine, the permissible limit by the U.S. Food and Drug Administration[Citation20] for scombroid fish and/or products.
TABLE 1 Biogenic amines concentration (mg/kg) in barramundi fillets in different packaging films (n = 3)
FIGURE 1 Concentration (mg/kg) of histamine a: putrescine, b: cadaverine, c: and total biogenic amines, d: in barramundi fillets packed at different films (n = 3). Symbols: ![]()
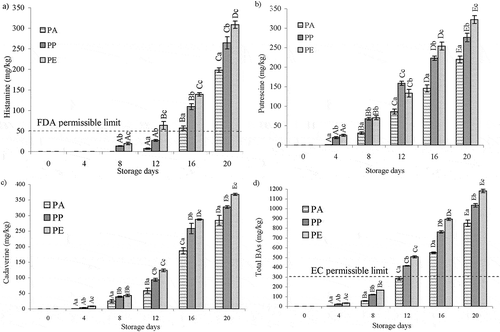
The presence of cadaverine and putrescine may synergistically enhance histamine toxicity by inhibiting histamine-metabolizing enzymes such as diamineoxidase and histamine methyltransferase.[Citation21] A significant increase (p < 0.05) was observed in the content of cadaverine and putrescine throughout storage. These two amines were the two most abundant amines found in all the packaged fillets. At the end of storage, the values of cadaverine and putrescine were 284.5 and 219.8 mg/kg for PA, 327.0 and 276.1 mg/kg for PP, and 367.7 and 321.9 mg/kg for PE. The values for PE-packed fillets were significantly (p < 0.05) higher than those of the two other packages. ( and ).
All biogenic amines except agmatine, were determined on day 8, although their levels were low in PE packages. The content of 2-phenylethylamine in PE packed was significantly (p < 0.05) higher than that in PP and PA packed at the end of storage. The tryptamine concentration in PE packed was revealed after 8 days and in PA and PP after 12 days and reached a maximum on the 16th day and thereafter, declined in all cases. Spermidine and spermine were present at low concentrations, and these levels are in agreement with the results reported for aerobically stored gilt-headed sea bream[Citation22] and for various sea fish species, including salmon, rockfish, lobster, and shrimp.[Citation23] The maximum recommended level of tyramine in food is 100–800 mg/kg;[Citation24] however, it is not specific for seafood. Tyramine content was present in low concentrations of 42.4, 30.5, and 33.1 mg/kg after 16 days in samples packed with PA, PP, and PE packaging materials, respectively, but decreased subsequently in all the samples. These values were three to four times higher than those recorded for freshwater carp (<10 mg/kg) by Krizek et al.[Citation25] Significant differences (p < 0.05) were observed in agmatine content among samples stored in the three different packaging materials throughout the storage duration (). A significant difference (p < 0.05) was observed between the concentration of total biogenic amines in the PA-packed fillet and PP- and PE-packed fillets. These values reached the rejection point (>300 mg/kg) as suggested by European Community (EC)[Citation26] after 12 days in PP and PE and after 16 days in PA films ().
TPC
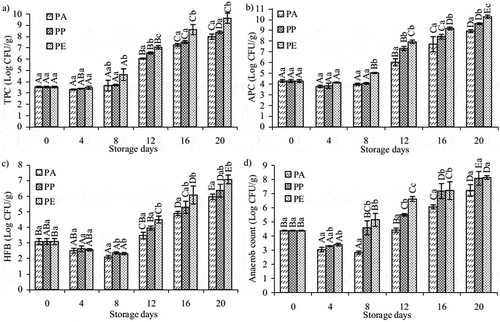
The changes in total aerobic microorganisms are shown in . The initial TPC of barramundi fillets was 3.52 log colony forming units (CFU)/g, considered as acceptable bacterial load and exceeded the acceptable limit of 6 log CFU/g[Citation18] on the 12th day of storage. The number of total aerobic microorganisms was higher in PE- and PP-packed fillets at initial value compared with PA packed, which had the lowest count. Lowest TPC was obtained from barramundi fillets kept in PA, indicating that the presence of CO2 in the pack and its low transmission rate during storage inhibited the growth of TPC, preventing spoilage extending the shelf life of the fish.
FIGURE 2 Microbial TPC (a) APC, (b) HFB, (c) and anaerobic bacteria, d: changes in barramundi fillets packed at different films (n = 3). Symbols: ![]()
Psychrotrophic Bacteria
The changes in the psychrotrophic bacteria count of the barramundi fillets packed in the packages (PA, PP, and PE) are shown in . In all of the cases, after 8 days of storage, the psychrotrophic bacteria in PE-packed fillets were significantly (p < 0.05) different from those in PP- and PA-packed (). The low permeability of PA limits the transmission of CO2 from the environment and maintains its level in the headspace. Significant reduction (p < 0.05) was observed in the present study in the growth rates of psychrotrophic in PA-packed barramundi fillets compared with those with high PP and PE. The number of psychrotrophic bacteria exceeded 7 log CFU/g (the limit of psychrotrophic bacteria)[Citation27,Citation28] for the PP- and PE-packed after 12 days; however, it took 16 days to reach this value for the PA-packed.
HFB
Changes in the number of HFB of barramundi stored in PA-, PP-, and PE-packed materials are shown in . HFB increased in all treatments with increasing storage time. Ozogul et al.[Citation29] reported that the histamine content of sardine stored at 4°C increased rapidly when the number of HFB in nylon-PE bags reached above 5 log CFU/g. There was a significant difference (p < 0.05) in the HFB between PA and other packed samples; however, no significant difference (p > 0.05) was seen between the PP- and PE-packed. Rodriguez-Jerez et al.[Citation30] and Pacheco-Aguilar et al.[Citation31] mentioned that most microorganisms described as histamine formers belong to the Enterobacteriaceae family, and similar to other Proteobacteria, they are gram-negative facultative anaerobes. Gram-negative bacteria have been identified as the main bacterial groups responsible for histamine formation in seafood; however, gram-positive bacteria have also been implicated.[Citation32] Some gram-positive bacteria, such as lactic acid bacteria, exhibit facultative anaerobic behavior and are able to produce histamine.[Citation33]
Anaerobic Bacteria
The number of anaerobic bacteria decreased during the first 4 days of storage (), probably because of the inhibitory effect of CO2 concentration at 8°C. All samples packed in PA had significantly (p < 0.05) lower anaerobic bacteria than PP and PE samples during the 12 days of storage. However, there was no significant difference (p > 0.05) between PP and PE during these days. From the 4th day, because of facultative anaerobic behavior,[Citation34] an exponential increase was observed in growth of anaerobic bacteria and subsequently reached the same level for TPC and HFB.
TVB-N
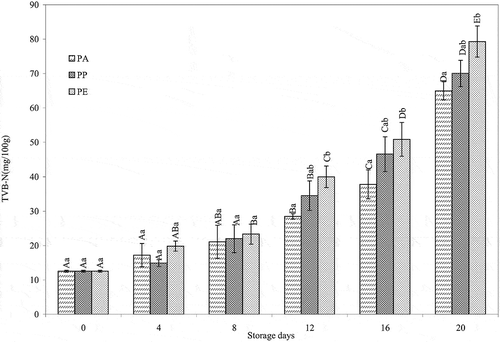
The concentration of TVB-N in freshly caught fish is typically between 5 and 20 mg N/100 g, whereas the acceptable limit for marine fish is 30–35 mg N/100 g of flesh.[Citation35,Citation36] No regulation has been specified for freshwater and brackish water fish. The concentrations of TVB-N present in the barramundi fillets packed in PA, PP, and PE are shown in . At the beginning of storage, the TVB-N value was 12.55 mg N/100 g of flesh of barramundi fillets. The release of total volatile bases increased to 28.47, 34.53, and 39.99 mg N/100 g in PA, PP, and PE films after 12 days of storage. Significant differences (p < 0.05) were found between the barramundi fillet stored in PA, PP, and PE after 8 days of storage. However, there were no significant differences (p > 0.05) between the TVB-N values at different stages of storage of the barramundi fillets in PP and PE. Because TVB-N is produced mainly by bacterial decomposition of fish flesh,[Citation35, Citation37–Citation39] the higher values of TPC of the sample in PE bags after 12 days of storage (7.03 log CFU/g) could account for the higher TVB-N values in packed samples.
FIGURE 3 TVB-N changes in barramundi fillets packed at different films (n = 3). Symbols: ![]()
Headspace Gas Composition in MAP
Gaseous environment within a modified atmosphere pack is not static, probably due to the permeability of packaging material, microbial growth, and respiration of the product or the absorption of gas by the food.[Citation40] The gas composition of each package changes significantly (p < 0.05) within the storage period. The changes in O2 and CO2 concentration in barramundi fillets packed in PA, PP, and PE films are shown in . A rapid increase in the concentration of O2 during the 4th day of storage might be due to high pressure of O2 outside the package and leakage into the package, after which, the O2 concentration gradually decreases until the 12th day and suddenly drops because of microbial growth and permeability of packaging material to 3.6, 1.2, and 1.1% in PA-, PP-, and PE-packed, respectively, at the end of the storage period.
FIGURE 4 Changes in the headspace composition of O2 and CO2 in barramundi fillets packed at different films (n = 3). Different lower case (for the same gas and different days) indicated significant difference (p < 0.05).
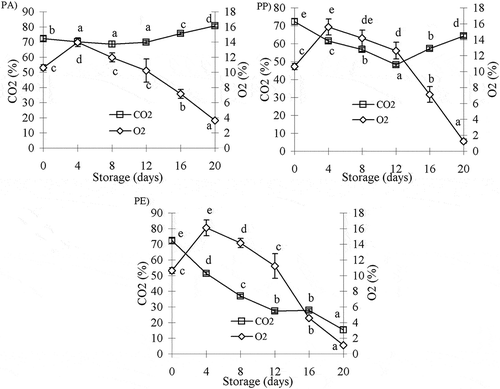
The changes in O2 and CO2 concentration were lower for barramundi fillets stored in PA due to low permeability compared with changes in PP- and PE-packed (). A significant increase in CO2 concentration was observed (p < 0.05) after 12 days in all packages, except in the PE packs wherein the CO2 concentration dropped significantly (p < 0.05) to 15.33% because of low permeability and a high population of microflora (9.61 log CFU/g) at the end of the storage period. This result is as anticipated because of the absorption of CO2 fillets,[Citation41] but this decline cannot be monitored just for microbial growth. During storage, the majority of microorganisms utilize available oxygen in the headspace, whereas some microflora, such as B. thermosphacta and lactic acid bacteria, produce CO2 as a metabolic product.[Citation41]
CONCLUSIONS
The results of this research have highlighted that among three different packaging materials (PA, PP, and PE) for the barramundi fillets, the concentration of total biogenic amines, individual important amines, that is, histamine, putrescine, and cadaverine, TVB-N, and microbial count in PA film was significantly lower than PP and PE films. Shelf life evaluation of the barramundi fillets stored at 8°C for 20 days showed that significant differences existed among the various packaging films on the basis of the TVB-N and microbiological parameters considered. The correlations found among microbial changes in barramundi fillets during storage and biogenic amines formation, highlighted that biogenic amines were principally increased when the count of HFB were increased over the time.
REFERENCES
- Piringer, O.G.; Baner, A.L. Plastic Packaging Materials for Food-Barrier Function, Mass Transport; Qual Assur Legis Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000.
- Lee, D.S.; Yam, K.L.; Piergiovanni, L. Food Packaging Science and Technology; Boca Raton, FL: CRC Press, 2008.
- Han, J.H.; Zhang, Y.; Buffo, R. Surface Chemistry of Food, Packaging, and Biopolymer Materials. Innovations in Food Packaging. Elsevier Science & Technology Books, 2005; 517.
- Hu, W.; Tanaka, S.; Uchino, T.; Hamanaka, D.; Hori, Y. Effects of Packaging Film and Storage Temperature on the Quality of Fresh Ginseng Packaged in Modified Atmosphere. Journal of the Faculty of Agriculture, Kyushu University 2004, 49, 139–147.
- Exama, A.; Arul, J.; Lencki, R.W.; Lee, L.Z.; Toupin, C. Suitability of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables. Journal of Food Science 1993, 58, 1365–1370.
- Zhang, M.; Xiao, G.; Peng, J.; Salokhe, V.M. Effects of Modified Atmosphere Package on Preservation of Strawberries. International Agrophysics 2003, 17, 143–148.
- Workneh, T.S.; Osthoff, G.; Steyn, M.S. Effect of Modified Atmosphere Packaging on Microbiological, Physiological, and Chemical Qualities of Stored Carrot. Journal of Food Technology in Africa 2004, 6, 138–143.
- Kim, J.G.; Luo, Y.; Gross, K.C. Effect of Package Film on the Quality of Fresh-Cut Salad Savoy. Postharvest Biology and Technology 2004, 32, 99–107.
- Ming, X.; Weber, G.H.; Ayres, J.W.; Sadine, W.E. (1997) Bacteriocins Applied to Food Packaging Materials to Inhibit Listeria Monocytogenes on Meats. Journal of Food Science 1997, 62, 413–415.
- Moore, M.E.; Han, I.Y.; Acton, J.C.; Ogale, A.A.; Barmore, C.R.; Dawson, P.L. Effects of Antioxidants in Polyethylene Film on Fresh Beef Color. Journal of Food Science 2003, 68, 99–104.
- Atrea, I.; Papavergou, A.; Amvrosiadis, I.; Savvaidis, I.N. Combined Effect of Vacuum-Packaging and Oregano Essential Oil on the Shelf-Life of Mediterranean Octopus (Octopus Vulgaris) from the Aegean Sea Stored at 4°C. Food Microbiology 2009, 26, 166–172.
- Fernández, K.; Aspe, E.; Roeckel, M. Shelf-Life Extension on Fillets of Atlantic Salmon (Salmo Salar) Using Natural Additives, Superchilling, and Modified Atmosphere Packaging. Food Control 2009, 20, 1036–1042.
- Bakar, J.; Yassoralipour, A.; Bakar, F.A.; Rahman, R.A. Biogenic Amine Changes in Barramundi (Lates Calcarifer) Slices Stored at 0°C and 4°C. Food Chemistry 2010, 119, 467–470.
- Yassoralipour, A.; Bakar, J.; Rahman, R.A.; Abu Bakar, F. Biogenic Amines Formation in Barramundi (Lates Calcarifer) Fillets at 8°C Kept in Modified Atmosphere Packaging with Varied CO2 Concentration. LWT–Food Science and Technology 2012, 48, 142–146.
- AOAC. Official Methods of Analysis of AOAC International, Horwitz, W.; Ed.; Association of Official Analytical Chemists: Gaithersburg, MD, 2000.
- Niven, C.F.; Jeffrey, M.B.; Corlett, D.A. Differential Plating Medium for Quantitative Detection of Histamine-Producing Bacteria. Applied Environmental Microbiology 1981, 41, 321–322.
- Perez-Alonso, F.; Aubourg, S.P.; Rodríguez, Ó.; Barros-Velazquez, J. Shelf Life Extension of Atlantic Pomfret (Brama Brama) Fillets by Packaging Under a Vacuum-Skin System. European Food Research and Technology 2004, 218, 313–317.
- Ravi Sankar, C.N.; Lalitha, K.V.; Jose, L.; Manju, S.; Gopal, T.K.S. Effect of Packaging Atmosphere on the Microbial Attributes of Pearlspot (Etroplus Suratensis Bloch) Stored at 0–2°C. Food Microbiology 2008, 25, 518–528.
- Goulas, A.E.; Kontominas, M.G. Effect of Salting and Smoking-Method on the Keeping Quality of Chub Mackerel (Scomber Japonicus): Biochemical and Sensory Attributes. Food Chemistry 2005, 93, 511–520.
- FDA. Scombrotoxin (Histamine) Formation, Fish and Fishery Products Hazards and Controls Guide, 3rd Ed; Silver Spring, MD: Food and Drug Administration, Office of Seafood 2001; 73–93.
- Antoine, F.R.; Wei, C.I.; Otwell, W.S.; Sims, C.A.; Littell, R.C.; Hogle, A.D., Marshall, M.R. Analysis of Biogenic Amines and Their Precursor Free Amino Acids in Mahi Mahi (Coryphaena Hippurus). Journal of Food Biochemistry 2002, 26, 131–152.
- Koutsoumanis, K.; Lampropoulou, K.; Nychas, G.J. Biogenic Amines and Sensory Changes Associated with the Microbial Flora of Mediterranean Gilt-Head Sea Bream (Sparus Aurata) Stored Aerobically at 0, 8, and 15°C. Journal of Food Protection 1999, 62, 398–402.
- Mietz, J.L.; Karmas, E. Polyamine and Histamine Content of Rockfish, Salmon, Lobster, and Shrimp As An Indicator of Decomposition. Journal Association of Official Analytical Chemists 1978, 61, 139–145.
- Brink, B.; Damink, C.; Joosten, H.M.; Huis, V.J.H. Occurrence and Formation of Biologically Active Amines in Foods. International Journal of Food Microbiology 1990, 11, 73–84.
- Křı́žek, M.; Vácha, F.; Vorlová, L.; Lukášová, J.; Cupáková, Š. Biogenic Amines in Vacuum-Packed and Non-Vacuum-Packed Flesh of Carp (Cyprinus Carpio) Stored at Different Temperatures. Food Chemistry 2004, 88, 185–191.
- European Community (EC) CD 91/493/EEC of 22 J. Laying Down the Health Conditions for the Production and the Placing on the Market of Fishery Products. Official Journal of the European Communities 1991, 268, 15–32.
- Arashisar, S.; Hisar, O.; Kaya, M.; Yanik, T. Effects of Modified Atmosphere and Vacuum Packaging on Microbiological and Chemical Properties of Rainbow Trout (Oncorynchus Mykiss) Fillets. International Journal of Food Microbiology 2004, 97, 209–214.
- ICMSF. International Commission on Microbiological Specifications for Foods; Kluwer Academic/Plenum Publishers: Springer, London, 2002.
- Özogul, F.; Polat, A.; Özogul, Y. The Effects of Modified Atmosphere Packaging and Vacuum Packaging on Chemical, Sensory, and Microbiological Changes of Sardines (Sardina Pilchardus). Food Chemistry 2004, 85, 49–57.
- Rodriguez-Jerez, J.J.; Lopez-Sabater, E.I.; Roig-Sagues, A.X.; Mora-Ventura, M.T. Histamine, Cadaverine, and Putrescine Forming Bacteria from Ripened Spanish Semipreserved Anchovies. Journal of Food Science 1994, 59, 998–1001.
- Pacheco-Aguilar, R.; Lugo-Sanchez, M.E.; Villegas-Ozuna, R.E.; Robles-Burgueno, R. Histamine Quantification in Monterey Sardine Muscle and Canned Products from Northwestern Mexico. Journal of Food Composition Analysis 1998, 11, 188–195.
- Kim, S.H.; Price, R.J.; Morrissey, M.T.; Field, K.G.; Wei, C.I.; An, H. Occurrence of Histamine-Forming Bacteria in Albacore and Histamine Accumulation in Muscle at Ambient Temperature. Journal of Food Science 2002, 67, 1515–1521.
- Landete, J.M.; de las Rivas, B.; Marcobal, A.; Munoz, R. Molecular Methods for the Detection of Biogenic Amine-Producing Bacteria on Foods. International Journal of Food Microbiology 2007, 117, 258–269.
- Debevere, J.; Boskou, G. Effect of Modified Atmosphere Packaging on the TVB/TMA-Producing Microflora of Cod Fillets. International Journal Food Mcrobiology 1996, 31, 221–229.
- Huss, H.H. Assurance of Seafood Quality. FAO Fisheries Technical Paper 1994, 334, 32–35.
- Connell, J.J. Methods of Assessing and Selecting for Quality. Control of Fish Quality; Springer: Berlin, 1990.
- Tejada, M.; Huidobro, A. Quality of Farmed Gilthead Seabream (Sparus Aurata) During Ice Storage Related to the Slaughter Method and Gutting. Europeon Food Research Technology 2002, 215, 1–7.
- Koutsoumanis, K.; Nychas, G.J. Application of a Systematic Experimental Procedure to Develop a Microbial Model for Rapid Fish Shelf Life Predictions. International Journal of Food Microbiology 2000, 60, 171–184.
- Kyrana, V.R.; Lougovois, V.P. Sensory, Chemical, and Microbiological Assessment of Farm-Raised European Sea Bass Dicentrarchus Labrax Stored in Melting Ice. International Journal of Food Science and Technology 2002, 37, 319–328.
- Esmer, O.K.; Irkin, R.; Degirmencioglu, N.; Degirmencioglu, A. The Effects of Modified Atmosphere Gas Composition on Microbiological Criteria, Color, and Oxidation Values of Minced Beef Meat. Meat Science 2011, 88, 221–226.
- Koutsoumanis, K.; Stamatiou, A.P.; Drosinos, E.H.; Nychas, G.J.E. Control of Spoilage Microorganisms in Minced Pork by a Self-Developed Modified Atmosphere Induced by the Respiratory Activity of Meat Microflora. Food Microbiology 2008, 25, 915–921.
