ABSTRACT
Bromelia pinguin L. is a natural source of bioactive compounds. The main purpose of this research was to isolate and characterize bioactive proteins from its fruit. B. pinguin proteins were fractionated by gel filtration chromatography, and analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The antibacterial activity of the proteins was analyzed against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923, and the enzymatic activity was evaluated by protease activity and trypsin inhibitions assays. Protein fraction obtained by gel filtration chromatography exhibited antibacterial activity against E. coli (minimum inhibitory concentration [MIC] 0.3492 mg/mL) and S. aureus (MIC 0.6845 mg/mL). The proteolytic activity of the fraction was 0.985 Ucas/mL. The substrate-sodium dodecyl sulfate-polyacrylamide gel electrophoresis assay detected protease inhibitors with molecular weights of 43 and 74 kDa. Antibacterial studies of E.coli and S. aureus were determined by comparing the protein fraction with different antibiotics. The antibacterial activity of proteins extracted from the pulp of the fruit of Bromelia pinguin L. could be related to the presence of enzymes, protease inhibitors and peptides.
Introduction
The continuous use of antibiotics has resulted in multi-resistant bacterial strains all over the world and, as expected, hospitals have become breeding grounds for human associated microorganisms. What is more, the same time-bomb effect is slowly developing with animal-associated pathogens in commercially driven activities, such as aquaculture and confined poultry breeding, where the indiscriminate use of antibiotics is perceived as essential for the survival of these industries. Consequently, there is an urgent need to search for alternatives to synthetic antibiotics. The discovery of two classes of antimicrobial peptides, non-ribosomally synthesized[Citation1] present in bacteria, lower eukaryotes, and plants, as well as ribosomally synthesized peptides of wider distribution, provided a new therapeutic strategy to fight microorganisms. Thus, priorities over the next decade should focus on the development of alternative drugs and/or the recovery of natural molecules that would allow the consistent and proper control of pathogen-caused diseases. Ideally, these molecules should be as natural as possible, with a wide range of action over several pathogens, easy to produce, and less prone to resistance induction.[Citation2]
Plants produce antimicrobial peptides (AMPs) in all organs either constitutively or in response to microbial infection; however, information about the expression of AMPs in epidermal cells is limited. As plants also biosynthesize protective secondary metabolites, AMPs may not be as crucial for the first line of defense as in animals.[Citation3] Plant AMPs were assigned to different classes according to their tertiary structures. The most common classes are thionins, defensins, and lipid transfer proteins. Plant AMPs share the following important features: They are small cationic peptides with molecular masses of 2–10 kDa. The structures of these small peptides are stabilized through formation of 2–6 disulfide bridges. The activities of plant AMPs are primarily directed against fungal, oomycete, and bacterial microorganisms, but certain members of a class can be directed against other targets, including herbivorous insects.[Citation4]
Fruit juices exhibit significant antibacterial effect, the activity being associated with mineral content and biologically active constituents. Hence, these fruit juices with the properties of bioavailability and retention of certain minerals, proteins, and polyphenolic compounds can be recommended for their use as an alternative anti-infective agent in natural medicine for the treatment of infectious diseases.[Citation5] The use of herbal medicines as dietary supplements to fight or prevent common diseases is increasing. These herbal products have fewer side effects and are easily available at affordable cost.[Citation6] Bromelia enguin L. fruit is an ovoid pointed berry of up to 5 cm length, the unripe fruit is green, changing to yellow after ripening. Before consumption, the fruits are peeled and commonly heated or roasted to inactivate pinguinain, which can damage palate tissue; this protease has been proposed as a meat tenderizer and for use in the preparation of biologic detergents.[Citation7] The juice of the fruit has been used as a refreshing beverage as well as an anthelmintic agent.[Citation8] The proteic fraction obtained from the juice of Bromelia pinguin L fruits has shown activity against Lumbricus terrestris and Trichomonas vaginalis.[Citation9] In addition, extracts of the pulp of fruit have shown activity against Candida albicans.[Citation10] The research was carried out to isolate and characterize bioactive proteins from the pulp of Bromelia enguin L. fruit, which have antibacterial activity, proteolytic activity, and proteases inhibitory activity.
Materials and Methods
Fruit Collection and Storage
Ripen fruits of Bromelia pinguin L. were collected in the municipality of Tizimin, Yucatán, México, during the month of January 2013. Plants were identified on the field and their samples were collected and brought to the laboratory for final identification with the help of literature.[Citation11] After collection, the fruits were selected, washed, and sanitized in chlorinated water and kept at –80°C. An extractor was used to obtain the pulp which was stored at –20°C until protein extraction.
Protein Extraction
Protein extraction was performed according to Brito-Argáez et al.[Citation12] Briefly, the pulp from B. pinguin L. fruits was mixed with Tris-HCl buffer (100 mM, pH 7.5) with cysteine (10 mM) and leupeptin (1.5 μg/mL) 1:1 (w/v). The pulp was manually homogenized for 5 min at 4°C and filtered through a cotton mesh. The filtrate was centrifuged at 16,000 × g for 30 min at 4°C. The recovered supernatant is the crude protein extract (CPE), which was then ultracentrifugated at 100,000 × g or 45 min. The supernatant recovered in this stage is the soluble protein extract (SPE); finally, the pellet re-suspended in Tris-HCl buffer is the membrane protein extract (MPE). The extracts were stored at –20°C until analysis. Protein content in CPE, SPE, and MPE was estimated using a dye-binding colorimetric assay for measuring total protein concentration.[Citation13] In this assay, Coomassie Brilliant blue binds to the proteins in the fractions, as well as to standards produced from bovine serum albumin stock (1 mg/mL) to generate a curve from 0–1 mg/mL.
Determination of Protein Profile and Molecular Weight
Denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Bizani et al.[Citation14] For the preparation of the samples, 5 μg of each extract were taken and diluted in 6 μl of loading buffer (5×); distilled water was added to achieve a final volume of 30 µL. The samples were incubated for 5 min at 95°C in order to favor the denaturation of proteins. Gels composed of two phases were used. The first was prepared with 4% of acrylamide in Tris-HCl buffer (1.0 M, pH 6.8), the second was prepared with 15% of acrylamide in Tris-HCl buffer (1.5 M, pH 8.8). Gels were run at 100 V for 2 h at room temperature. In order to obtain a good definition of bands with high molecular weight, the method of silver nitrate staining was employed, and for bands with low molecular weight, the method of Coomassie blue staining was used.[Citation15] The relative molecular weight of the polypeptides of samples was determined by plotting a standard curve of log MW versus Rf for known samples (ladder), and reading off the log MW of the sample after measuring distance migrated on the same gel.
Gel Filtration Chromatography (GFC) and Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
The protein extracts were fractionated by GFC. Briefly, 10 mL of extract was placed into a Sephadex G-25 column (0.5 cm diameter × 70 cm length) which had been previously equilibrated with 100 mM Tris-HCl buffer (pH 7.5). The volume of sample injected was 1.0 mL at a protein concentration of 1.1683 mg/mL. The flow rate employed was 4 mL/min at 4°C. Fractions of 1.0 mL were collected and read at 280 nm. Samples were dissolved in deionized water and injected in a preparative HPLC (Agilent, Model 1110) reverse-phase column (C18 Hi-Pore RP-318, 250 × 10 mm BIO-RAD column).[Citation16] The injection volume was 100 μL, and the sample concentration was 1 mg/mL. Elution was achieved by a linear gradient of acetonitrile in water (0–30% in 50 min) containing 0.1% trifluoroacetic acid at a flow rate of 4 mL/min at 30°C. Elution was monitored at 280 nm.
Culture Preparation
Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 stock cultures were grown in Luria Broth (LB) and sub-cultured every 4 weeks to maintain viability. To obtain working cultures, a loopful of culture was inoculated into LB and incubated for 24 h at 37°C. After incubation, the cultures were diluted to 1 × 106 CFU/mL.
Disk Diffusion Test for Antibacterial Activity
Disk diffusion test[Citation17] was conducted using sterile Petri dishes containing Muller Hinton-Agar. The bacterium was spread over the plates. A sterile blank paper disc (0.625 cm in diameter) with Tris-HCl buffer was placed on the agar. 59 μL of sample at a concentration of (70 mg/mL) was added to one of the disks. Ampicillin (5 mg/mL) was added to the control disc. The plate was incubated at 37°C for 24 h. A transparent ring around the paper disc signified antibacterial activity.
Micro-Broth Dilution Assay
Sterile 96-well microtiter plates with a well capacity of 300 μL were used for micro-broth dilution assay. A total volume of 250 μL, consisting of 125 μL of double strength LB, 100 μL of sample and 25 μL of inoculum (1 × 106 CFU/mL), was used to determine minimum inhibitory concentrations of samples. Microtiter plates were covered with a sterile lid and incubated for 24 h at 37°C and absorbance (630 nm) of each well was read at 0, 3, 6, 12, and 24 h with a microtiter plate spectrophotometer Thermo Scientific Multiskan FC. Waltham, Massachusetts, USA. Finally 100 μL of triphenyltetrazolium chloride (2% w/v) were added to each plate and read at 485 nm. The micro-broth dilution assays[Citation17] were performed in triplicate.
In Vitro Antibacterial Studies
Kinetic studies of the strains exposed to different antimicrobial agents were performed as reported by Schneider et al.[Citation18] with a slight modification. Briefly, bacterial strains were grown overnight in LB medium and diluted in NaCl (0.85%) to an optical density at 600 nm of approximately 1. Total extracts and different antimicrobial agents were added in concentrations corresponding to 2× of its MIC to obtain a final bacterial OD 600 of approximately 0.1. The final mixtures were incubated at 37°C and the OD 600 of the samples was determined every hour for a total time of 6 h.
Proteolytic Activity Assay
This assay was performed according to Abreu-Payrol et al.[Citation19] In a test tube 100 μL of sample was mixed with 1100 μL of substrate (casein 1%, w/v) in Tris-HCl buffer (0.05 mM, pH 8.0) with cysteine (5.0 mM). The mixture was incubated at 37°C for 20 min, and then quenched by adding 1800 μL of trichloroacetic acid (5%, w/v). Blanks were prepared by mixing 100 μL of the sample, 1800 μL of trichloroacetic acid (5%, w/v) and 1100 μL of substrate. The test tubes were then centrifuged at 7000 × g for 20 min. Finally the absorbance of the supernatant was measured at 280 nm. Each assay was performed in triplicate. The proteolytic activity was expressed in caseinolytic units (Ucas), defined as the change in absorbance units at 280 nm which produces 1 mL of enzyme solution, due to the digestion products of casein, soluble in trichloroacetic acid to 5% (w/v) per minute in Tris-HCl buffer (0.05 mM, pH 8.0).
Characterization of Proteinases or Proteinaceous Proteinase Inhibitors by Substrate-Gel Electrophoresis
Electrophoretic separation of proteinases or proteinaceous proteinase inhibitors in the samples was performed using SDS-PAGEs followed by immersion of the gel in (1) a protein substrate solution for detection of proteinases or (2) an appropriate proteinase solution, and then in a protein substrate solution for detection of proteinase inhibitors.[Citation20]
Results and Discussion
Determination of Protein Profile and Molecular Weight
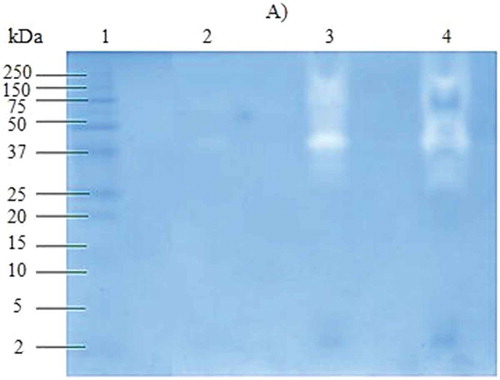
Three types of protein extracts were obtained from the pulp of Bromelia pinguin L. fruit which were denominated: CPE, SPE, and MPE. The highest concentration of protein was found in CPE (1.22 mg/mL), followed by SPE (1.17 mg/mL), and MPE (0.79 mg/mL). CPE and SPE showed a similar electrophoretic profile, indicating that most of the proteins present in CPE are soluble, in contrast to those associated with membrane (). For CPE and SPE the pattern of the gel showed two groups of distinctive bands, the first one with high molecular weights ranging from 17 to 142 kDa and the second one with low molecular weights ranging from 3 to 8 kDa. One distinctive band was detected in the three protein extracts with a molecular weight of 23 kDa.
Figure 1. SDS-PAGE patterns of proteins extracted from Bromelia pinguin L. fruit pulp. Lane 1: molecular marker; Lane 2: crude protein extract; Lane 3: soluble protein extract; Lane 4: membrane protein extract. A: Silver staining; B: Coomassie staining.
The fruits of Bromelia pinguin contain a protease of low molecular weight called pinguinain (crude extract [CE] 3.4.99.18) which is immunologically related to fruit.[Citation21] Abreu-Payrol et al.[Citation7] reported the presence of four cysteine endopeptidases with weights ranging from 23.23 to 23.68 kDa in fruits of Bromelia pinguin from Cuba. There are three potential roles for cysteine endopeptidases in plant defense: perception of the pathogen, activation of downstream signaling pathways, and execution of a defense response.[Citation22] This protease family has drawn attention as a potential pharmaceutical drug target in diseases characterized by excessive extracellular matrix degradation such as in osteoporosis, arthritis, vascular diseases, and cancer. They have also been identified as critical components of growth, cell differentiation, signaling, and host invasion of various human and livestock pathogens (pathogenic parasites causing malaria, Chagas’ disease, and schistosomiasis), as well as major allergens.[Citation23,Citation24]
GFC and RP-HPLC
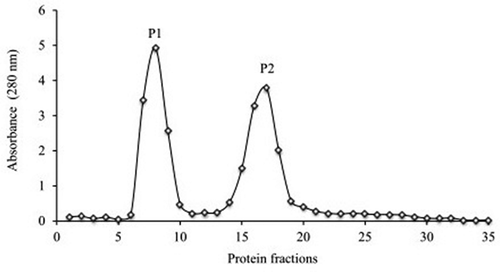
The chromatography elution profile () showed the fractionation of SPE into two peaks. A total of 35 fractions (1 mL) were collected at a flow of 0.25 mL/min. The first peak (P1) consisted of fractions 6–10 and the second one (P2) consisted of fractions 14–20. Only P1 exhibited protein content with 0.0776 mg/mL. Therefore, it refers to the presence of other components in the SPE, such as phenolic compounds which absorb at the same wavelength (280 nm).[Citation25] For P1 the SDS-PAGE gel pattern showed two groups of distinctive bands, the first one with high molecular weights ranging from 17 to 142 kDa and the second one with low molecular weights ranging from 3 to 8 kDa. For P2, in the SDS-PAGE gel pattern no bands were observed.
Figure 2. Chromatography elution profile of soluble protein extract of Bromelia pinguin fruit pulp, on fractionation using gel filtration chromatography.
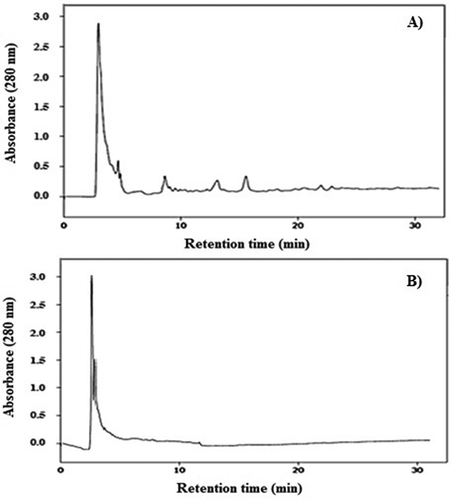
Additionally, SPE and P1 were injected into a RP-HPLC column () in order to evaluate whether an adequate purification of the extract was performed, given that the electrophoretic profiles of the two samples were similar. In the chromatogram corresponding to SPE, peaks were observed distributed between the retention times of 2–22 min, while in the chromatogram corresponding to P1, peaks were observed between the retention times of 2 to 12 min. This shows that CFG effectively removes the non-proteic components from SPE. This is the first report of purification of proteins present in the fruit of Bromelia pinguin.
Disk Diffusion Test for Antibacterial Activity
Antimicrobial activity was assessed using the following rating system: (1) <10.0 mm zone of inhibition may be expressed as inactive; (2) 10.0–13.0 mm zone of inhibition is partially active; (3) 14.0–19.0 mm zone of inhibition is active; (4) >19.0 mm zone of inhibition is very active.[Citation26] The diameter of the inhibitory zone of PSE on E. coli ATCC 25922 and S. aureus ATCC 25923 is presented in . The results presented in are averages of three trials of disk diffusion tests. PSE have significant antibacterial effect on both microorganisms with diameters of inhibitory zone of 1.2 and 1.3 mm for E. coli ATCC 25922 and S. aureus ATCC 25923, respectively. Thus, PSE was partially active against both microorganisms.
Table 1. Extension of zone of bacteria growth inhibition around filter papers with samples in mm.
Subsequently, GFC antimicrobial activity was evaluated for P1 and P2. P1 was partially active against both microorganisms and P2 was inactive. In order to confirm that the antimicrobial activity was due to the presence of proteins, P1 was subjected to two treatments. In the first treatment, the sample was heated at 95°C for 30 min in order to denature the proteins. In the second, proteinase K (20 mg/mL) was added in order to break peptide bonds. After the thermic treatment, P1 remained partially active against both microorganisms (). These results suggest that proteins are exceptionally heat-stable. The stability of proteins at high temperatures is due to many factors[Citation27] which include the co-occurrence of metabolites and sugars and the presence of polar amino acids. Specific polar residues (Asp, Glu, Lys, Arg, Tyr) can enhance the occurrence of intra-subunit ion-pair formation which confers thermal stability to the proteins. This kind of proteases has great potential in the food, biotechnology, and pharmaceutical industries given their activity over a wide range of temperatures and pH. In contrast, after the hydrolytic treatment with proteinase K, P1 was found to be inactive against both microorganisms (). These results suggest that the antibacterial activity of P1 is caused by the proteins extracted from the B. pinguin fruit pulp.
Micro-Broth Dilution Assay
The results of the micro-broth dilution assay showed that P1 inhibited the growth of both strains; E. coli ATCC 25922, with 0.279 mg/mL and S. aureus ATCC 25923 with 0.594 mg/mL. The MIC values for the ATCC strains exposed to ampicillin were 0.008 and 0.014 mg/mL for E. coli ATCC 25922 and S. aureus ATCC, respectively. Pío-León et al.[Citation28] evaluated the antibacterial activity against E. coli ATCC 29213 and S. aureus ATCC 29212 of methanolic extracts (ME), and fractions obtained with ethyl acetate (EaF) and water (AqF) from B. pinguin fruit pulp.[Citation28] The MICPlease spell out MIC. values were 16 mg/mL (CE); 4 mg/mL (EaF) and 16 mg/mL (AqF) against E. coli, and 16 mg/mL (CE); 8 mg/mL (EaF) and 16 mg/mL (AqF) against S. aureus. According to these authors the observed antibacterial activity of extract/fractions of B. pinguin fruit could be associated with the content of phenolics. Fruits are rich in phenolics, tannins, and flavonoids[Citation29] In the present study, the MIC values were significantly lower indicating that proteins of B. pinguin fruit pulp are more active against microorganisms than phytochemicals.
In Vitro Antibacterial Studies
In this study, growth kinetic studies of E.coli and S. aureus were determined by analyzing and comparing protease inhibitors (PI) with four antibiotics (ampicillin, tetracycline, rifampicin, and vancomycin). This study, unlike an MIC assay, allows the determination of the bactericidal activity of P1.[Citation30] The killing kinetics of P1 against tested strains is shown in . P1 exhibited bactericidal effect at 0.558 mg/mL concentration against E. coli ATCC 25922 and at 1.188 mg/mL concentration against S. aureus ATCC 25923.
Figure 4. Time kill studies of E. coli ATCC 25922. A: and S. aureus ATCC 25923; B: exposed to ampicillin (AMP); tetracycline (TET); rifampicin (RIF); vancomycin (VAN); and Peak one (P1).
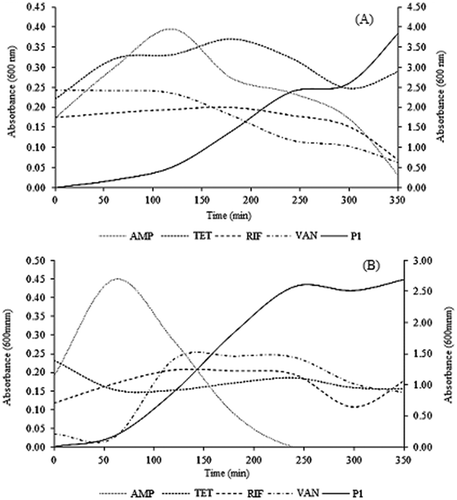
Growth inhibition of P1 against E. coli ATCC 25922 could be compared with the mechanism of inhibition of rifampicin. Rifampicin is active against both gram-positive and gram-negative bacteria and its mechanism of action is based on its ability to bind to and inactivate the bacterial DNA-dependent RNA polymerase, a mode of action not shared by any other antibiotic in use.[Citation31] Growth inhibition of S. aureus ATCC 25923 showed that P1 is similar to that reported for tetracycline. The tetracyclines are a family of antibiotics that inhibit protein synthesis by preventing the attachment of aminoacyl-tRNA to the ribosomal acceptor (A) site. Tetracyclines are broad-spectrum agents, exhibiting activity against a wide range of gram-positive and gram-negative bacteria, atypical organisms such as chlamydiae, mycoplasmas, and rickettsiae, and protozoan parasites.[Citation32]
Characterization of Proteases by Substrate-Gel Electrophoresis
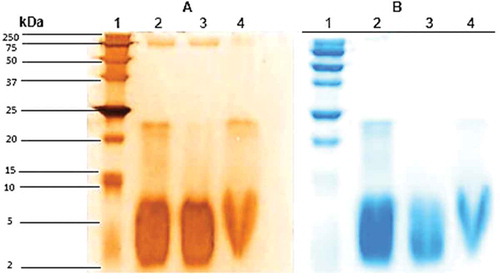
Zymography technique facilitated the detection of proteolytic bands that contrasted very well in the blue background of undegraded protein substrate in stained polyacrylamide gel. showed discrete lytic zones that represent the presence of proteolytic enzymes. Comparatively, discrete proteolytic bands were noticed in the gel copolymerized with casein as substrate, where lytic zones (white bands) were observed in the gel. The SPE and peak one obtained by GFC had similar profiles. A zone of proteolytic activity observed within a range of 30–250 kDa. Proteases may be contributing to the antibacterial activity of the SPE and P1. The proteases can be found in the vacuole,[Citation33] lysosome,[Citation34] and chloroplasts,[Citation35] and have also been found extracellular, in the apoplast.[Citation36] The proteases catalyze the cleavage of peptide bonds of proteins or polypeptide chains. This feature plays an important role in many biological processes such as protein degradation, and protein processing, as well as plant defense against pathogens.[Citation37]
Characterization of Proteinaceous PI by Substrate-Gel Electrophoresis
Other compounds related to plant defense against herbivores and pathogens are PI, which are constitutively expressed in seeds, tubers, and in other storage organs, and are induced in injured plants.[Citation38] Because PI can affect bacterial growth, the presence of such compounds was determined in this study. In , a substrate-SDS-PAGE gel showed PI. SPE has a greater content of this type of proteins with molecular weights ranging from 57–200 kDa and from 29–50 kDa. Meanwhile, in P1 only two bands were observed with weights of 43 and 75 kDa; these proteins could be inhibitors. Proteases are essential in the development and growth of microorganisms; some pathogens are known to produce extracellular proteases, which may be involved in disease development. Plants synthesize PI to respond to the attack of microorganisms and suppress proteolytic enzyme activity, which results in a potent inhibition of the growth of a variety of bacterial and fungal strains.[Citation39]
Conclusion
Antibacterial protein fraction specific to Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 was obtained by GFC from fresh fruit of Bromelia pinguin L. Protein fraction inhibited the growth of E. coli with 0.279 mg/mL and S. aureus with 0.594 mg/mL. In vitro antibacterial studies indicated that the protein fraction acted as rifampicin against E. coli and as tetracycline against S. aureus. SDS-PAGE analysis indicated the presence of enzymes, PI and peptides in the protein fraction. This kind of molecules plays an important role in plant defense process against pathogens. The results indicate that proteins from the pulp of B pinguin could be antimicrobial agents, naturally occurring and non-toxic, which may be used for the treatment of human diseases caused by pathogenic bacteria due to the fact that their mechanisms of action are similar to that exhibited by antibiotics which are chemically synthesized and pharmaceutically employed.
Funding
This research forms part of the project 4577.12-P “Análisis del extracto proteico de Bromelia pinguin con posible aplicación terapéutica y/o biotecnológica,” financed by Tecnológico Nacional de México.
Additional information
Funding
References
- Hancock, R.E.W.; Chapple, D.S. Peptide Antibiotics (Minireview). Antimicrobial Agents Chemotherapy 1999, 43, 1317–1323.
- Marshall, S.H.; Arenas, G. Antimicrobial Peptides: A Natural Alternative to Chemical Antibiotics and a Potential for Applied Biotechnology. Electronic Journal of Biotechnology 2003, 6, 271–284.
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329.
- Stotz, H.U.; Waller, F.; Wang, K. Innate Immunity in Plants: The Role of Antimicrobial Peptides. In Antimicrobial Peptides and Innate Immunity, Progress in Inflammation Research, Hiemstra, P.S; Zaat, S.A.J.; Eds.; Springer: Switzerland, 2013; 29–51.
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic Resistant Bacteria. Brazilian Journal of Microbiology 2000, 31, 247–256.
- Bansode, D.S.; Chavan, M.D. Studies on Antimicrobial Activity and Phytochemical Analysis of Citrus Fruit Juices Against Selected Enteric Pathogens. International Research Journal of Pharmacy 2012, 3, 122–126.
- Abreu-Payrol, J.; Obregon, W.D.; Trejo, S.A.; Caffini, N.O. Purification and Characterization of Four New Cysteine Endopeptidases from Fruits of Bromelia Pinguin L. Grown in Cuba. Protein Journal 2008, 27, 88–96.
- Abreu-Payrol, J.; Miranda-Martínez, M.; Griset, T.C.; Osmaida, C.G. Preliminary pharmacological activity of the Bromelia Pinguin L. fruit. Revista Cubana de Farmacia 2001, 35, 56–60.
- Abreu-Payrol, J.; González-Mosquera, D.M.; Meneses, A.; Cruz-de-la-Cruz, M.E.; Banze, F.; Miranda-Martínez, M.; Ros-López, O. Determination of pharmacognostical and bromatological parameters and antiparasitic activity of Bromelia Pinguin fruit preparation. Acta Farmacéutica Bonaerense 2005, 24, 377–382.
- Camacho-Hernández, I.L.; Chávez-Velázquez, J.A.; Uribe-Beltrán, M.D.J.; Ríos-Morgan, A.; Delgado-Vargas, F. Antifungal Activity of Fruit Pulp Extract from Bromelia Pinguin. Fitoterapia 2002, 73, 411–413.
- Mares, M.; Simón, F.; Martínez, G.M.; Sánchez, R.F.S. Frutales tropicales de Tabasco. Universidad Juárez Autónoma de Tabasco: Villahermosa, Mexico, 2000.
- Brito-Argáez, L.; Lizama, G.; Souza, R.; Ortiz-Vázquez, E. Antibacterial Activity of the Protein Fractions of B. Pinguin Fruit and Seed Against Pathogen Bacteria, 111th General Meeting of ASM, New Orleans, LA, 2011; A1–2008.
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry 1976, 72, 24248–24254.
- Bizani, D.; Motta, A.; Morrissy, J.; Terra, R.; Souto, A.Y.; Brandelli, A. Antibacterial Activity of Cerein 8A, a Bacteriocin-Like Peptide Produces by Bacillus cereus. International Microbiology 2005, 8, 125–131.
- Bollag, D.; Rozycki, M.; Edelstein, S. Protein Methods; Wiley Press: New York, NY, 1996; 127.
- Segura-Campos, M.R.; Chel-Guerrero, L.; Betancur-Ancona, D. Purification of Angiotensin I-Converting Enzyme Inhibitory Peptides from a Cowpea (Vigna Unguiculata) Enzymatic Hydrolysate. Process Biochemistry 2011, 46, 864–872.
- Qaiyumi, S. Macro- and Microdilution Methods of Antimicrobial Susceptibility Testing. In Antimicrobial Susceptibility Testing Protocols, Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C.; Eds; CRC Press Taylor & Francis Group: New York, NY, 2007; 75–80.
- Schneider, T.; Kruse, T.; Wimmer, R.; Wiedemann, I.; Sass, V.; Pag, U.; Jansen, A.; Nielsen, A.; Mygind, P.; Raventós, D.; Gammelgaard, L.; Sahl, H.; Kristensen, H. Plectasin, a Fungal Defensin, Targets the Bacterial Cell Wall Precursor Lipid II. Science 2010, 328, 1168–1172.
- Abreu-Payrol, J.; Obregón, W.D.; Natalucci, C.L.; Caffini, N.O. Reinvestigation of the Proteolytically Active Components of Bromelia Pinguin Fruit. Fitoterapia 2005, 76, 540–548.
- García-Carreño, F.L.; Dimes, L.E.; Haard, N.F. Substrate-Gel Electrophoresis for Composition and Molecular Weight of Proteinases or Proteinaceous Proteinase Inhibitors. Analitycal Biochemestry 1993, 214, 65–69.
- Rowan, A.D.; Buttlet, D.J.; Barrett, A.J. The Cysteine Proteinases of the Pineapple Plant. Biochemistry Journal 1990, 266, 869–875.
- Van der Hoorn, R.A.L.; Jones, J.D.G. The Plant Proteolytic Machinery and Its Role in Defence. Current Opinion in Plant Biology 2004, 7, 400–407.
- Lecaille, F.; Kaleta, J.; Brömme, D. Human and Parasitic Papain-Like Cysteine Proteases: Their Role in Physiology and Pathology and Recent Developments in Inhibitor Design. Chemical Reviews 2002, 102, 4459–4588.
- McKerrow, J.H.; Caffrey, C.; Kelly, B.; Loke, P.; Sajid, M. Proteases in Parasitic Diseases. Annual Review of Pathology 2006, 1, 497–536.
- How, J.S.L. Removal of Phenolic Compounds from Soy Protein Extracts Using Activated Carbon. Journal of Food Science 2006, 47, 933–940.
- Valgas, C.; Machado de Souza, S.; Smânia, E.F.A.; Smânia, A.J. Screening Methods to Determine Antibacterial Activity of Natural Products. Brazilian Journal of Microbiology 2007, 38, 369–380.
- Unsworth, L.D.; Van Der Ost, J.; Koutsopoulos, S. Hyperthermopholic Enzymes—Stability, Activity, and Implementation Strategies for High Temperature Applications. FESB Journal 2007, 274, 4044–4056.
- Pío-León, J.F.; López-Angulo, G.; Paredes-López, O.; Uribe-Beltrán, M.; Díaz-Camacho, S.P.; Delgado-Vargas, F. Physicochemical, Nutritional, and Antibacterial Characteristics of the Fruit of Bromelia Pinguin L. Plant Food Human Nutrition 2009, 64, 181–187.
- Rios, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. Journal of Ethnopharmacology 2005, 100, 80–84.
- Aiyegoro, O.A.; Afolayan, A.J.; Okoh, A.I. In Vitro Antibacterial Time Kill Studies of Leaves Extracts of Helichrysum Longifolium. Journal of Medicinal Plant Research 2009, 3, 462–467.
- Padayachee, T.; Klugman, K.P. Molecular Basis of Rifampin Resistance in Streptococcus Pneumonia. Antimicrobial Agents and Chemotherapy 1999, 43, 2361–2365.
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiology and Molecular Biology Reviews 2001, 65, 232–260.
- Cueva, R.; Garcia-Alvarez, N.; Suarez-Rendueles, P. Yeast Vacuolar Aminopeptidase Yscl. Isolation and Regulation of the Apel (LAP4) Structural Gene. FEBS Letters 1989, 259, 125–129.
- Davies, D.R. The Structure and Function of the Aps. Annual Review of Biophysics and Biophysical Chemistry 1990, 19, 189–215.
- Bushnell, T.P.; Bushnell, D.; Jagendorf, A.T. A Purified Zinc Protease of Pea Chloroplasts, EPI, Degrades the Large Subunit of Ribulose-1, 5-Bisphosphate CarboxyIase/Oxygenase. Plant Physiology 1993, 103, 585–591.
- Xia, Y.; Suzuki, H.; Borevitz, J.; Blount, J.; Guo, Z.; Patel, K.; Dixon, R.A.; Lamb, C. An Extracellular Aspartic Protease Functions in Arabidopsis Disease Resistance Signaling. EMBO Journal 2004, 23, 980–988.
- Guevara, M.G.; Oliva, C.R.; Huarte, M.; Daleo, G.R. An Aspartic Protease with Antimicrobial Activity Is Induced After Infection and Wounding in Intercellular Fluids of Potato Tubers. European Journal of Plant Pathology 2002, 108, 131–137.
- Conconi, A.; Smerdson, M.J.; Howe, G.A.; Ryan, C.A. The Octadecanoid Signalling Pathway in Plants Mediates a Response to Ultraviolet Radiation. Nature 1996, 383, 826–829.
- Kim, M.H.; Park, S-C.; Kim, J-Y.; Lee, S.Y.; Lim, H.T.; Cheong, H.; Hahm, K-S.; Park, Y. Purification and Characterization of a Heat-Stable Serine Protease Inhibitor from the Tubers of New Potato Variety “Golden Valley.” Biochemical and Biophysical Research Communications 2006, 346, 681–686.