ABSTRACT
Resistant starch has received much attention for both its potential health benefits and functional properties. The present study revealed that the culinary banana can be a potential source for production of resistant starch type III. The results showed that resistant starch yield can be increased using debranching enzyme (Pullulanase EC 3.2.1.41) and hydrothermal process. The three repeated autoclaving and cooling (hydrothermal) cycles followed by storage at –20ºC, increased the resistant starch yield from 12.3 to 26.4%. The use of debranching enzyme and subsequent retrogradation at –20ºC increased the resistant starch yield up to 31.2%. The modified resistant starch when compared to culinary banana starch evinced better stability. The modified resistant starch was analyzed using scanning electron microscopy, Fourier transform infrared, x-ray diffraction, and thermogravimetric diffraction analysis to determine the structural changes which proved that the modification occurred substantially.
Introduction
Starch is the storage polysaccharides of green plants and is a major dietary component in all human populations.[Citation1] It is composed of a number of monosaccharide or sugar (glucose) linked together with α-D-(1-4) and/or α-D-(1-6) linkages.[Citation2] Starch is deposited in the fruit in the form of granules, partially crystalline, whose morphology, chemical composition, and super molecular structure are characteristics of each particular plant species. Starch owes much of its functionality to two major high-molecular-weight carbohydrate components viz., amylose and amylopectin. Amylose is essentially linear polymer in which glucose residues are α-D-(1-4) linked typically constituting 15 to 20% of starch. Amylopectin, the major component of starch is a larger branched molecule with α-D-(1-4) and α-D-(1-6) linkages. This biopolymer constitutes an excellent raw material to modify food texture and consistency.[Citation3] The amount of starch is not only important for the texture of a given food product and its type is equally critical.[Citation4]
Starch, on the basis of its digestibility, has been classified into three groups viz., readily digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS).[Citation5] RDS is the starch fraction that causes an increase in blood glucose level immediately after ingestion, whereas SDS is the starch fraction that is digested completely in the small intestine at a lower rate as compared to RDS. RS is the portion of starch and/or starch hydrolysis products that escape digestion in the small intestine and enters the colon for fermentation.[Citation2] Extensive studies have shown that RS has physiological functions similar to those of dietary fiber.[Citation6] RS appears to be highly resistant to mammalian enzyme and may be classified as a component of fiber on the basis of the definitions of dietary fiber given by the American Association of Cereal Chemist (AACC).[Citation7]
The diversity of the modern food industry and the enormous variety of food products being produced require starches that can tolerate a wide range of processing techniques and preparation conditions.[Citation8] These demands are met by modifying native starches with chemical, physical, and enzymatic methods which may lead to the formation of indigestible residues. The availability of such starches, therefore, deserves consideration. Four forms of RS are distinguished: RS type I is defined as physically inaccessible starch for instance in grains; type II is granular starch in raw potato and bananas; type III is retrograded starch, arising after hydrothermal treatment of starch; and type IV is considered to be a chemically modified starch.[Citation9] Among these four types, RS type III is particularly interesting because it preserved its nutritional characteristics when added as an ingredient in cooked food. RS type III is produced by gelatinization, which is a disruption of granular structure by heating starch with excess water, and then retrogradation occurs.
The generation of RS after hydrothermal treatment is mainly due to increase interactions between starch polymers. The degree of formation of RS in food depends not only on the type of incorporated starch and the processing conditions but is also influenced by the duration and conditions of storage.[Citation10] As stated by Fuentes-Zaragoza et al.[Citation11] unripe banana is considered as RS-richest non-processed food. The content of RS in unripe banana ranges between 47–57% and because of this fact several studies have suggested that consumption of unripe banana results in beneficial effects to human health. Rodríguez-Ambriz et al.[Citation12] found a total starch content of 73.4% and RS content of 17.5% for unripe banana, so it may be considered an alternative source of RS and dietary fiber and when the unripe fruit is cooked, RS is rendered digestible.
The global trends in rising levels of obesity, diabetes, and cardiovascular diseases have renewed research interest in the dietary intake of fat, protein, and carbohydrates to maintain good health. The World Health Organization (WHO) and Food and Agricultural Organization (FAO) of the United Nations stated that globally overweight populations are a bigger problem than under nourishment and recommended people in industrialized countries to base diet on low glycemic index foods to prevent most common disease of affluence.[Citation13] One of the most important objectives in the dietary treatment of diabetes patients is to maintain their blood glucose level, avoid obesity, and achieve optimal lipids level. Food containing RS generally give a low glycemic response because RS is not digested in the small intestine and instead it passes into the large intestine where it is fermented.[Citation2]
Many tropical countries have plant species which can be used as a good source of starch and unfortunately some of them have not been exploited and one such plant species is kachkal (Musa ABB), the only culinary banana found in the entire Assam and North-East India.[Citation14] Starch, being the principal component of culinary banana, can be considered as a resource for production of modern forms of consumption like processed snacks and precooked products. Therefore, in the present study an effort has been made to isolate starch from culinary banana and modifying it into RS in order to find an alternative route to increase value addition of the culinary banana by providing RS of high quality food properties necessary for a well-balanced diet. It is expected that the utilization of RS from culinary banana should not only expand the market but also provide a solution to those who want to consume food with low glycemic index value.
Material and methods
Unripe culinary bananas at optimum harvesting stage (50 days after emergence of flower) were harvested and collected from Tezpur University campus, Tezpur, Assam. Prior to the starch isolation, the samples were thoroughly cleaned under running tap water followed by rinsing with distilled water and pat dried using a clean cloth. All the chemicals required for present study were of high purity analytical grade supplied by Sigma-Aldrich, USA, HiMedia, India and Merck, India.
Starch isolation and chemical analysis
The starch was isolated following the method described by Bello-Perez et al.[Citation15] and the chemical analyses viz., moisture content, ash, crude fiber, fat, and protein content were determined according to the Association of Official Analytical Chemists (AOAC) methods.[Citation16] The pH of starch dispersion (8% w/v) was measured by using a pH meter. Total amylose content was determined as per the method described by McGrance et al.[Citation17] The dried defatted starch (20 mg) was dissolved in 8 mL 90% dimethylsulfoxide in screw cap vials. The suspension was homogenized vigorously for 20 min followed by heating at 85°C for 15 min. The mixture was cooled and volume was made to 25 mL with distilled water and 1 mL of diluted solution was further mixed with 40 mL distilled water to which 5 mL of potassium iodide solution (0.0025 M iodine and 0.0065 M KI) was added and finally the volume was adjusted to 50 mL. The absorbance was taken at 600 nm using spectrophotometer after allowing the samples to stand for 15 min in dark at room temperature. The amylose content was calculated from standard curve prepared using pure potato amylose type III.
The RS content in the culinary banana starch was determined by an enzymatic method.[Citation16] The samples were incubated with pancreatic α-amylase and amyloglucosidase (AMG) for 16 h at 37°C. During this time the non-RS was solubilized and hydrolyzed to glucose by the combined action of the two enzymes. The reaction was terminated by the addition of an equal volume of ethanol and the RS was recovered as a pellet on centrifugation. This was then washed twice with ethanol (50 % v/v) and centrifuged. The RS in the pellet was dissolved in 2 M KOH by vigorously stirring on an ice-water bath. This solution was neutralized with acetate buffer and the starch was quantitatively hydrolyzed to glucose with AMG. The glucose was quantified with glucose oxidase/peroxidase reagent (GOPOD), which gave a measure of the RS content of the sample.
Functional properties
Water holding capacity, starch swelling power, and solubility
Water holding capacity was determined as described by Hallgren.[Citation18] The percentage solubility and swelling power were calculated using the Eqs. (1) and (2):
Freeze-thaw stability and paste clarity
Methods described by Jeong and Lim[Citation19] and Bello-Perez et al.[Citation15] were used for freeze-thaw stability and paste clarity properties, respectively.
Pasting properties
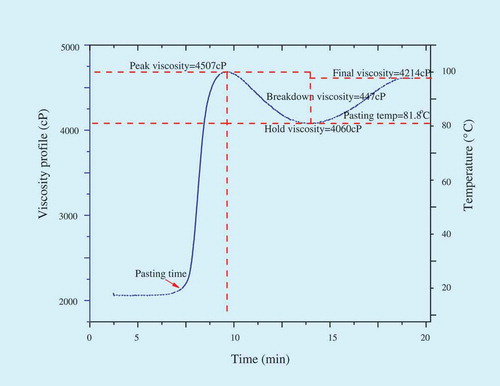
Pasting properties of starches were evaluated in rapid visco-analyzer (RVA; -4, Newport Scientific, Sydney, Australia). An 8% slurry was given a programmed heating and cooling cycle set for 23 min, where the sample was held at 30°C for 1 min, heated to 95°C for 7.5 min, further held at 95°C for 5 min before cooling to 50°C within 7.5 min, and holding at 50°C for 2 min. The speed was 960 rpm for the first 10 s and 160 rpm for the remaining period of the experiment. Peak viscosity (PV), hold viscosity (HV), final viscosity (FV), breakdown viscosity (BV), setback viscosity (SV), and pasting temperature (PT) of starches were measured and all measurements were repeated three times.
Structural analysis of starch
X-ray diffraction (XRD)
The XRD was obtained from a D/max 2500 X-ray diffractometer (Rigaku Miniflex, Japan) at room temperature using CuKα radiation (λ = 0.15418 nm). A conventional x-ray tube was set to 30 kv acceleration potential and 15 mA current. Data were collected from 2θ of 5 to 50º (θ being the angle of diffraction) with a scan speed of 8º at 2θ/min. The starch sample was dried at 50ºC to constant moisture (10%) in a vacuum oven and 50 mg sample was added into the slide for packing prior to x-ray scanning.
Fourier transforms infrared (FT-IR) spectra
An infrared spectrum of starch was measured using KBr disk method. The dry sample was blended with KBr in a ratio starch/KBr 1:4. The blend was pressed to obtain a pellet and introduced in the spectrometer (Nicolet Instruments 410 FT-IR equipped with KBr optics and a DTGS detector, Thermo Scientific, USA). Each spectrum was analyzed in the range of resolution from 400–4000 cm–1 with a resolution of 4 cm–1 and altogether 64 scans were collected.
Morphological analysis by scanning electron microscopy (SEM)
Starch granules were observed under a scanning electron microscope (JEOL JSM 6390 LV, USA) operating at an accelerating voltage of 15 kv. A small portion of starch sample was assembled on metallic stubs with double sided tape and coated with a thin layer of gold. Magnification was taken at 500× and both shape and size of the starch granules were observed.
Thermal characteristics by thermogravimetric analysis (TGA)
Thermal degradation behaviour of starch was evaluated using TGA (Shimadzu, TGA-50, North America). The thermal stability of each sample was conducted at 25 to 600°C with constant heating rate of 10°C/min under nitrogen atmosphere.
Development of type III RS from culinary banana starch
Autoclaving and cooling method
The RS samples were prepared following the method of Berry[Citation20] with slight modification by suspending 10 and 20% (w/v) of culinary banana starch in 1000 mL of water and autoclaving using 15 psi pressure at 120°C for 30 min followed by cooling at 4°C for 24 h. After three cycles of autoclaving and cooling, the samples were freeze-dried (LDF-5512, Daihan Lab Tech Co., Ltd., South Korea) and ground into fine particles by using mechanical grinder (Fritsch, Germany) and passed through 100 mesh screen sieve and stored until further analysis. The effect of storage temperature on RS content was studied at two different storage temperatures of 4°C and –20°C for 24 h.
Enzyme debranching method
RS was developed following the method of RS production by enzyme debranching[Citation21] by suspending culinary banana starch (20%) in sodium acetate buffer (pH 5.0) and slurry was given repeated thermal and enzymatic treatment. The samples were subjected to thermal treatment up to 100°C for 15 min and further diluted using 10% sodium acetate buffer and mixed thoroughly. For the enzyme debranching process, pullulanase (EC 3.2.1.41) from Klebsiella pneumoniae (5%) was added to the homogenized suspension of starch gel and the hydrolysis reaction was maintained for 24 h at 60°C. The thermal treatment at 100°C for 15 min was repeated in between enzyme debranching process and the developed RS was freeze dried, ground, and stored for further analysis.
Chemical analysis of RS
Moisture content, ash, crude fiber, fat, and protein contents were determined according to AOAC methods.[Citation16] The pH of modified starch dispersion (8% w/v) was measured by using a pH meter. The amylose content in developed RS was measured following the method described by McGrance et al.[Citation17] and the RS content in the starch modifications was determined by an enzymatic method.[Citation16]
Results and discussion
Chemical composition of culinary banana starch
The yield of starch isolated from culinary banana was 16.00% with a purity of 96.00% (). Waliszewski et al.[Citation22] isolated starch from valery variety of bananas and the yield of starch reported by authors was much higher (33.8%). Bello-Perez et al.[Citation23] reported yields of starch from macho and criollo banana at 43.8 and 11.8%, respectively. The marked difference in the starch yield between the two varieties may be ascribed to the texture of banana fruit and its maturity stages. The moisture content of culinary banana starch was found to be 10.90% and is somewhat higher than those reported by Perez-Sira[Citation24] for plantain starch and the difference may be due to the highly humid atmospheric condition of North-East India. However, according to the reports of Mweta et al.[Citation25] the moisture content of starch ranged from 8.96 to 11.93% and falls within the acceptable range for storage and marketing without deterioration in quality of starch. The data of the present study () revealed that the culinary banana starch contained 0.35% ash, 0.31% protein, 0.27% fiber, and 0.50% fat. The content of ash reported in present study was higher than that of plantain starch (0.02%) reported by Perez-Sira.[Citation24] The high ash content in culinary banana starch may be indicative of presence of more minerals like potassium and magnesium. A result of protein content is comparable with results of Mweta et al.[Citation25] in case of cassava starch. The presence of fat is another plausible reasons for its resistance to amylolysis and cause formation of amylose-lipid complex.[Citation26] The pH value obtained for the culinary banana starch was recorded to be 6.70 which is within the pH range of 3–9 obtained for most starches used in the pharmaceutical, cosmetics, and food industries.[Citation27] As most normal starches contain 20–30% amylose and 34.10% amylose content in the present study of culinary banana starch revealed a non-waxy starch type. Amylose content in valery banana starch (40.7%)[Citation22] was different for cavendish banana starch (19.5%).[Citation28] The high amylose starch is much more resistant to digestive enzymes than the low amylose starch. The RS content in the starch sample studied was 18.88% which was on the higher side as compared to the content of 17.5% in banana flour reported by Ovando-Martinez et al.[Citation29] Khawas et al.[Citation30] reported that culinary banana is an excellent source of starch which corroborates the present findings.
Table 1. Chemical compositions (% dry weight basis) of culinary banana starch.
Functional properties of culinary banana starch
Water holding capacity, starch swelling power, and solubility
The present study revealed that water holding capacity of culinary banana starch () increased with rise in temperature. The maximum water holding capacity (42.24%) was observed at 90ºC and the results are in accordance with the findings of Bello-Perez et al.[Citation31] for “criollo” and “macho” starches. The swelling behavior of starch is an indication of the water absorption characteristics of the granules during heating. Generally, the solubility and swelling profiles show a general trend of increase with increase in temperature and expansion of starch mainly depends on degree of gelatinization[Citation32] and results are presented in . The starch sample swelled slowly up to 70°C and above it the starch granules swelled rapidly due to the breakage of intermolecular hydrogen bonds in amorphous region.[Citation23] However, the starch exhibited a restricted swelling pattern, which may be attributed to fat content, as fats are known to inhibit swelling by forming insoluble complexes with the linear fraction of starch.[Citation33] Similar range of swelling power was also reported for kernel starch[Citation34] and white and yellow plantain starches.[Citation35] Lower swelling power of culinary banana starch is also a reflection of more stable granular structure within the starch molecule and a similar pattern was also observed for solubility () and the result of the swollen starch granules allowed amylose exudation. The maximum solubility (9.00%) was observed at 90°C which is lower than corn starch (15.80%) at the same temperature.[Citation36] The lower values of solubility of starches at low temperatures might be due to the semi-crystalline structure of the starch granules and the hydrogen bonds formed between hydroxyl groups within the starch molecules. As the temperature increased, the solubility also increased due to breaking of starch granules and exposure of hydrophilic groups to water.[Citation37]
Table 2. Water holding capacity, swelling and solubility profile of culinary banana starch (%).
Freeze-thaw stability
Exudation of water from frozen gels (syneresis) indicates the retrogradation behavior of the cooked starch pastes. Freeze-thaw stability measures the amount of water released from the gels during storage by degree of syneresis and is an important factor to be considered when formulating refrigerated and frozen foods.[Citation38] Culinary banana starch gel was unstable during different freezing and thawing cycles and released 24.13–42.58% of water (). The amount of water separated from the gels during freezing increased with storage time and it suggests that banana starch is not desirable for frozen products.
Figure 2. A: Freeze-thaw stability of culinary banana starch and B: paste-clarity of culinary banana starch.
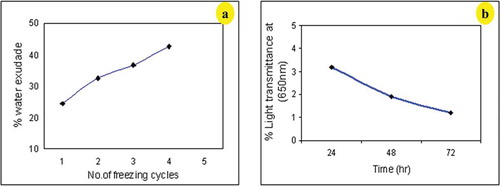
The low freeze-thaw stability of culinary banana starch might be due to the amylose and amylopectin content. Baker and Rayas-Duarte[Citation38] have reported this behavior for corn starches, mentioning low freezing-thawing gel stability for corn and amaranth starches.
Starch paste clarity
Starch gel clarity is a much desirable functionality of starches for its utilization in food industries since it directly influences brightness and opacity in foods. The starch sample experienced low paste clarity (3.2–1.2% light transmittance) during storage time () and the results are in line with previous reports of Bello-Pérez et al.[Citation31] that banana starch forms an opaque gel and with increase in storage period the transmittance value decreases. This reduction in transmittance is due to retrogradation tendency of starch pastes which means that under refrigerated condition banana starch have tendency to retrograde. Opaqueness of starch paste has been attributed to various factors such as granule swelling, granule remnants, leached amylose, and amylopectin, amylose-amylopectin chain-length, intra- or intermolecular bonding and presence of lipids.[Citation39] Since the paste clarity of culinary banana starch is very low, it could be used in food products that do not require transparency.
Pasting properties
Pasting properties of culinary banana starch are illustrated in . The starch sample exhibited a high (81.80°C) PT which indicates the starch is highly resistant toward swelling and it favorably supports our findings for swelling power. The PT is an indication of the gelatinization temperature of the starches; which indicates culinary banana starches have high gelatinization temperature. The culinary banana starch showed a PV of 4507 cP and reflects the ability of starch granules to swell freely before their physical breakdown.[Citation40] PV occurs at the equilibrium point between granule swelling and polymer leaching, which causes an increase in viscosity and granule rupture and polymer alignment because of mechanical shear. The starch exhibited high SV during cooling indicating that it retrograded highly which might be due to the effect of amylose and amylopectin contents since the starch with high amylose could undergo the retrogradation process faster than the starch with low amylose content.[Citation41] During the holding temperature at 95°C accompanied with shear, a decrease in the viscosity of the starch pastes was observed, resulting in the breakdown of some swollen starch granules. The culinary banana starch also experienced a low breakdown (447 cP) viscosity which is also indicative of lower degree of swelling and subsequent disintegration. Kayitsu et al.[Citation40] reported that the high PT coupled with high PV of the starches exhibited more resistance to swelling and rupturing.
Structural analysis; XRD
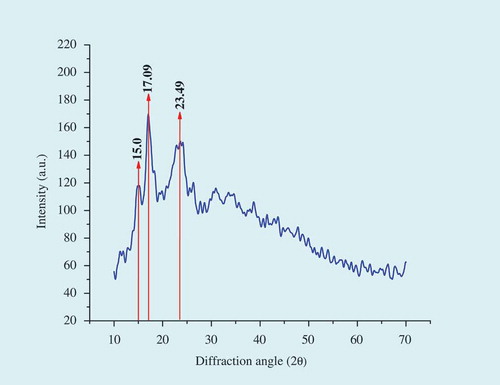
XRD is one of the most effective methods for evaluating the structure of starch and determining the crystalline form of starch.[Citation42] XRD provides an elucidation of the long range molecular order, typically termed as crystalline, which is due to the ordered arrays of double helices formed by the amylopectin side chains.[Citation43] The culinary banana starch exhibited strong diffraction peaks at 15.0 and 17.09° (2θ) and one very broad peak at 23° (2θ). The wide angle x-ray diffractogram revealed that culinary banana starch () is a mixture between the A- and B-type polymorphs. According to the report of Yu et al.[Citation44] C-type starch pattern has been considered a mixture of both A and B-types because its XRD pattern can be resolved as a combination of the previous two. The results of XRD reported in the present study is in line with Chang et al.[Citation45] and Waliszewskiet al.[Citation22] as they have also assigned a C-type diffraction pattern for banana starches. The % crystallinity of starch sample was recorded to be 27.45% which is comparatively higher than the reported values of crystallinity index of different varieties of banana starches studied by Soares et al.[Citation46]
FT-IR spectra
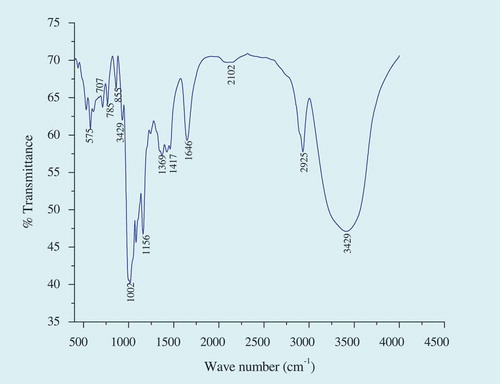
The FT-IR spectrum of culinary banana starch is illustrated in and the characteristics absorption bands appeared at 575, 785, 855, 929, cm–1 and may be attributed to anhydroglucose ring stretching vibrations. The carbohydrate nature of the starch sample was confirmed by the spectra observed near the wave numbers 1156, 1369, 1417, 1646, 2925, and 3429 cm–1.[Citation47] An extremely broad band appeared at 3429 cm–1 is attributed to hydrogen bonded hydroxyl groups. The sharp band observed at 2925 cm–1 is a characteristic of O-H and H-C-H bond stretching associated with the methine ring hydrogen atom.[Citation48] The band at 1646 cm–1 is related to COO- stretching vibration in a carbohydrate group.[Citation49,Citation50] Peaks observed at 1417 and 1369 cm–1 were attributable to the bending modes of H–C–H and C–H symmetric bending of CH3[Citation49,Citation51] and the signal at 1156 cm–1 could be attributed to C-O bond stretching.[Citation47] The additional characteristics absorption bands at 929, 855, 785, 707, and 575 cm–1 are due to the entire anhydroglucose ring stretching vibrations.[Citation47] The peak observed at around 1002 cm–1 is associated with the crystalline and amorphous structure of starch.[Citation49,Citation50] The FT-IR spectra observed in the present study resemble the spectra obtained for plantain and banana starches studied by various authors.[Citation24,Citation47]
Morphological analysis by SEM
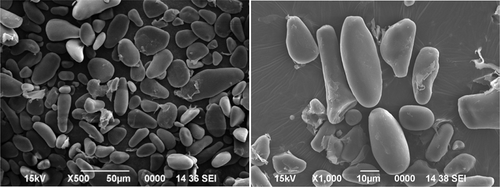
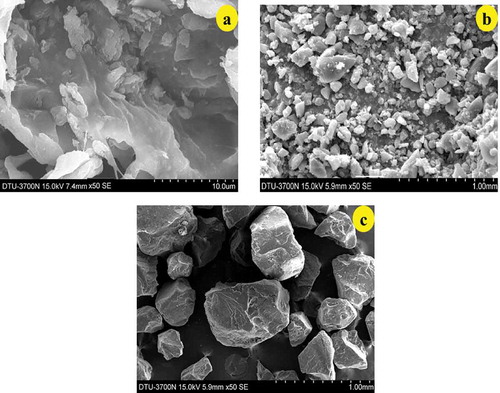
The scanning electron micrographs () of culinary banana starch revealed that starch granules appeared as a mixture of spherical and elliptical shaped with granule size ranged from 7.55–68.00 µm. Eggleston et al.[Citation52] reported that the plantain starch had a broad range of granule size (7.8–61.3 μm) and is lower than our findings for culinary banana starch granules. The surface of the starch sample appeared to be smooth as reported by Kayiasu et al.[Citation40] and indicated that the isolation process was efficient and did not cause damage to starch granules.
Thermal stability by TGA
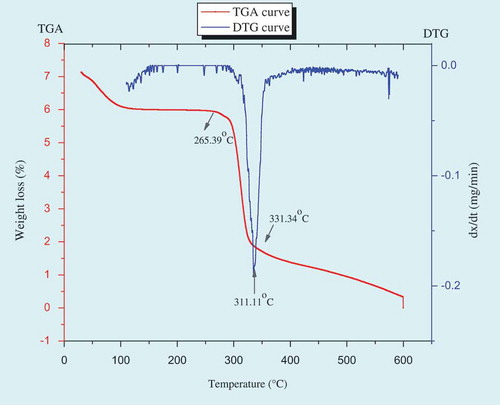
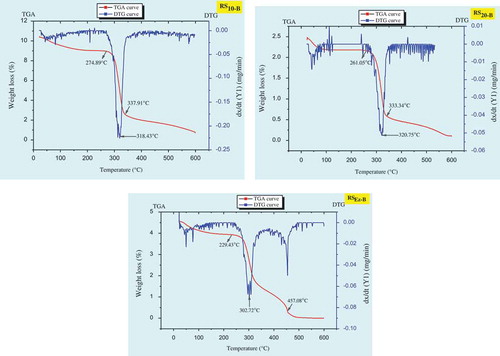
The thermal stability of culinary banana starch with increasing temperature at 10°C/min was evaluated by TG and the corresponding thermogravimetric derivative (DTG) curves as illustrated in . TGA curve revealed that thermal degradation of starch showed three distinct weight losses, the first weight loss initiated at 25.15–247.35°C corresponds to the initial vaporization of residual water. The second weight loss at around 265.39–331.34°C is due to the pyrolysis of starch and the weight loss observed at the temperature range of 360–480°C may be ascribed to decomposition of cellulose, hemicelluloses, and lignin present in the starch sample.[Citation53] The region at which second degradation or pyrolysis of starch occurred, revealed a sharp and well-defined peak of DTG curve (), and on further increase in temperature showed asymmetric appearance (shoulder formation). It may be ascribed to the second degradation stage which is quite complex and probably that may be divided into substages which were specifically defined by the formation of shoulder at the end of second decomposition process. The temperature and degradation rate increased with increase in heating rate which is a characteristic behavior for the thermally stimulated processes in the solid state.[Citation54]
Development of type III RS from culinary banana starch
The production of RS type III by autoclaving and cooling is mainly due to the retrogradation of the amylose fraction (). The four sets of RS obtained after autoclaving followed by cooling cycle were coded as RS10-A (10% starch, 4°C storage), RS10-B (10% starch, –20°C storage), RS20-A (20% starch, 4°C storage), and RS20-B (10% starch, –20°C storage). The generation of RS type III by hydrothermal treatment depends mainly on the number of heating and cooling cycles, starch type, storage time, and temperature, etc. The amylose content and the amount of water are directly correlated to the yield of RS.[Citation55] The effect of starch concentration and the number of autoclaving and cooling cycles on the yield of RS type III are presented in . The present study showed that the yield of RS increased with the increase in starch concentration and likewise the yield of RS content also increased with the number of cycles. However, the effect of starch concentration on RS yield was considerably lower than the number of autoclaving and cooling cycles. After the third autoclaving and cooling cycle, the RS content increased to 23.31%. This gradual rise in the yield of RS after repeated heating and cooling cycles might be associated with a decrease in the hydrolysis limit of pancreatic α-amylase and the increased RS type III formation. Haralampu[Citation55] determined RS values between 2.5 and 21.3% for diverse starch sources and with the Berry method,[Citation20] the RS values ranged between 2.8–31%[Citation56] which favorably supports our findings.
Table 3. Effect of starch concentration and number of autoclaving and cooling cycles on RS content.
Effect of enzyme concentration on starch debranching
An increased degree of debranching enables the chains to align and aggregate and hence, form perfectly crystalline structures and thereby leading to the formation of more RS.[Citation57] As illustrated in , RS obtained by enzyme debranching method were coded as RSEz-A (4°C storage) and RSEz-B (–20°C storage). Berry[Citation20] reported that debranching of potato amylopectin with pullulanase before subjecting it to heating and cooling cycles substantially increased the RS type III content; and attributed to an increase in the content of linear starch chains resulting from debranching. The different enzyme concentrations used in this study showed that lower the concentration levels used, the lower is the amount of RS produced. This evidenced that the substrate needed higher enzyme concentration for starch debranching and maximum RS (31.17%) yield was obtained when debranched with 5% enzyme concentration.
Effect of storage temperature on RS content
Both the RS obtained from hydrothermal treatment after three cycles of autoclaving and cooling, and enzyme debranching method were subjected to two different storage temperatures (4°C and –20°C) and its effect on the yield of RS type III are presented in . The results revealed that low temperature storage enhanced the formation of RS and gradually increased at short storage duration. The RS obtained from 10% starch increased from 12.30% (stored at 4°C) to 15.43% (stored at –20°C) and likewise RS obtained from 20% starch increased to 26.42% when stored at –20°C. The RS obtained using enzyme debranching showed very little increase in the RS content from 30.21 to 31.17% with respect to storage condition. This supports the general behavior that RS yield increases during storage, especially during low-temperature storage.
Table 4. Effect of storage temperature on resistant starch content.
Chemical analysis
The chemical analysis of RS obtained after undergoing various treatments was performed () and moisture content of RS obtained at various isolating conditions differed significantly. It ranged from 4.65 to 5.97% in all the samples which are in the considerable range for long term storage of product without any microbial decomposition. The variation in the moisture content might be attributed to the linear chains produced during the different treatments, which sometimes increased water binding properties.[Citation58] The ash content varied from 0.14 to 0.22% in all the samples and not much significant difference was observed among the treatments given. The plausible reason may be the temperature used during autoclaving process is not high enough to digest the minerals present in the banana starch.[Citation59] The content of crude fiber in all the RS obtained showed significant difference among the samples. The highest crude fiber recorded was 6.15% in case of enzymatically debranched RS stored at –20°C and the minimum was 4.23% in case of RS obtained by autoclaving and cooling method followed by storage at 4°C.
Table 5. Chemical compositions (% dry weight basis) of culinary banana RS.
The fat content varied from 0.11–0.78% and did not have significant difference among the samples obtained by autoclaving and cooling cycle stored at 10°C and unlike for samples stored at –20°C. On the other hand, enzymatically debranched RS showed statistical difference within it. Protein varied from 0.21–0.46% with significant difference among enzyme and thermally treated samples. The protein content obtained after the autoclaving and cooling cycle was comparatively lesser than RS obtained from enzyme debranching and could be ascribed to the heat treatment given during autoclave cycle which denatures proteins and saponifies lipids and may become solubilized.[Citation59] The results of chemical analysis obtained in the present study are in line with the findings of Aparicio-Saguilan et al.[Citation59] in the case of RS obtained from linterized banana.
SEM
The modified RS (RS10-B, RS20-B, and RSEz-B) were studied under electron microscope in order to evaluate the microstructure and also examined how the treatments affected the microstructure (, , ). The RS obtained by hydrothermal treatment (autoclaving and cooling) of 10% starch sample and stored for 24 h () is not prominent in its structure as did not undergo significant morphological changes compared to RS obtained from 20% starch (). The structure of starch is hazy and there was no proper conversion of RS from starch samples. On the other hand, with an increase in the concentration of starch sample from 10 to 20%, the structural difference was evident and the modification of starch to RS with higher percentage (20%) was more noticable and clearly visible. This could be due to partial gelatinization of starch which occurs appropriately during retrogradation process for RS with higher amount of starch.[Citation60] The structural morphology of RS obtained after enzyme debranching is illustrated in and the modification of starch to RS was more distinguished and composed of round to irregular shaped granules. The enzymatically modified starch changes the composition and the structure.[Citation61]
FT-IR spectra of RS
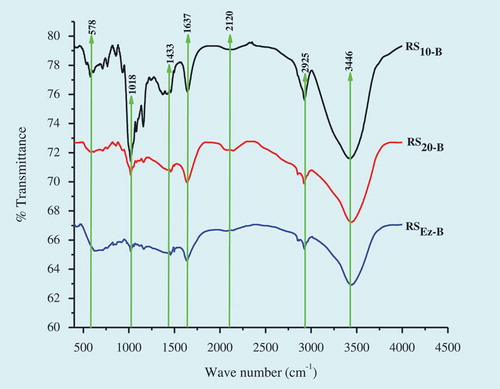
The changes in molecular structure during the starch modification process were studied using FT-IR (). When FT-IR spectra of RS were compared to the spectra of culinary banana starch (), it revealed that the bands in case of RS10-B were almost similar to the spectra of starch sample before retrogradation which suggests that the molecular structure of RS10-B was not altered during retrogradation process. Seven major spectra were observed in the region of 500–3500 cm–1 in all the RS studied and located at 3446, 2925, 2120, 1637, 1433, 1018, and 578 cm–1. But the bands were sharper in case of RS10-B and decreased in RS20-B and further decreased in RSEz-B. The sharp band observed at 1018 cm–1 may be related to the crystalline starch and water content. Stretching vibrations of the hydrogen bonded hydroxyl groups at 3446 cm–1, O-H and H-C-H bond at 2925 cm–1, COO- at 1637 cm–1, bending modes of H–C–H at 1433 cm–1, and anhydroglucose ring at 578 cm–1 bring the visible difference in spectral bands during retrogradation and modified structurally.[Citation62]
TGA
The TGA results are illustrated by a three step weight loss curves of TG and its derivative DTG (). The results of both the starches (i.e., culinary banana starch and RS) were compared with TGA-DTG curves and RS showed endothermic peak at around 237.91°C (RS10-B), 334.34°C (RS20-B), and 457.08°C (RSEz-B) which were comparatively higher to that of starch sample and may be because of the treatment given in order to modify the starch to RS. The different treatments (hydrothermal and enzyme debranching) given to the starch sample for modification to RS may lead to the formation of crystallites and amounts of double helices with different stabilities.[Citation63] The endothermic transition of starch during modification are generally influenced by the interactions between amylose-amylose, amylose-amylopectin, and amylose-lipid content.[Citation58] From the DTG curve () it is evident that, the sharp interval of weight loss indicates the presence of large amounts of compounds such as homopolysaccharides.[Citation64] The RSEZ-B showed faster rate of decomposition at higher temperature and with higher initial degradation temperature 457.08°C caused lower weight loss compared to RS10-B and RS20-B and may be because of crystal formation during starch modification in RSEZ-B was different.[Citation63] Our findings are in line with the reports of Zhou et al.[Citation63] in case of RS obtained from rice starch.
Conclusion
The isolated starch of culinary banana modified to RS type III by hydrothermal and enzyme debranching treatment revealed marked changes in physicochemical, functional, morphological, and thermal properties when compared among them. The yield of isolated starch was 16% with a purity of 96% and the amount of RS content was 18.88%. The isolated starch experienced a restricted swelling and solubility profile and was unstable during freezing and thawing cycles. The starch exhibited a high PT (81.80ºC) indicating high resistance toward swelling. The XRD study clearly revealed that the culinary banana starch is a mixture of A and B-type polymorphs and FT-IR spectra evinced the various functional groups present and suggest C-type starch with a mixture of spherical and elliptical granules. TGA behavior showed pyrolysis of starch occurred between the temperature range of 265.39–331.34°C. The modification of starch to RS occurred due to retrogradation of the amylose fraction and temperature and storage conditions enhanced its formation. Upon analysis of modified by SEM, FT-IR, and TGA/DTG revealed various significant morphological changes and observed with increase in starch concentration and elicited prominent modifications in enzyme debranched RS. Debranching enzyme treatment revealed higher rate of initial decomposition with lower weight loss due to crystal formation and modification indicated structural changes. Therefore it is credible to justify that culinary banana is an excellent source of RS and may be utilized as an alternative source of nutraceutical ingredient for preparing low glycemic functional foods.
Funding
The financial help received from Ministry of Defense and GoI, New Delhi is duly acknowledged.
Additional information
Funding
References
- French, D. Organization of Starch Granules. In Starch: Chemistry and Technology, 2nd Ed, Whistler, R.L.; BeMiller, J.N.; Paschall, E.F.; Eds.; Academic Press: New York, London, 1984; 183–247.
- Sajilata, M.G; Singhal, R.S.; Kulkarni, P.K. Resistant Starch—A Review. Comprehensive Reviews in Food Science and Food Safety 2006, 5 (1), 1–17.
- Suma, P.F.; Urooj, A. Isolation and Characterization of Starch from Pearl Millet (Pennisetum Typhoidium) Flours. International Journal of Food Properties 2015, 18 (12), 2675–2687.
- Biliaderis, C.G. The Structure and Interactions of Starch with Food Constituents. Canadian Journal Physiology and Pharmacology 1991, 69 (1), 60–78.
- Englyst, H.N.; Cummings, J.H. Digestion of the Polysaccharides of Potato in the Small Intestine of Man. American Journal of Clinical Nutrition 1987, 45 (2), 423–431.
- Eerlingen, R.C.; Delcour, J.A. Formation, Analysis, Structure and Properties of Type III Enzyme Resistant Starch. Journal of Cereal Science 1995, 22 (2), 129–138.
- A.A.C.C. American Association of Cereal Chemist. Approved Methods of the AACC, 10th Ed; AACC: St. Paul, MN, 2000.
- Visser, R.G.F.; Suurs, L.C.J.M.; Bruinenberg, P.M.; Bleeker, I.; Jacobsen, E. Comparison Between Amylose-Free and Amylose Containing Potato Starches. Starch-Starke 1997, 49 (11), 438–443.
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. European Journal of Clinical Nutrition 1992, 46 (2), 33–50.
- Goni, I.; Garcia-Diz, L.; Manas, E.; Saura-Calixto, F. Analysis of Resistant Starch: A Method for Foods and Food Products. Food Chemistry 1996, 56 (4), 445–449.
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sanchez-Zapata, E.; Perez-Alvarez, J.A. Resistant Starch as Functional Ingredient: A Review. Food Research International 2010, 43 (4), 931–942.
- Rodriguez-Ambriz, S.L.; Islas-Hernandez, J.J.; Agama-Acevedo, E.; Tovar, J.; Bello-Perez, L.A. Characterization of a Fiber-Rich Powder Prepared by Liquefaction of Unripe Banana Flour. Food Chemistry 2008, 107 (4), 1515–1521.
- W.H.O. Food and Agriculture Organization/World Health Organization. Carbohydrates in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation; FAO: Rome, 1998.
- Khawas, P.; Dash, K.K.; Das, A.J.; Deka, S.C. Modeling and Optimization of the Process Parameters in Vacuum Drying of Culinary Banana (Musa ABB) Slices by Application of Artificial Neural Network and Genetic Algorithm. Drying Technology: An International Journal 2015, DOI:10.1080/07373937.2015.1060605
- Bello-Pérez, L.A.; Meza-León, K.; Contreras-Ramos, S.; Paredes-López, O. Functional Properties of Corn, Banana, and Potato Starch Blends. Acta CientificaVenezolana 2001, 52 (1), 62–67.
- A.O.A.C. Association of Official Analytical Chemists Official Methods of Analysis of the Association of Official Analytical Chemists, 18th Ed; AOAC International: Washington, DC, 2010.
- McGrance, S.J.; Cornell, H.J.; Rix, J.C. A Simple and Rapid Colorimetric Method for the Determination of Amylose in Starch Products. Starch/Starke 1998, 50 (4), 158–163.
- Hallgren, L. Physical and Structural Properties of Cereals, Sorghum in Particular, in Relation to Milling Methods and Product Use, Vol. 1. Carlsberg Research Laboratory, Technical University of Denmark: Copenhagen, 1985.
- Jeong, H.-Y.; Lim, S.-T. Crystallinity and Pasting Properties of Freeze-Thawed High Amylose Maize Starch. Starch/Starke 2003, 55 (11), 511–517.
- Berry, C.S. Resistant Starch: Formation and Measurement of Starch that Survives Exhaustive Digestion with Amylolytic Enzymes During the Determination of Dietary Fiber. Journal of Cereal Science 1986, 4 (4), 301–314.
- Morales-Medina, R.; Muñio, M.D.M.; Guadix, E.M.; Guadix, A. Produciton of Resistant Starch by Enzymatic Debranching in Legume Flours. Carbohydrate Polymers 2014, 101 (30), 1176–1183.
- Waliszewski, K.N.; Aparicio, M.A.; Bello, L.A.; Monroy, J.A., Changes of Banana Starch by Chemical and Physical Modification. Carbohydrate Polymers 2003, 52 (3), 237–242.
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Sánchez-Hernández, L.; Paredes-López O. Isolation and Partial Characterization of Banana Starches. Journal of Agricultural and Food Chemistry 1999, 47 (3), 854–857.
- Perez-Sira, E. Characterization of Starch Isolated from Plantain (Musa Paradisiaca Normalis). Starch/Starke 1997, 49 (2), 45–49.
- Mweta, D.E.; Maryke, T.L.; Elizma, K; Ibrahim, R.M.B.; John, D.K.S. Some Properties of Starches from Cocoyam (Colocasia Esculenta) and Cassava (Manihote Esculenta Crantz). Grown in Malawi. African Journal of Food Science 2008, 2, 102–111.
- Bertolini, C.A.; Creamer, K.L.; Eppink, M.; Boland, M. Some Rheological Properties of Sodium Caseinate-Starch Gels. Journal of Agricultural and Food Chemistry 2005, 53 (6), 2248–2254.
- Omojola, M.O.; Akinkunmi, Y.O.; Olufunsho, K.O.; Egharevba, H.O.; Martins, E.O. Isolation and Physicochemical Characterization of Cola Starch. African Journal of Food, Agriculture, Nutrition, and Development 2010, 10 (7), 2884–2990.
- Ling, L.-H.; Osman, E.M.; Fernandes, J.B.; Reilly, P.J. Physical Properties of Starch from Cavendish Banana Fruit. Starch/Starke 1982, 34 (6), 184–188.
- Ovando-Martinez, M.; Sayago-Ayerdi, S; Agama-Acevedo, E.; Goni, I.; Bello-Perez, L.A. Unripe Banana Flour as an Ingredient to Increase the Indigestible Carbohydrates of Pasta. Food Chemistry 2009, 113 (1), 121–126.
- Khawas, P.; Das, A.J.; Sit, N.; Badwaik, L.S.; Deka, S.C. Nutritional Composition of Culinary Musa ABB at Different Stages of Development. American Journal of Food Science and Technology 2014, 2 (3), 80–87.
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Sayago-Ayerdi, S.G.; Moreno-Damian, E.; Figueroa, J.D.C. Some Structural, Physicochemical, and Functional Studies of Banana Starches Isolated from Two Varieties Growing in Guerrero, Mexico. Starch/Starke 2000, 52 (2–3), 68–73.
- Kannadhason, S.; Muthukumarappan, K. Effect of Starch Sources on Properties of Extrudates Containing DDGS. International Journal of Food Properties 2010, 13 (5), 1012–1034.
- Betancur, A.D.A.; Guerrero, C.A.L.; Matos, C.R.I.; Ortiz, D.G. Physicochemical and Functional Characterization of Baby Lima Bean (Phaseoluslunatus) Starch. Starch/Starke 2001, 53 (5), 219–226.
- Cai, J.; Cai, C.; Man, J.; Xu, B.; We, C. Physicochemical Properties of Ginkgo Kernal Starch. International Journal of Food Properties 2015, 18 (2), 380–391.
- Nwokocha, L.M.; Williams, P.A. Some Properties of White and Yellow Plantain (Musa Paradisiaca Normalis) Starches. Carbohydrate Polymers 2009, 76 (1), 133–138.
- Thomas, D.J.; Atwell, W.A. Gelatinization, Pasting, and Retrogradation. In Starches: Practical Guides for the Food Industry, Thomas, D.J.; Atwell, W.A.; Eds.; Eagan Press: St Paul, MN, 1999; 25–29.
- Eliasson, A.C.; Gudmundsson, M. Starch: Physicochemical and Functional Aspects. In Carbohydrates in Food, Eliasson, A.C. Ed.; Marcel Dekker: New York, 1996, 431–503.
- Baker, L.A.; Rayas-Duarte, P. Freeze-Thaw Stability of Amaranth Starch and the Effects of Salt and Sugars. Cereal Chemistry 1998, 75 (3), 301–307.
- Jacobson, M.R.; Obanni, M.; BeMiller, J.N. Retrogradation of Starches from Different Botanicals. Cereal Chemistry 1997, 74 (5), 511–518.
- Kayisu, K.; Hood, L.F.; Vansoest, P.J. Characterization of Starch and Fiber of Banana Fruit. Journal of Food Science 1981, 46 (6), 1885–1890.
- Goheen, S.M.; Wool, R.P. Degradation of Polyethylene-Starch Blends in Soil. Journal of Applied Polymer Science 1991, 42 (10), 2691–2701.
- Blazek, J.; Gilberta, E.P. Application of Small-Angle X-Ray and Neutron Scattering Techniques to the Characterization of Starch Structure: A Review. Carbohydrate Polymers 2011, 85 (2), 281–293.
- Perez, S.; Bertoft, E. The Molecular Structures of Starch Components and Their Contribution to the Architecture of Starch Granules: A Comprehensive Review. Starch/Starke 2010, 62 (8), 389–420.
- Yu, H.; Cheng, L.; Yin, J.; Yan, S.; Liu, K.; Zhang, F.; Xu, B.; Li, L. Structure and Physicochemical Properties of Starches in Lotus (Nelumbonucifera Gaertn.) Rhizome. Food Science and Nutrition 2013, 1 (4), 273–283.
- Chang, S.M.; Li, C.Y.; Yang, C.C., X-Ray Diffraction Patterns of Some Taiwan Native Starches. Bulletin of the Institute of Chemistry. Academia Sinica 1991, 38, 91–98.
- Soares, C.A.; Peroni-Okita, F.H.; Cardoso, M.B.; Shitakubo, R., Lajolo, F.M.; Cordenunsi, B.R. Plantain and Banana Starches: Granule Structural Characteristics Explain the Differences in Their Starch Degradation Patterns. Journal of Agricultural and Food Chemistry 2011, 59 (12), 6672–6681.
- Sanchez-Rivera, M.M.; Flores-Ramirez, I.; Zamudio-Flores, P.B.; Gonzalez-Soto, R.A.; Rodriguez-Ambriz, S.L.; Bello-Perez, L.A. Acetylation of Banana (Musaparadisiaca L.) and Maize (Zea Mays L.) Starches Using a Microwave Heating Procedure and Iodine as Catalyst: Partial Characterization. Starch/Starke 2010, 62 (3–4), 155–164.
- Gallant, D.J.; Bonchet, B.; Baldwin, P.M. Microscopy of Starch: Evidence of a New Level of Granule Organization. Carbohydrate Polymers 1997, 32 (3–4), 177–191.
- Zeng, J.; Li, G.; Gao, H.; Ru, Z. Comparison of A and B Starch Granules from Three Wheat Varieties. Molecules 2011, 16, 10570–10591.
- Fan, D.; Ma, W.; Wang, L.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W. Determination of Structural Changes in Microwaved Rice Starch Using Fourier Transform Infrared and Raman Spectroscopy. Starch/Starke 2012, 64 (8), 598–606.
- Van Soest, J.J.G.; De Wit, D.; Tournois, H.; Vliegenthart, J.F.G. Retrogradation of Potato Starch as Studied by Fourier Transform Infrared Spectroscopy. Starch/Starke 1994, 46 (12), 453–457.
- Eggleston, G.; Swennen, R.; Akoni, S. Physicochemical Studies on Starches Isolated from Plantain Cultivars, Plantain Hybrids, and Cooking Bananas. Starch/Starke 1992, 44 (4), 121–128.
- Vega, D.; Villar, M.A.; Failla, M.D.; Vallés, E.M. Thermogravimetric Analysis of Starch-Based Biodegradable Blends. Polymer Bulletin 1996, 37 (2), 229–235.
- Jankovi, B. Thermal Characterization and Detailed Kinetic Analysis of Cassava Starch Thermo-Oxidative Degradation. Carbohydrate Polymers 2013, 95 (2), 621–629.
- Haralampu, S.G. Resistant Starch: A Review of the Physical Properties and Biological Impact of RS3. Carbohydrate Polymers 2000, 41 (3), 285–292.
- Pomeranz, Y.; Sievert, D. Purified Resistant Starch Products and Their Preparation. WO 1990015147 A1. University of Washington, December 13, 1990.
- Guraya, H.S.; James, C.; Champagne, E.T. Effect of Enzyme Concentration and Storage Temperature on the Formation of Slowly Digestible Starch from Cooked Debranched Rice Starch. Starch/Starke 2001, 53 (3–4), 131–139.
- Shin, M.; Woo, K.; Seib, P.A. Hot-Water Solubilities and Water Sorptions of Resistant Starches at 25°C. Cereal Chemistry 2003, 80 (5), 564–566.
- Aparicio-Saguilan, A.; Flores-Huicochea, E.; Tovar, J.; García-Suáreza, F.; Gutiérrez-Meraza, F.; Bello-Pereza, L.A. Resistant Starch-Rich Powders Prepared by Autoclaving of Native and Lintnerized Banana Starch: Partial Characterization. Starch/Starke 2005, 57 (9), 405–412.
- Ovando-Martinez, M.; Whitney, K.; Reuhs, B.L.; Doehlert, D.C.; Simsek, S. Effect of Hydrothermal Treatment on Physicochemical and Digestibility Properties of Oat Starch. Food Research International 2013, 52 (1), 17–25.
- Leszczynski, W. Resistant Starch—Classification, Structure, Production. Polish Journal of Food and Nutrition Sciences 2004, 13/54 (SI 1), 37–50.
- vanSoest, J.J.G.; Tournois, H.; Wit de, D.; Vliegenthart, J.F.G. Short-Range Structure in (Partially) Crystalline Potato Starch Determined with Attenuated Total Reflectance Fourier-Transform IR Spectroscopy. Carbohydrate Research 1995, 279, 201–214.
- Zhou, Y.; Meng, S.; Chen, D.; Zhu, X.; Yuan. H. Structure Characterization and Hypoglycemic Effects of Dual Modified Resistant Starch from Indica Rice Starch. Carbohydrate Polymers 2014, 103, 81–86.
- Di-Medeiros, M.C.; Pascoal, A.M.; Batista, K.A.; Bassinello, P.Z.; Lião, L.M.; Leles, M.I.; Fernandes, K.F. Rheological and Biochemical Properties of Solanumlycocarpum Starch. Carbohydrate Polymers 2014, 104, 66–72.