ABSTRACT
PR5-like protein and osmotin-like protein PR-5 are osmotin-like proteins found in sweet pepper and eggplant, respectively. These belong to the family of 5 pathogenesis-related proteins, which are considered a plentiful source of allergens. Recently, we established an assay to quantify protein NP24, an osmotin-like protein of tomato, using absolute quantification method with a stable isotope-labeled internal standard peptide (GQTWVINAPR[13C6,15N4]) and liquid chromatography/tandem mass spectrometry. In this study, the peaks of GQTWVINAPR derived from PR5-like protein and osmotin-like protein PR-5 were detected by multiple reaction monitoring. The results indicated that the assay could be applied for the quantification of these proteins.
Introduction
PR5-like protein (B2CZJ9: UniProtKB accession number) shown in is an osmotin-like protein found in sweet pepper (Capsicum annuum L.). The amino acid sequence of PR5-like protein is very similar to those of osmotin (P14170, 91.5% identity), an osmotic stress-induced protein in tobacco (Nicotiana tabacum L.),[Citation1] and protein NP24 (P12670, 96.4% identity), an osmotin-like protein in tomato (Lycopersicon esculentum Mill.).[Citation2,Citation3] Osmotin and osmotin-like proteins belong to the family of 5 pathogenesis-related proteins (PRs), which are widely viewed as an abundant source of allergens.[Citation4] The gene encoding osmotin-like protein PR-5 (A7VMZ7) in eggplant (Solanum melongena L.) is reported as a fragment, and its full-length sequence has not been determined. Its protein sequence is very similar to those of osmotin and other osmotin-like proteins. The similarity between osmotin and three osmotin-like proteins may reflect the fact that sweet pepper,[Citation5] eggplant, tomato, and tobacco all belong to the same family, i.e., Solanaceae.
Figure 1. Amino acid sequences of osmotin and osmotin-like proteins. Italicized/bold/underlined, AQUA peptide; italicized/bold, identical amino acids among all proteins; italicized, identical amino acids among all proteins except osmotin-like protein PR-5.
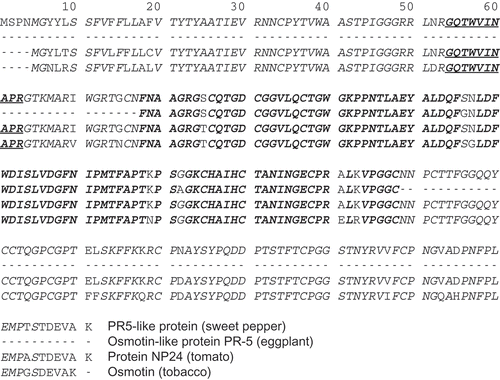
Osmotin showed immunoglobulin E (IgE) binding in 22 of 117 serum samples from patients who were sensitised to tomato and apple.[Citation6] According to a study[Citation7] reporting allergenic activity of tomato cultivars against subjects with a tomato allergy, 4 of 12 subjects also exhibited binding of IgE to NP24. These results indicate that osmotin and NP24 are potential allergens, which suggests that PR5-like protein and osmotin-like protein PR-5 are latent allergens of sweet pepper and eggplant, respectively, based on their sequence similarity to osmotin and NP24.
We previously quantified NP24 in tomato fruit using the absolute quantification (AQUA) method with a stable isotope-labeled internal standard (SIIS) peptide and liquid chromatography/tandem mass spectrometry (LC/MS/MS).[Citation8] In this study, we determined the applicability of the assay to the quantification of PR5-like protein in sweet pepper fruit and osmotin-like protein PR-5 in eggplant fruit.
Materials and Methods
Materials
Ammonium bicarbonate, iodoacetamide, and trichloroacetic acid were purchased from Wako Pure Chemical Industries (Osaka, Japan). Dithiothreitol was purchased from Nacalai Tesque (Kyoto, Japan) and Wako Pure Chemical Industries. GQTWVINAPR[13C6,15N4] (SIIS) and trypsin were purchased from Thermo Fisher Scientific (MA, USA). Guanidinium chloride and β-mercaptoethanol were purchased from Sigma-Aldrich (MO, USA). The LC/MS-grade solvents acetonitrile, formic acid, and water were used for LC/MS/MS. Sweet pepper and eggplant were purchased from local stores. PR5-like protein, osmotin-like protein PR-5, NP24, and osmotin sequences were retrieved from UniProtKB (http://www.uniprot.org/). These sequences were digested in silico and used to generate lists of tryptic peptides using PeptideCutter (web-based software, http://web.expasy.org/peptide_cutter/).
Trichloroacetic Acid/Acetone Extraction
Sweet pepper and eggplant protein extractions were performed as follows;[Citation8] a whole sweet pepper fruit (39 g, edible part) was symmetrically cut into eight pieces and one piece (4 g) was cut into smaller pieces and ground into a fine powder in liquid nitrogen with a mortar and pestle. The skin (1 g) collected from a sweet pepper fruit and skin (1.2 g) from an eggplant fruit were also ground into a fine powder in liquid nitrogen with a mortar and pestle. The powders (100 or 50 mg) derived from the whole sweet pepper fruit or the skins of sweet pepper and eggplant were suspended in 1 or 0.5 mL, respectively, of 10% (w/v) trichloroacetic acid in acetone mixed with 2% (v/v) β-mercaptoethanol, and stored at –20°C overnight. The suspensions were centrifuged at 5000 × g for 30 min at 4°C, and the supernatants were discarded. Cold acetone (1 or 0.5 mL) was added to the pellets from whole sweet pepper fruit or skins, respectively, centrifuged at 5000 × g for 10 min at 4°C, and the supernatants were discarded. This washing procedure with acetone was repeated twice and then the pellets were gently dried.
Trypsin Digestion
Sweet pepper and eggplant protein extracts obtained using trichloroacetic acid/acetone treatment were digested with trypsin.[Citation8] These extracts were resuspended in 100 μL of 50 mM ammonium bicarbonate and 6 M guanidinium chloride, mixed with 5 μL of 200 mM dithiothreitol in 50 mM ammonium bicarbonate, and boiled for 10 min. For protein alkylation, the mixtures were supplemented with 4 μL of 1 M iodoacetamide in 50 mM ammonium bicarbonate and incubated under shading for 1 h at room temperature. The samples were then supplemented with 40 μL of 200 mM dithiothreitol in 50 mM ammonium bicarbonate and incubated for 1 h at room temperature. These mixtures were diluted with 50 mM ammonium bicarbonate (851 μL), and the protein concentration was examined using the Quick Start Bradford Protein Assay (Bio-Rad Laboratories, CA, USA) with γ-globulin (Bio-Rad Laboratories) as a protein standard. Aliquots (50 μL) of diluted solution derived from whole sweet pepper fruit or sweet pepper and eggplant skins were supplemented with 25 μL of 4 or 10 μg/mL trypsin in 50 mM ammonium bicarbonate, respectively, and 25 μL of 80 fmol/μL GQTWVINAPR[13C6,15N4] in 50 mM ammonium bicarbonate, and incubated overnight at 37°C. The additive contents of trypsin followed the manufacturer’s instructions.
Sweet Pepper Heat Treatment
Three whole sweet pepper fruits were symmetrically cut into two pieces. One piece (16, 23, and 23 g, edible parts) from each fruit was heated in a microwave oven (NE-T155; Panasonic, Osaka, Japan) for 90 s at a high frequency power output of 500 W and at an oscillatory frequency of 2450 MHz. The heated and non-heated pieces (14, 23, and 18 g, edible parts) were treated following the procedure described in the “Trichloroacetic Acid/Acetone Extraction” and “Trypsin Digestion” sections. The weights of the powders derived from heated or non-heated pieces used for protein extraction were 50 or 100 mg, respectively, and the concentration of trypsin solution for digestion was 10 μg/mL.
LC/MS/MS Analysis
The LC/MS/MS apparatus used for this study was an ACQUITY UPLC connected to an XEVO TQD equipped with a ZSpray ion source (Waters, MA, USA). A capillary voltage of 3 kV, collision energy of 20 V, cone gas flow of 50 L/h, cone voltage of 36 V, desolvation gas flow of 1000 L/h, and desolvation temperature of 500°C were used. Trypsin digestion solutions (100 μL) were added with 100 μL of acetonitrile with 0.2% formic acid and centrifuged at 16,000 × g for 10 min at 4°C. The supernatants (5 μL) were loaded onto a SeQuant ZIC-HILIC guard column (20 mm × 2.1 mm, Merck, Darmstadt, Germany) followed by a SeQuant ZIC-HILIC column (150 mm × 2.1 mm, Merck) at 30°C. The analyte was eluted at a flow rate of 0.1 mL/min with an isocratic mobile phase (water with 0.1% formic acid/acetonitrile with 0.1% formic acid, 60:40). Multiple reaction monitoring (MRM) was performed using a mass spectrometer as follows: the dwell time for the MRM transitions of 571.3+2 to 669.4+1 and 576.3+2 to 679.4+1 for quantification was set to 210 ms, and the other (571.3+2 to 855.5+1, 571.3+2 to 956.5+1, 576.3+2 to 865.5+1, and 576.3+2 to 966.5+1) for identification was set to 20 ms. In addition to the MRM experiment, total ion scans with a mass range from m/z 50 to 2000 with a scan time duration of 0.4 s/scan were carried out. The acquisition and analysis of data were performed using MassLynx software (v. 4.1, Waters).
Results and Discussion
LC/MRM Analyses of Osmotin-Like Proteins
Our assay for NP24 contained in tomato fruit uses GQTWVINAPR (amino acid numbers 54–63, ) as an AQUA peptide,[Citation8] and this peptide is generated from PR5-like protein by trypsin digestion in silico; accordingly, the assay can hypothetically be applied to the quantification of PR5-like protein in sweet pepper fruit. However, the osmotin-like protein PR-5 sequence in the region corresponding to the peptide sequence is not known. If this protein possesses the sequence that produces GQTWVINAPR by trypsin activity similar to NP24, then our method can be applied.
and show the total ion and MRM chromatograms of the tryptic digest of whole sweet pepper protein extracts containing the SIIS peptide, respectively. The peaks corresponding to native and SIIS peptides were sharp and distinctly observed near a retention time of 5.6 min on the MRM chromatogram (). This result demonstrates that the quantification method used for NP24 in tomato fruit can be used to assay PR5-like protein in sweet pepper fruit.
Figure 2. A: Total ion and B: MRM chromatograms of tryptic digestion products from the whole sweet pepper protein extract. B: Solid (native) and dotted (SIIS) lines indicate data monitored with MRM transitions for quantification. The enlarged area of the chromatogram (5.4–5.9 min) correspond to GQTWVINAPR and GQTWVINAPR[13C6,15N4].
![Figure 2. A: Total ion and B: MRM chromatograms of tryptic digestion products from the whole sweet pepper protein extract. B: Solid (native) and dotted (SIIS) lines indicate data monitored with MRM transitions for quantification. The enlarged area of the chromatogram (5.4–5.9 min) correspond to GQTWVINAPR and GQTWVINAPR[13C6,15N4].](/cms/asset/7d4265d3-b461-428b-b2f3-c11e5e9cae12/ljfp_a_1154571_f0002_b.gif)
Since the peak on the MRM chromatogram for the quantification of the whole eggplant fruit protein extract was subtle (data not shown) and the assay could not be evaluated, the digest of the eggplant skin protein extract was used. This extract provided a total ion chromatogram () and a satisfactory peak on the MRM chromatogram (), similar to that of sweet pepper. The peaks of the expected y ions corresponding to WVINAPR (571.3 > 855.5 in ) and TWVINAPR (571.3 > 956.5 in ) produced by the fragmentation of [GQTWVINAPR+2H]2+ were observed at the same retention time, which supports the existence of GQTWVINAPR in the sequence of osmotin-like protein PR-5 as well as osmotin and other osmotin-like proteins. Therefore, we postulated that osmotin-like protein PR-5 possesses the sequence that generates GQTWVINAPR by trypsin digestion.
Figure 3. A: Total ion and B and C: MRM chromatograms of tryptic digestion products from eggplant skin protein extract. B: Solid (native) and dotted (SIIS) lines indicate data monitored with MRM transitions for quantification. The enlarged area of the chromatogram (5.4–5.9 min) correspond to the expected GQTWVINAPR and GQTWVINAPR[13C6,15N4]. C: 571.3 > 855.5 and 571.3 > 956.5 exhibit data detected with MRM transitions for the identification of GQTWVINAPR, 571.3+2 to 855.5+1 and 571.3+2 to 956.5+1, respectively.
![Figure 3. A: Total ion and B and C: MRM chromatograms of tryptic digestion products from eggplant skin protein extract. B: Solid (native) and dotted (SIIS) lines indicate data monitored with MRM transitions for quantification. The enlarged area of the chromatogram (5.4–5.9 min) correspond to the expected GQTWVINAPR and GQTWVINAPR[13C6,15N4]. C: 571.3 > 855.5 and 571.3 > 956.5 exhibit data detected with MRM transitions for the identification of GQTWVINAPR, 571.3+2 to 855.5+1 and 571.3+2 to 956.5+1, respectively.](/cms/asset/48acee7e-5a64-44d0-8ebd-d1b3bd39d799/ljfp_a_1154571_f0003_b.gif)
Osmotin-Like Protein Content
Based on this postulation, we assayed osmotin-like proteins of sweet pepper and eggplant fruits. These proteins were successfully quantified in skin samples (). However, the proteins in the endocarp of the sweet pepper and the endocarp and placenta of the eggplant could not be determined, because they were below the limit of quantification. According to a previous study,[Citation2] the concentration of NP24 is greater in the skin of tomato (18.3 μg/g fresh weight) than in its endocarp (5.8 μg/g fresh weight) and locular gel (0 μg/g fresh weight). Therefore, osmotin-like proteins of these Solanaceae fruits is thought to predominate in the skin.
Table 1. Osmotin-like protein concentration in sweet pepper and eggplant skins
Influence of Heat Treatment
Next, the influence of heat treatment on the ability to assay PR5-like protein contained in sweet pepper fruit was investigated (). Three whole sweet peppers (A, B, and C, listed in ) were cut into two halves. One half was fully heated in a microwave oven. PR5-like protein contained in these heat-treated and untreated samples were analysed using the AQUA-based method. There were no significant differences (p > 0.05) evaluated by a Student’s t-test between the contents of heated and non-heated pieces derived from same sweet pepper fruit. This result indicated that heat treatment does not affect the detection ability of this analytical method for sweet pepper.
Table 2. PR5-like protein concentration in heated and non-heated sweet peppers
Conclusion
In this study, the peak of GQTWVINAPR derived from sweet pepper and the presumptive peak from eggplant were observed on the MRM chromatograms measured by the LC/MS/MS analysis using an AQUA peptide designed to assay NP24, an osmotin-like protein contained in tomato fruit. The results of this research indicated that this analytical assay could be applied to the quantification of PR5-like protein in sweet pepper fruit and osmotin-like protein PR-5 in eggplant fruit. This method based on AQUA technology is thought to be generally useful for the quantification of osmotin-like proteins.
Funding
This work was supported in part by a grant from the research project ‘‘A Scheme to Revitalise Agriculture and Fisheries in Disaster Area through Deploying Highly Advanced Technology (NouEi 2-02)” from the Ministry of Agriculture, Forestry, and Fisheries.
Additional information
Funding
References
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A.; Pfankoch, E.; Regnier, F.E.; Bressan, R.A. Characterization of Osmotin: A Thaumatin-Like Protein Associated with Osmotic Adaptation in Plant Cells. Plant Physiology 1987, 85, 529–536.
- Pressey, R. Two Isoforms of NP24: A Thaumatin-Like Protein in Tomato Fruit. Phytochemistry 1997, 44, 1241–1245.
- Izli, N.; Isik, E. Color and Microstructure Properties of Tomatoes Dried by Microwave, Convective, and Microwave-Convective Methods. International Journal of Food Properties 2015, 18, 241–249.
- Breiteneder, H. Thaumatin-Like Proteins—A New Family of Pollen and Fruit Allergens. Allergy 2004, 59, 479–481.
- Gogus, F.; Ozel, M.Z.; Keskin, H.; Yanık, D.K.; Lewis, A.C. Volatiles of Fresh and Commercial Sweet Red Pepper Pastes: Processing Methods and Microwave Assisted Extraction. International Journal of Food Properties 2015, 18, 1625–1634.
- Sharma, P.; Singh, A.K.; Singh, B.P.; Gaur, S.N.; Arora, N. Allergenicity Assessment of Osmotin, a Pathogenesis-Related Protein, Used for Transgenic Crops. Journal of Agricultural and Food Chemistry 2011, 59, 9990–9995.
- Dölle, S.; Lehmann, K.; Schwarz, D.; Weckwert, W.; Scheler, C.; George, E.; Franken, P.; Worm, M. Allergenic Activity of Different Tomato Cultivars in Tomato Allergic Subjects. Clinical & Experimental Allergy 2011, 41, 1643–1652.
- Ippoushi, K.; Sasanuma, M.; Oike, H.; Kobori, M.; Maeda-Yamamoto, M. Absolute Quantification of Protein NP24 in Tomato Fruit by Liquid Chromatography/Tandem Mass Spectrometry Using Stable Isotope-Labelled Tryptic Peptide Standard. Food Chemistry 2015, 173, 238–242.
