Abstract
Mulberry pestil and köme are Turkish traditional foods made from herle. Browning leads to quality losses in the herle, and its products. In this study, the effect of thermal treatment on the hydroxymethylfurfural formation and color values in the herle was evaluated. The herle samples were kept at different temperatures (60–110°C) and times (2–6 h). The hydroxymethylfurfural formation was found to increase with increasing time and temperature. The legal limit for hydroxymethylfurfural in the pestil (50 mg/kg) was exceeded at temperatures above 90°C. The lightness (L* value) of the sample decreased with the thermal treatment, indicating browning.
INTRODUCTION
Mulberry pestil and köme, traditional Turkish foods, have become commercial products within the last 10 years. Protected Geographical Indication (PGI) from Turkish Patent Institute was received for Gümüşhane mulberry pestil and köme (TPE, 2004).[Citation1] Herle made from mulberry, wheat flour, honey, sugars, and, milk is the main constituent of the pestil and köme. Mulberry wort is mixed with sugar, honey, milk, and water, and then the mixture is boiled. A paste of wheat flour and mulberry wort is added to the boiled mixture. The mixture is boiled in a vessel for 20–120 min, and mild gel occurs. This gel is called as herle. Hydroxymethylfurfurol (HMF) accumulation occurs during boiling treatment.[Citation2,Citation3]
Maillard and caramelization reactions are called as non-enzymatic browning reaction. Maillard reaction may take place during food processing or storage, particularly at high temperatures, in foods containing reducing sugars and amino acids. Caramelization reaction occurs when foods containing sugars are heated at high temperatures. HMF is an intermediate product of non-enzymatic browning reactions. HMF formation can lead to sensorial and nutritional losses in the food products.[Citation4] HMF, a quality indicator, can be used to evaluate the effects of processing and storage conditions on the quality of food products.[Citation4,Citation5] HMF and its derivatives may show toxic properties such as cytotoxic, genotoxic, nephrotoxic, mutagenic, and cancerogenic.[Citation6] Therefore, HMF accumulation in the foods should be monitored and kept down. Codex Alimentarius established a maximum level for HMF in the foods such as honey, fruit juice, and jam.
There were no standard process conditions for boiling treatment in the herle production. The boiling temperature and time vary with respect to factories. The HMF formation in the herle should be monitored, and the standard process conditions should be determined. In the literature, there is no available information on the HMF formation in the herle. The aim of this study was to investigate HMF formation in the herle treated at different temperatures and times. The effect of thermal treatment on the HMF formation, and the kinetic model for HMF formation in the herle were determined.
MATERIALS AND METHODS
Materials
The herle sample was provided from a factory in Gümüşhane (Kral Pestil and Köme). The herle containing invert sugar (30 g/100 g), honey (15 g/100 g), wheat flour (20 g/100 g), mulberry molasses (15 g/100 g), milk (3 g/100 g), and water (17 g/100 g) was prepared in a vacuum vessel at 80°C. Its HMF content was 1.25 mg/kg. The herle sample was taken into jar (100 g), and stored at 4°C.
Thermal Treatment
The sample jars were kept at different temperatures, ranging from 60–110°C. Heat treatment was applied in an oven (Daihan, WiseVen WOF, South Korea), for different time periods, ranging from 2–6 h. Four jars were used for each treatment. After the heat treatment, the jars were cooled to 4°C.
Proximate Analysis
Total soluble solids, moisture, protein, and ash contents were carried out in accordance with the methods of Turkish Standard (TS 3792).[Citation7] Total soluble solids were determined by an Abbe refractometer (ABBE 2WAJ, USA).
Physicochemical Analysis
The pH value of the samples was measured with a pH meter (Hanna HI 2221, USA).[Citation8] Titration acidity of the samples was determined by the alkali titration method.[Citation9] Color measurement was done with a chromometer (Minolta CR-300, Japan). L*, a*, and b* values were determined.[Citation10]
Sugar Analysis
Sugar analysis was carried out in accordance with the Turkish Standard (TS) method.[Citation11] Five grams of the homogenized sample was weighed, and dissolved in water (40 mL). The sample solution was taken into a flask (100 mL) containing methanol (25 mL). The volume of the flask was fulfilled with water. The sample solution was filtered via a filter (PTFE, 25 mm, 0.45 µm), and then it was injected to high performance liquid chromatography-refractive index (HPLC-RI) system (Schimadzu 20 A, Japan) with an amino column (ShodexAsahipak NH2P-50 4E, 250 mm × 4.6 mm, 5–7 μm). The mobile phase was acetonitrile and water (80:20), and flow rate was 1.3 mL.
HMF Analysis
HMF analysis was carried out in accordance with the Harmonized Methods of the International of Honey Commission with slight modifications.[Citation12] HPLC method was selected instead of White method. The reason of this choice is better precision of HPLC method for the determination of low concentrations.[Citation13]Five gram of the homogenized sample was weighed, and dissolved in water (25 mL). After Carrez I and II solutions were added, the sample solution was filtered via 0.45 μm filter. The sample solution was injected to high-performance liquid chromatography-diode array detector (HPLC-DAD) system (Agilent 1100, USA) with C18 column (Nucleosil, 250 mm × 4.6 mm, 5 μm). The isocratic mobile phase was water and methanol (90:10). The flow rate was 1 mL and the wavelength was 285 nm.
Statistical Analysis
One-way and two-way analysis of variance (ANOVA) analysis, least significant difference (LSD) test and Pearson correlation analysis were performed. All analyses were done in triplicate
RESULTS
Characterization of the Herle
shows proximate composition and physicochemical properties of the herle. The herle sample had high moisture content (60 g/100 g). The herle sample was rich in reducing sugars (>20 g/100 g). Its protein content was low (1.6 g/100 g). It had acidic pH value (pH ~ 4.00).
TABLE 1 Characterization of the herle sample
HMF Accumulation
After each treatment, the HMF contents of the samples were determined. The HMF contents of the herle samples are presented in . Two-way ANOVA results showed that treatments of both times and temperatures had significant effect on the HMF content of the herle samples (p < 0.001). An interaction term of time and temperature was also found to have significant effect on the HMF contents (p < 0.001). The HMF contents of the herle samples increased with increasing treatment time at each treatment temperature. An increase in the treatment temperature resulted in a rise in the HMF contents at each treatment time as well.
TABLE 2 HMF contents of the herle samples
The köme and pestil were produced from the studied herle and the samples were stored at the room temperature for 3 months. The HMF contents of the pestil and köme samples are presented in . Initial HMF contents of the köme and pestil samples were 22.45 and 24.56 mg/kg, respectively. The HMF contents of the samples increased to 25.27 and 27.26 mg/kg, respectively, at the end of the storage. One-way ANOVA results indicated that the storage time had significant effect on the HMF contents of the samples (p < 0.001). No significant difference was found between the 2nd and 3rd month of the pestil samples, and between the 1st and 2nd month of the köme samples.
TABLE 3 HMF contents of pestil and köme samples
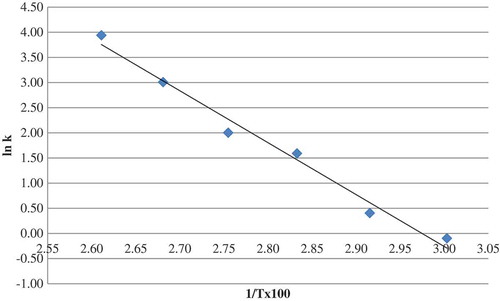
The HMF formation in the herle samples showed first order kinetic model at each temperature (data not shown). The reaction rate constant at each treatment temperature was calculated. As seen from , the reaction rate constant of the HMF accumulation in the herle samples increased with increasing temperature. Arrhenius equation was used to determine temperature dependence of the HMF formation and the activation energy of the reaction was calculated using Arrhenius plot (). The activation energy (AE) of the HMF accumulation in the herle samples was 20.5 kcal/mole. Temperature quotient (Q10) was determined for the temperature ranges of 60–70, 70–80, 80–90, 90–100, and 100–110°C. Q10 values were ranged from 1.009–1.025. These values indicated that the effect of the temperature on the HMF content was similar among the studied temperature ranges.
TABLE 4 Rate constants of the herle samples
k = rate constant; ko: pre-exponential factor; Ea: AE (kcal/mole); R: gas constant (1.987 kcal/mole K); T: temperature (Kelvin).
Color Values
The color values of the HMF samples are presented in . Two-way ANOVA results revealed that treatment time and temperature had significant effect on the color values of the herle samples (p < 0.001). An interaction term of time and temperature showed significant effect on the color values as well (p < 0.001). The L* values (lightness) decreased with increasing treatment time and temperature, whereas the a* (redness) and b* (yellowness) values increased.
TABLE 5 Color values of the herle samples
Pearson correlation analysis was applied to evaluate the correlation between the HMF formation and color values. A negative significant correlation was found between the HMF and L* values (r: –0.890, p = 0.01) where a positive significant correlation was found between the HMF and both a* value (r: 0.958, p = 0.01) and b* value (r: 0.619, p = 0.01). These results indicated that a reduction in the L* value and an increase in the a* and b* values can be related to a rise in the HMF value.
DISCUSSION
Analysis results showed that the herle sample had the highest content of reducing sugars and the lowest level of protein. These results can be attributed to the formulation of the herle. The composition of the herle makes it susceptible to browning reactions. Maillard reaction may be the main browning reaction in the herle. The Maillard reaction is favored in foods at temperatures above 50°C and at a pH 4–7.[Citation14] Caramelization reaction occurs when sugars are heated at high temperatures . In this study, the studied temperatures (60–110°C) and the pH value of the herle may favor the Maillard reaction.
In this study, the effect of treatment time and temperature on the HMF formation was evaluated. Temperature is one of the factors affecting HMF formation. The HMF content of the herle sample showed increasing trend with increasing temperature. The HMF value of the herle increased with increasing treatment time as well. Our findings were comparable with the literature. Turhan et al.[Citation3] studied the effect of heat treatment on the HMF formation in honey, and they found that the HMF formation increased with increasing treatment time and temperature. Storage temperature and time were reported to have significant effect on the HMF formation in the molasses samples made from grape, mulberry, and carob.[Citation15,Citation16] The maximum level for HMF in the pestil was 50 mg/kg in the Turkish Standard.[Citation17] When the herle was heated at temperatures above 90°C, the legal limit for the pestil was exceeded. Therefore, the boiling of the herle should be done at temperatures below 90°C.
HMF formation in the herle sample showed first order kinetic. In the literature, the reaction degree for the HMF formation was reported to be ranged from zero to second order.[Citation18] The first order kinetic was reported for cookies,[Citation19] and honey,[Citation3] whereas zero order kinetic was for Zile molasses.[Citation15] The herle sample showed higher AE value than cookies (2.2 kcal/mole), and lower AE value than Zile molasses (30.9 kcal/mole). These differences may be explained by the composition and water activity of the products. The reaction constants increased with increasing temperature. These results indicated higher HMF formation at high temperatures. Our results were comparable with the literature. The rate constants of the jam samples exhibited increasing trend with increasing temperature.[Citation20]
Color is an important quality parameter of the foods, determining consumer preference. Color is also an indicator for food deterioration. Color was expressed with L* (lightness), a* (redness), and b* (yellowness) values. Treatment time and temperature were found to have negative effect on the L* value of the herle. A reduction in the L* value indicated browning reaction. Similar results were reported for Zile molasses. The L* value of the Zilemolasses made from grape juice was found to decrease with increasing storage temperature and storage time.[Citation15]
Color changes and HMF content in numerous food systems (fruit juices, jam, honey, and baked foods, etc.) have been determined.[Citation4,Citation21] However, few studies focused on the relationship between color changes and HMF content. In our study, Pearson correlation analysis showed that there was a significant correlation between HMF accumulation and color values. The lightness (L* value) of the herle significantly decreased with the HMF accumulation, whereas the redness (a* value) significantly increased with the HMF accumulation. These results revealed that HMF formation may be proposed to develop a model explaining color changes during browning reactions. More studies are needed.
CONCLUSION
The effect of thermal treatment on the HMF formation in the herle was evaluated. Our findings revealed that the boiling temperature of the herle should be below 90°C. The HMF accumulation in the herle showed first order kinetic. These results may help to develop standard process conditions for herle, which is the main constituent in the mulberry pestil and köme production.
FUNDING
This study was supported from Gumushane University Scientific Council (Project number: 2012.02.1716.1).
Additional information
Funding
REFERENCES
- TPE. Turkish Patent Institutue, 2004.
- Tetik, N.; Turhan, I.; Karhan, M.; Öziyici, H.R. Characterization and 5-Hydroxymethylfurfural Concentration in Carob Pekmez. Gida 2010, 35, 417–422.
- Turhan, I.; Tetik, N.; Karhan, M.; Gürel, F.; Tavukçuoğlu, H.R. Quality of Honeys Influenced by Thermal Treatment. LWT–Food Science and Technology 2008, 41, 1396–1399.
- Kuş, S.; Göğüş, F.; Eren, S. Hidroxymethyl Furfural Content of Concentrated Food Products. International Journal of Food Properties 2005, 8, 367–375.
- Khalafi, R.; Goli, S.A.H.; Isfahani, M.B. Characterization and Classification of Several Monofloral Iranian Honeys Based on Physicochemical Properties and Antioxidant Activity. International Journal of Food Properties. DOI:10.1080/10942912.2015.1055360
- Capuano, E.; Fogliano, C. Acrylamide and 5-Hydroxymethylfurfural (HMF): A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. LWT–Food Science and Technology 2011, 44, 793–810.
- TS 3792. Pekmez (Traditional Turkish Grape Juice Concentrate). Turkish Standard Institutue, 2008.
- TS 1728 ISO 1842. Fruit and Vegetable Products—Determination of pH. Turkish Standard Institutue, 2001.
- TS 1125 ISO 750. Fruit and Vegetable Products—Determination of Titratable Acidity. Turkish Standard Institutue, 2001.
- Quintas, M.; Brandao, T.; Silva, C. Modelling Colour Changes During Caramelisation Reaction. Journal of Food Engineering 2007, 83, 483–491.
- TS 13359. Determination of Fructose, Glucose, Saccharose, Turanose, and Maltose of Honey by High Performance Liquid Chromatography. Turkish Standard Institutue, 2008.
- Harmonised Methods of the International Honey Commission, 2001.
- Truzzi, C.; Illuminati, S.; Annibaldi, A.; Finale, C.; Rossetti, M.; Giuseppe, S. Determination of Very Low Levels of 5-(hydroxymethyl)-2-furaldehyde (HMF) in Natural Honey: Comparison Between the HPLC Technique and the Spectrophotometric White Method. Journal of Food Science 2012, 77, C784–C790.
- Ramirez-Jimenez, A.; Garcia-Villonova, B.; Guerra-Hernandez, E. Effect of Toasting Time on the Browning of Sliced Bread. Journal of the Food Science and Agriculture 2001, 81, 513–518.
- Tosun, I. Color Changes and HMF Accumulation in Zile Pekmezi During Storage. Grasas y Aceites 2004, 55, 259–263.
- Toker, O.S.; Doğan, M.; Ersöz, N.B.; Yilmaz, M.T. Optimazition of Content of 5-Hydroxmethylfurfural (HMF) Formed in Some Molasses Types: HPLC-DAD Analysis to Determine Effect of Different Storage Time and Temperature Levels. Industrial Crops and Products 2013, 50, 137–144.
- TS 12677. Spreaded Dried Mulberry. Turkish Standard Institute, 2000.
- Bozkurt, H.; Göğüş, F.; Eren, S. Kinetic Modelling of the Maillard Browning Reaction in Pekmez (Grape Molasses). Turkish Journal of Engineering and Environmental Science 1998, 22, 455–460.
- Ameur, L.A.; Trystram, G.; Birlouez-Aragon, I. Accumulation of 5-Hydroxmethylfurfural in Cookies During the Backing Process: Validation of Extraction Method. Food Chemistry 2006, 98, 790–796.
- Aslonova, D.; Bakkalbaşı, E.; Artık, N. Effect of Storage on 5-Hydroxymethylfurfural (HMF) Formation and Color Change in Jam. International Journal of Food Properties 2010, 23, 904–912.
- Rada-Mendoza, M.; Sanz, M.L.; Olano, A.; Villamiel, M. Formation of Hydroxymethyl Furfural and Furosine During the Storage of Jams and Fruit-Based Infant Foods. Food Chemistry 2004, 85, 605–609.