ABSTRACT
The effect of heating time (30, 45, and 60 min) and temperature (45, 65, and 85°C) on the extraction yield (total soluble solids) of Ulam raja (Cosmos caudatus) was studied using face-centered composite design-response surface methodology. Total phenolic content and antioxidant activity (TEACDPPH) of samples obtained by aqueous extraction under different temperature and time were determined using Folin–Ciocalteau and 2,2-diphenyl-1-picrylhydrazyl scavenging activity assays, respectively. The yield, total phenolic content, and TEACDPPH of Ulam raja extracts ranged as 3.0–4.2 °Brix, 36.09–37.76 mg gallic acid equivalent/g, and 121.17–146.84 µmol Trolox equivalent/g of plant material on dry basis, respectively. Yield and TEACDPPH of samples were significantly influenced (p < 0.05) by the extraction conditions. Meanwhile, no significant effect (p > 0.05) on total phenolic content was observed. Extraction of Ulam raja at 85°C for 30 min resulted in higher yield and TEACDPPH of the extract. The chromatographic and spectral data confirmed the presence of several flavonoids in the lyophilized Ulam raja extract, i.e., quercitrin, catechin, and rutin, and their quantities were also reported as 36.9, 25.0, and 8.2 mg/g of Ulam raja extract, respectively.
Introduction
Ulam raja (Cosmos caudatus), which is originally from tropical Central America, is an annual aromatic herb that can be found in tropical regions.[Citation1] Fresh leaves of Ulam raja are served as fresh salad together with rice. Traditionally, the Malays consume this herb because of its antioxidant activities and many health beneficial effects.[Citation2] In addition, it has a strong aroma, which makes it a unique appetizer and flavoring in Malay’s traditional dishes.[Citation1] Ulam raja is believed to possess strong antioxidant activities.[Citation1–Citation9] According to a report,[Citation1] a variety of antioxidant compounds are responsible for the health beneficial properties of Ulam raja. It was ranked as the first in terms of antioxidant activity among the five popular Ulam in Malaysia, i.e., “selom” (Oenanthe javanica), curry leaf (Murraya koenigii), “pegaga” (Centella asiatica), Ulam raja, and the seeds of “petai” (Parkia speciosa).[Citation10] According to a study by Rafat et al., Ulam raja showed significant superoxide dismutase and 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity among plants such as lemon grass, garlic, turmeric, and “Hempedu bumi.”[Citation11]
Phytochemicals such as proanthocyanidins, quercetin glycosides such as quercetin-3-O-glucoside, quercetin-3-O-arabinofuranoside, quercetin-3-O-rhamnoside, quercetin-3-O-glucoside, quercetin deoxyl-hexose, chlorogenic, neochlorogenic, cryptochlorogenic acid, caffeic acid, ferulic acid, and (+)catechin were detected in Ulam raja.[Citation1,Citation12,Citation13] In addition, Ulam raja was also found to carry antifungal and antibacterial activities.[Citation14–Citation16] There are evidences that show it has antidiabetic activities by showing a good inhibitory against carbohydrate modulating enzymes which are connected to glucose absorption in the intestine.[Citation17,Citation18] Ulam raja can also stimulate bone formation and improve the dynamic and cellular bone histomorphometry parameters.[Citation19,Citation20] A recent report indicated that Ulam raja supplementation in mice can lead to extrahepatic organs protection from xenobiotic and oxidative injury.[Citation21]
However, this health-enhancing salad is no longer consumed frequently with rice due to the changes in the eating behavior of society, especially due to the influence of Western dietary pattern among younger generation. Hence, it is crucial to find new approaches in consuming Ulam raja in order to ensure that the coming generations will not be missing the beneficial health effects of Ulam raja. In our previous work, the potential of incorporating Ulam raja extract (UREX) into beef patty was examined as a new approach to promote the consumption of this health-enhancing salad and to improve the frozen storage stability of the beef patty as well.[Citation22]
Despite the relatively large number of studies performed on antioxidant activity, only a few of them examined the effect of extraction conditions on the yield and antioxidant compounds of UREX.[Citation23,Citation24] Extraction is the critical first step in the analysis of plants, because it is necessary to extract the chemical components from the plant materials for further separation and characterization.[Citation25] A number of methods have been proposed for the extraction of phenolic compounds from plant materials.[Citation24,Citation25] Water is a non-hazardous and cheap extraction solvent and is widely used to efficiently extract phenolic compounds from plant materials.[Citation1,Citation25–Citation30] Therefore, in the present study we aimed to optimize the aqueous extraction of Ulam raja by using a face-centered composite design-response surface methodology (FCCD-RSM) to investigate the influence of time and temperature on its total phenolic content (TPC) and antioxidant activity. Finally, selected marker compounds of the lyophilized UREX were identified and quantified using high-performance liquid chromatography (HPLC).
Experimental
Materials
DPPH, 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), gallic acid, and sodium carbonate were purchased from Sigma Aldrich Chemicals (St. Louis, MO, USA). Folin–Ciocalteu phenol reagent from R&M Chemicals (Essex, UK), hydrochloric acid (HCl) from Lab Scan Asia Co., Ltd. (Bangkok, Thailand), and glacial acetic acid obtained from QRëC Chemical Co., Ltd. (Chonburi, Thailand). Methanol was purchased from Systerm (Analytical grade, Selangor, Malaysia) and Merck (HPLC grade, Darmstadt, Germany).
Preparation of Dried Ulam raja
Fresh Ulam raja (Cosmos caudatus) leaves were purchased from wet-market in Penang. Ulam raja leaves were washed thoroughly with tap water and visibly damaged leaves were removed before rinsed with deionized water. Then, the leaves were stripped and dried at 45°C for 48 h in a convection dryer (AFOS, Mini Kiln, East Yorkshire, UK), as described by Zin et al.[Citation31] The fully dried leaves were kept in a sealable polyethylene bags and store at 4°C until further study.
Extraction and Measurement of Total Soluble Solids (TSS)
Extraction was conducted according to the method as described by Wong et al., with some modifications.[Citation5] Prior to the extraction, dried Ulam raja leaves were ground into powder using a dry blender (Panasonic, MX335, Malaysia). Thereafter, 3.5 g of the powdered sample was added into 25 mL of deionized water (14% w/v) for extraction. The mixture was incubated in a water bath at 45, 65, or 85°C for 30, 45, or 60 min with occasional shaking. This ratio (14%) was selected based on preliminary trials which led to a TSS as high as that of 25% (w/v) while taking shortest time for the filtration process. A study by Liyana-Pathirana et al. showed that the rate of extraction of thermally stable antioxidants at elevated temperature can be higher than the rate of decomposition of less soluble antioxidants.[Citation32] Various studies showed that phenolic decomposition mostly occur at high temperature, e.g., 100°C or above depending on their structure.[Citation33–Citation37] Thus, the heating temperature was limited to 85°C in order to minimize the detrimental heating effect on TPC.
UREX was obtained by filtering the aqueous mixture through a Büchner funnel (Duran Group, Germany) to remove most of the solid powder and then through Whatman No. 1 filter paper. The yield was expressed as TSS[Citation38,Citation39] based on the degree of brix (°Bx) by using a digital refractometer (Atago 3830/PAL-3, Tokyo, Japan). The more soluble solids extracted, the more yield of extraction would be achieved.
TPC
TPC of UREX was determined using Folin–Ciocalteu method as described by Wong et al.[Citation5] An aliquot (100 µL) of an extract was mixed with 2.5 mL of Folin–Ciocalteu phenol reagent (10× dilution). Mixture was incubated for 5 min at room temperature before adding 2.5 mL of saturated sodium carbonate solution. Mixture was incubated for 1 h at room temperature. Measurement of absorbance was carried out in ultraviolet-visible (UV-Vis) spectrophotometer (Shimadzu, UV-1650 PC, Nakagyo-ku, Japan) at wavelength of 765 nm. Samples were analyzed in triplicates and results were averaged. The TPC of UREX was calculated using gallic acid calibration curve (five different concentrations within the range of 1.7–3.0 mM of gallic acid, RCitation2 ≥ 0.98). TPC was expressed as mg gallic acid equivalents (GAE) per gram of plant material on dry basis (mg GAE/g db).
DPPH Free Radical Scavenging Assay (TEACDPPH)
The DPPH free radical scavenging activity of UREX was determined using UV-1650 PC UV–Vis spectrophotometer according to the method as described by Reihani et al. with slight modifications.[Citation10] Initial absorbance of DPPH solution (0.1 mM) was measured at 515 nm and the absorbance was monitored throughout the period of assay at this wavelength. An aliquot (20 µL) of UREX (with appropriate dilution, if necessary) was added to 1.5 mL of methanolic DPPH solution. The change in absorbance at 515 nm was monitored at 30 min intervals until the reaction curve reaches the plateau. Samples were analyzed in triplicates and results were averaged. The antioxidant activity (TEACDPPH) was expressed as µmol Trolox equivalent per gram of plant material on dry basis (µmol TE/g db).
Design and Statistical Analysis of Experiment
FCCD-RSM (Design expert version 6.0.10 software, Stat-Ease, Inc., Minneapolis, MN, USA), was used to investigate the effect of two independent variables, i.e., extraction temperature (X1) and heating time (X2) on the extraction yield. The levels of each factor were chosen based on preliminary experiment. The FCCD consisted of 4 = (22) factorial points with 4 = 2 × 2 star points, and 5 points at the center. Total number of runs was 13. Experimental data were analyzed in order to fit the following regression model with interaction terms:
where, Y is the response, β0 is the intercept of the model, βi is the linear coefficient, βij is the interaction coefficient between the regression factors and Xi, Xj are regression factors. Analysis of the experimental design data and calculation of predicted responses were carried out using Design Expert software version 6.0 (Stat-Ease).
An experiment with a factorial design of type 2 × 2 was carried out to study the effect of two factors, extraction time (X1) and extraction temperature (X2), on two different responses (TPC and TEACDPPH). Two different extraction times (30 and 60 min) and two different extraction temperatures (65 and 85°C) were selected based on the extraction conditions that provide the top two highest and lowest extraction yield (). Three replicates were tested for each sample. Hence, the total number of sample runs was 12. Analysis of variance (ANOVA) and Duncan’s test for multiple comparisons were used for analyzing the data. SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used to complete the statistical tests.
Table 1. The results of 13-run with observed responses and predicted values for extraction yield.
HPLC Analysis
Preparation of Lyophilized UREX Powder
An extract solution (14% g/mL) obtained from optimized conditions was lyophilized under 40°C temperature and 100 millitorr pressure for 72 h (Millrock Technology, LD53, Kingston, NY, USA) for further storage and analysis. This process was done in order to extend the shelf life of aqueous UREX and ease its storage and use. The lyophilized UREX powder was vacuum packed and stored at −18°C (Toshiba, GR-M48MP, Minato-ku, Japan) until further analysis.
Quantitative Analysis of Antioxidants in Ulam raja by HPLC
UREX sample (10 mg) was dissolved in 1 mL deionized water in a HPLC vial (Agilent, Avondale, PA, USA) and vortexed (Gilson GVLab, Germany) to ensure complete dissolution. The solution was then filtered through a 0.45 μm membrane filter (Whatman, 13 mm GD/X PTFE Filtration Media, Wycombe, UK) prior to HPLC analysis. The chromatographic system was coupled with a computer, a HPLC pump (Waters Corporation, Delta 600 with 600 Controller, Milford, MA, USA) and a photodiode array detector (Waters Corporation, Waters 996, Milford, MA, USA). The sample (10 μL) was injected onto a reverse phase column (Phenomenex, Luna C18 column, 5 μm, 250 mm × 4.6 mm, Torance, CA, USA) and eluted with a mobile phase containing 0.1% aqueous formic acid (Solvent A) and acetonitrile (Solvent B) at a flow rate of 1 mL/min. The eluents were detected by gradient elution () at 280 nm. Stock standard solution (consists of catechin, quercitrin, and rutin) at 1 mg/mL were prepared with deionized water. Working standard solutions of the mixture were prepared by diluting stock standard solution to give final concentrations of 20–140 mg/L (RCitation2 ≥ 0.99) in deionized water. Calibration curves were obtained from the working standard solutions.
Table 2. HPLC gradient program for marker compounds.
Results and Discussion
Yield of Extraction
In order to investigate the optimum conditions for extraction, the effect of two variables (extraction temperature and heating time) on yield (based on TSS) was analyzed by using a two-level factorial design with 13 runs. The ANOVA revealed that the models adequately fitted the experimental data for yield and temperature, time and the interaction terms between temperature and time exhibited strong significant effect (p < 0.05) on yield. This significant interaction implies that variables depended on each other, and variation in response at different levels of a factor was not the same at all levels of another factor. shows the results of FCCD for 13-runs including actual and predicted values of yield in different conditions. The yield of extraction ranged from 3–4.2 °Bx, and the highest yield (4.2 °Bx) was obtained at 85°C/30 min. The data were fitted to a second order polynomial model as given by Eq. (2).
The value of the coefficient of determination was quite high (RCitation2 = 0.83).Thus, the obtained regression model for yield can be considered as satisfactory. In other words, 0.83 of the total variation could be explained by the defined model. The regression coefficient in the fitted model was used to measure the relative contribution of each parameter to the response. The effect of time (X1) and temperature (X2) on yield (Y) is shown in a three dimensional response surface plot (). It is clearly illustrated that by applying higher heating temperature and shorter heating time maximum yield was achieved. In general, regression coefficient with a positive sign in the fitted model implies the ability of the factors to increase the response, while a negative sign implies the ability of the factors to decrease it.[Citation40] Although the effect of different solvent ratio is widely investigated,[Citation1,Citation23,Citation24,Citation41] there are no much studies on the effect of extraction parameters such as time and temperature on Ulam raja. Depending on the plants chemical structure, various extraction parameters exhibit different effects on yield and antioxidant compounds of the extract.
Figure 1. Three-dimensional response surface plot for yield as a function of temperature and heating time of extraction.
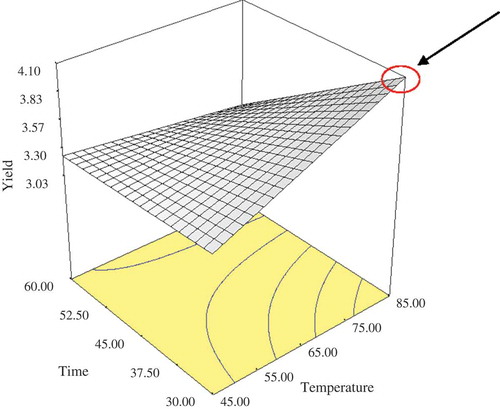
Michiels et al.[Citation42] reported that change in temperature can influence the yield of extraction of some plants (orange, broccoli, apple, and leek). In their study two different non-aqueous solvents (methanol-based and acetone-based) mixtures were used. Nevertheless, by using different solvents no significant impact (p > 0.05) was observed on the quantity of the extracted phenolics (expressed as mg chlorogenic acid equivalents per gram of fresh weight) from orange, leek, and broccoli. On the other hand, an increase in temperature from 25 to 70°C had improved the extraction yield from orange (21% more) but led to a decrease in extraction yield from leek (10% less).
In another study on optimization of extraction of phenolic compounds from wheat, solvent concentration (methanol) and heating temperature significantly affected the response (phenolic compounds) while the extraction time showed no significant contribution to the response.[Citation32] Parameters such as solvent polarity, extraction time, and temperature can affect the extraction efficiency of a compound either independently or interactively.[Citation43] Therefore, depending on the structure and plant matrices various trends might be observed in extraction efficiency, phenolic compounds, and antioxidant activities of plant extracts treated using different extraction methods.
TPC
Results of TPC values of extracts treated under different conditions revealed that neither temperature nor time had significant impact (p > 0.05) on the TPC of the extracts. Moreover, interaction terms between temperature and time did not show any significant influence (p > 0.05) on the TPC of the extracts. The average values of TPC of the extracts treated under different conditions are shown in . A report by Michiels et al.[Citation42] showed that heating time had no effect on the quantity of phenolic compounds extracted from apples and broccoli, while heating temperature had a significant influence (p < 0.05) on the phenolic extractability. In another report, time and interaction between time and temperature were either weak or posed no influence on the quantity of TPC of the extracts, even though temperature had a significant influence (p < 0.05) on the TPC.[Citation44] Gonzalez-Montelongo et al. in their study on the methanolic extracts of banana peel reported that heating time slightly influenced the extraction efficiency of total phenolics of banana peel. Nevertheless, no influence was observed by heating temperature.[Citation45] In another study on optimization of extraction of bio-active compounds from Parkia speciosa, incubation time had a significant impact on TPC.[Citation46] Zhao et al. studied the effect of different extraction conditions on phenolic compounds and antioxidant activity of Pyracantha fortuneana, and indicated that different solvent ratio (60–80% ethanol), time (60–150 min), pH (2–4), and temperature (20–60°C) significantly affected the TPC, antioxidant activity, and chromatogram profiling of the plant extract. They showed that ethanol at 51°C with a pH solution of 3.2 can lead to optimized extraction of the plant.[Citation47]
Table 3. Average values of total phenolic content (TPC) and antioxidant activities (TEACDPPH) of the extracts under different extraction conditions.
As stated earlier, various factors are generally influential on the TPC extraction. In some plants heat treatment might affect the phenolics extractability and, therefore, partial destruction of phenolic compounds can occur.[Citation34–Citation37,Citation48] Solvent polarity and also plant cell wall structure can cause different behavior of the response to time and temperature variables. In this study, extraction conditions had no significant influence (p > 0.05) on the TPC of the extracts. This may be due to the fact that Folin–Ciocalteu reagent detects not only polyphenolic compounds, but also other biological substances which are reactive toward this reagent such as amino acids, carbohydrates, and ascorbic acid.[Citation49–Citation51] Escarpa et al. also reported the poor specificity of this assay.[Citation50]
DPPH Free Radical Scavenging Assay
In this study, DPPH scavenging activity was expressed as a quantity relative to that of Trolox (TEAC) that leads to convenient comparison of antioxidant activity of bio-active compounds from other studies. The stability and ease of handling of DPPH radical enabled its wide use for the determination of free radical scavenging activities of different synthetic and natural antioxidant compounds. Both extraction variables, i.e., temperature and time, had significant (p < 0.05) effect on DPPH scavenging activities of the extracts. However, interaction terms between temperature and time had no significant (p > 0.05) influence on the antioxidant activities of the extracts. The highest DPPH scavenging activity () was shown in extracts obtained from the highest yield (85°C for 30 min). This is in accordance with a report by Michiels et al. which indicated that temperature can affect the yield of extraction and antioxidant activities (DPPH and ORAC) of some fruits and plants.[Citation42]
In another study that was conducted on the effect of extraction parameters on antioxidant activity of Ulam raja, the highest value for total antioxidant compound was observed at 80ºC for 30 min; that is very similar to our results.[Citation52] In addition, the best combination of extraction time and temperature for roasted yerba mate (Ilex paraguariensis) were found to be 10 min at 90°C for highest extraction of antioxidants.[Citation53] Likewise, Chen et al. worked on extraction of a tea carbohydrate that presents a considerable antitumor activity and verified that the best experimental conditions were an extraction temperature of 90°C and extraction time of 30 min.[Citation54] Even though, Spigno et al. reported that time, temperature, and interaction terms between time and temperature had no significant impact on the antioxidant activities of grape stalks.[Citation44]
In spite of yield and DPPH scavenging activity, TPC was not affected by extraction conditions in this study. This implies that no correlation existed between DPPH scavenging and TPC, i.e., p = 0.17, which is more than 0.05. It could be due to the fact that not all phenolic compounds can scavenge DPPH radical possibly due to steric hindrance. Besides, some non-phenolic compounds such as amino acids and ascorbic acid might be present which can react with free radicals.[Citation5,Citation10,Citation49]
Preliminary and Quantitative Analysis of Antioxidants in UREX
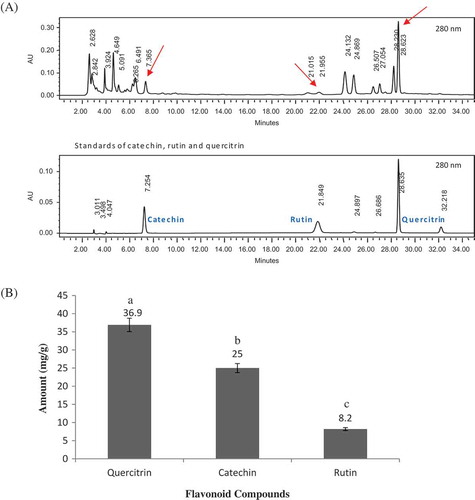
A wide variety of antioxidants in Ulam raja were attributed to a number of proanthocyanidins that existed as dimers through hexamers, quercetin glycosides, chlorogenic, neochlorogenic, cryptochlorogenic acid, catechin, kaempferol, myricetin, luteolin, and apigenin.[Citation1,Citation3] Chromatographic profiles of UREX samples showed the presence of three types of flavonoids, i.e., catechin, quercetin-3-L-rhamnoside (quercitrin), and quercetin-3-rutinoside (rutin; ).
Figure 2. A: HPLC chromatograms of standards versus UREX (at wavelength of 280 nm); B: Quantified constituents of UREX: Average amount (in mg/g of UREX). Error bars indicate mean values ± standard deviation of three replicates. Different letters on top of each bar indicate significant different (p < 0.05) from other bars.
Quercetin, which is from the class of flavonols, is the most abundant flavonoid.[Citation55] It is an aglycon or aglucone by itself without carbohydrate moiety in its structure. However, it is normally found as glycone or carbohydrate conjugate in many plants.[Citation55–Citation57] Quercitrin and rutin are examples of quercetin glycon conjugates that were identified in UREX. A study by Mediani et al. assessed the effect of different drying methods (i.e., freeze, air, and oven drying) on the phytochemicals of UREXs.[Citation12] Their results indicated that extracts from lyophilized Ulam raja had a high amount of flavonoids and flavonoid glycosides than those from air or oven dried Ulam raja. In addition, high amount of chlorogenic acid, α- and β-glucose were identified in lyophilized Ulam raja, but were absent in the extracts from air or oven dried Ulam raja.
The concentration of each flavonoid compounds are illustrated in . As can be seen, quercitrin with an average concentration of 36.90 mg/g of UREX appeared to be the most abundant flavonoid determined by HPLC in this study. In the study by Andarwulan et al. the sum of flavonoid compounds in ethanolic UREXs was reported as 52.2 ± 4.1 mg/100 g of fresh Ulam raja. The quercetin content in the ethanolic UREXs was the highest, i.e., 51.3 ± 4.1 mg/100 g of fresh Ulam raja, followed by kaempferol (0.90 ± 0.04 mg/100 g) and traces (less than 0.02 mg/100 g) of apigenin, luteolin, and myrecitin.[Citation3] Nevertheless, by calculating the amount of sum of the flavonoids[Citation3] in dry basis (~3.72 mg/g dried Ulam raja), it can be seen that the quantity of quercetin rhamnoside or quercitrin in UREX is almost ten times higher, i.e., 36.90 mg/g UREX. This could be simply due to the difference in their process of extracting phenolic compounds. UREX was undergone an extra step of freeze drying of the soluble solids from the aqueous UREX. Onion, apple, red wine, and ginkgo biloba, which reported for being effective on some carcinogenesis markers, contain quercetin and its derivatives.[Citation58,Citation59] Kudolo suggested that as a principal polyphenol of ginkgo biloba, quercetin might be partly responsible for medicinal impact such as decrease in blood pressure.[Citation59] A recent study on separation of quercitrin from Lindera obtusiloba Blume indicated significant effects of quercitrin on antioxidant and anti-melanogenic activities in melanoma cells.[Citation60] It is proposed that UREX might also carry some functions due to its noteworthy content of quercitrin as its marker compound.
Conclusion
Optimized extract of Ulam raja was obtained at 85°C for 30 min with the highest yield and DPPH scavenging activity. However, TPC of the extracts showed no significant difference under applied extraction conditions. Quantification of main marker compounds of optimized lyophilized extract revealed that UREX contains a relatively high amount of quercitrin, catechin, and rutin that are all well known for their antioxidant properties and therapeutic benefits. Also it may provide the initial understanding of the possible dietary intake of these compounds. Nevertheless, more extensive characterization is required for identifying other antioxidant compounds and evaluating their therapeutic potential through in vitro and in vivo studies. Even though, the existence of flavonoid compounds with established health beneficial properties in such a considerable amount in UREX powder is a highlight of this study.
Acknowledgment
The authors gratefully acknowledge the research facilities by Dean of the School of Industrial Technology, Universiti Sains Malaysia, Penang.
Funding
The authors wish to extend their gratitude for the financial assistance from Universiti Sains Malaysia. RUI grant [1001/PTEKIND/815063] is gratefully acknowledged.
Additional information
Funding
References
- Shui, G.H.; Leong, L.P.; Wong, S.P. Rapid Screening and Characterisation of Antioxidants of Cosmos Caudatus Using Liquid Chromatography Coupled with Mass Spectrometry. Journal of Chromatography B 2005, 827, 127–138.
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidative Activities of Water Extracts of Some Malaysian Herbs. ASEAN Food Journal 2007, 14, 61–68.
- Andarwulan, N.; Batari, R.; Sandrasari, D.A.; Bolling, B.; Wijaya, H. Flavonoid Content and Antioxidant Activity of Vegetables from Indonesia. Food Chemistry 2010, 121, 1231–1235.
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidant Activity of Plants Methanolic Extracts Containing Phenolic Compounds. African Journal of Biotechnology 2009, 8, 484–489.
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chemistry 2006, 99, 775–783.
- Noriham, A.; Babji, A.S.; Aminah, A. Determination of Antioxidative Activities of Selected Malaysian Plant Extracts. ASEAN Food Journal 2004, 13, 193–199.
- Zainol, M.K.; Abdul-Hamid, A.; Yusof, S.; Muse, R. Antioxidative Activity and Total Phenolic Compounds of Leaf, Root, and Petiole of Four Accessions of Centella Asiatica (L.) Urban. Food Chemistry 2003, 49, 5165–5170.
- Mohd Zin, Z.; Abdul Hamid, A.; Osman, A. Antioxidative Activity of Extracts from Mengkudu (Morinda Citrifolia L.) Root, Fruit, and Leaf. Food Chemistry 2002, 78, 227–231.
- Ong, H.C.; Norzalina, J. Malay Herbal Medicine in Gemencheh Negeri Sembilan Malaysia. Fitoterapia 1999, 70, 10–14.
- Reihani, S.F.S.; Azhar, M.E. Antioxidant Activity and Total Phenolic Content in Aqueous Extracts of Selected Traditional Malay Salads (Ulam). International Food Research Journal 2012, 19, 1439–1444.
- Rafat, A.; Philip, K.; Muniandy, S. Antioxidant Properties of Indigenous Raw and Fermented Salad Plants. International Journal of Food Properties 2011, 14, 599–608.
- Mediani, A.; Abas, F.; Khatib, A.; Maulidiani, H.; Shaari, K.; Young, H.C.; Lajis, L.H. 1H-NMRH-based Metabolomics Approach to Understanding the Drying Effects on the Phytochemicals in Cosmos Caudatus. Food Research International 2012, 43, 763–770.
- Abas, F.; Shaari, K.; Lajis, N.H.; Israf, D.A.; Kalsom, Y.U. Antioxidative and Radical Scavenging Properties of the Constituents Isolated from Cosmos Caudatus Kunth. Natural Product Sciences 2003, 9, 245–248.
- Nurul, H.; Ruzita, A.; Aronal, A.P. The Antioxidant Effects of Cosmos Caudatus and Polygonum Minus in Refrigerated Duck Meatballs. International Food Research Journal 2010, 17, 893–904.
- Rasdi, N.H.M.; Samah, O.A.; Sule, A.; Ahmed, Q.U. Antimicrobial Studies of Cosmos Caudatus Kunth. (Compositae). Journal of Medicinal Plants Research 2010, 4, 669–673.
- Salehan, N.M.; Meon, S.; Ismail, I.S. Antifungal Activity of Cosmos Caudatus Extracts Against Seven Economically Important Plant. International Journal of Agriculture and Biology 2013, 15, 864–870.
- Bunawan, H.; Baharum, S.N.; Bunawan, S.N.; Amin, N.M.; Noor, N.M. Cosmos Caudatus Kunth: A Traditional Medicinal Herb. Global Journal of Pharmacology 2014, 8, 420–426.
- Loh, S.P.; Hadira, O. In Vitro Inhibitory Potential of Selected Malaysian Plants Against Key Enzymes Involved in Hyperglycemia and Hypertension. Malaysian Journal of Nutrition 2010, 17, 77–86.
- Mohamed, N.; Sahhugi, Z.; Ramli, E.S.M.; Muhammad, N. The Effects of Cosmos Caudatus (Ulam Raja) on Dynamic and Cellular Bone Histomorphometry in Ovariectomized Rats. BioMed Central 2013, 6, 233–239.
- Koleva, I.I.; Niederlander, H.A.G.; Van-Beek, T.A. An On-line HPLC Method for Detection of Radical Scavenging Compounds in Complex Mixtures. Analytical Chemistry 2000, 72, 2323–2328.
- Abdullah, A.; Dhaliwal, K.K.; Roslan, N.N.F.; Lee, C.H.; Kalaiselvam, M.; Radman, H.M.; Mohd Saad, Q.H.; Yusof, K.; Jaarin, K. The Effects of Cosmos Caudatus (Ulam Raja) on Detoxifying Enzymes in Extrahepatic Organs in Mice. Journal of Applied Pharmaceutical Science 2015, 5, 82–88.
- Reihani, S.F.S.; Tan, T.C.; Huda, N.; Easa, A.M. Frozen Storage Stability of Beef Patties Incorporated with Extracts from Ulam Raja Leaves (Cosmos Caudatus). Food Chemistry 2014, 155, 17–23.
- Mediani, A.; Abas, F.; Khatib, A.; Tan, C.H. Cosmos Caudatus as a Potential Source of Polyphenolic Compounds: Optimisation of Oven Drying Conditions and Characterisation of Its Functional Properties. Molecules 2013, 18, 10452–10464.
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seo, E.M. Effect of Solvents in Extracting Polyphenols and Antioxidants of Selected Raw Vegetables. Journal of Food Composition and Analysis 2011, 24, 506–515.
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, Isolation, and Characterization of Bioactive Compounds from Plants’ Extracts. The African Journal of Traditional, Complementary, and Alternative Medicines 2011, 8, 1–10.
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the Aqueous Extraction of Phenolic Compounds from Olive Leaves. Antioxidants 2014, 3, 700–712.
- Busnena, B.A.; Foudah, A.I.; Melancon, T.; El Sayed, K.A. Olive Secoiridoids and Semi Synthetic Bioisostere Analogues for the Control of Metastatic Breast Cancer. Bioorganic & Medicinal Chemistry 2013, 21, 2117–2127.
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B., III; Gualillo, O. Oleocanthal Inhibits Proliferation and MIP-1α Expression in Human Multiple Myeloma Cells. Current Medicinal Chemistry 2013, 20, 2467–2475.
- Obied, H.K.; Bedgood, D.R. Jr; Prenzler, P.D.; Robards, K. Bioscreening of Australian Olive Mill Waste Extracts: Biophenol Content, Antioxidant, Antimicrobial, and Molluscicidal Activities. Food and Chemical Toxicology 2007, 45, 1238–1248.
- Lima, C.F.; Valentao, P.C.R.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Water and Methanolic Extracts of Salvia Officinalis Protect HepG2 Cells from t-BHP Induced Oxidative Damage. Chemico-Biological Interactions 2007, 167, 107–115.
- Zin, Z.M.; Abdul Hamid, A.; Osman, A. Antioxidative Activity of Extracts from Mengkudu (Morinda Citrifolia L.) Root, Fruit, and Leaf. Food Chemistry 2002, 78, 227–231.
- Liyana-Pathirana, C.; Shahidi, F. Optimization of Extraction of Phenolic Compounds from Wheat Using Response Surface Methodology Food Chemistry 2005, 93, 47–56.
- Cheng, Y.; Xu, Q.; Liu, J.; Zhao, C.; Xue, F.; Zhao, Y. Decomposition of Five Phenolic Compounds in High Temperature Water. Journal of the Brazilian Chemical Society 2014, 25, 2102–2107.
- Pietrzak, W.; Nowak, R.; Olech, M. Effect of Extraction Method on Phenolic Content and Antioxidant Activity of Mistletoe Extracts from Viscum Album Subsp. Abietis. Chemical Papers 2014, 68, 976–982.
- Rebolva, Z. Effect of Temperature on the Antioxidant Activity of Phenolic Acids. Czech Journal of Food Science 2012, 30, 171–177.
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods 2014, 3, 66–81.
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. Journal of Agricultural and Food Chemistry 2007, 55, 330–335.
- Carvalho, G.B.M.; Silva, D.P.; Santos, J.C.; Filho, H.J.I.; Vicente, A.A.; Teixeira, J.A.; Felipe, M.d.G.A.; Silva, J.B.A. Total Soluble Solids from Banana: Evaluation and Optimization of Extraction Parameters. Applied Biochemistry and Biotechnology 2009, 153, 34–43.
- Endress, H.-U.; Mattes, F.; Norz, K. Pectins. In: Handbook of Food Science, Technology and Engineering, Hui, Y.H.; Eds.; CRC Press: Boca Raton, FL, 2006; 16.
- Wong, W.W.; Alkarkhi, A.F.M.; Easa, A.M. Effect of Extraction Conditions on Yield and Degree of Esterification of Durian Rind Pectin: An Experimental Design. Food and Bioproducts Processing 2010, 88, 209–214.
- Lee, T.K.; Vairappan, C.S. Antioxidant, Antibacterial, and Cytotoxic Activities of Essential Oils and Ethanol Extracts of Selected South East Asian Herbs. Journal of Medicinal Plant Research 2011, 5, 5284–5290.
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction Conditions Can Greatly Influence Antioxidant Capacity Assays in Plant Food Matrices. Food Chemistry 2012, 130, 986–993.
- Montgomery, D.C. Design and Analysis of Experiments; Wiley: New York, NY, 2001; 27–120.
- Spigno, G.; De Faveri, D.M. Antioxidants from Grape Stalks and Marc: Influence of Extraction Procedure on Yield, Purity, and Antioxidant Power of the Extracts. Journal of Food Engineering 2007, 78, 793–801.
- Gonzalez-Montelongo, R.; Lobo, M.G.; Gonzalez, M. The Effect of Extraction Temperature, Time, and Number of Steps on the Antioxidant Capacity of Methanolic Banana Peel Extracts. Search Results Separation and Purification Technology 2010, 71, 347–355.
- Gan, C.-Y.; Latiff, A.A. Optimisation of the Solvent Extraction of Bioactive Compounds from Parkia Speciosa Pod Using Response Surface Methodology. Food Chemistry 2011, 124, 1277–1283.
- Zhao, C.H.; Li, S.; Li, S.J.; Song, G.H.; Yu, L.J.; Zhang, H. Extraction Optimization Approach to Improve Accessibility of Functional Fraction Based on Combination of Total Polyphenol, Chromatographic Profiling, and Antioxidant Activity Evaluation: Pyracantha Fortuneana Fruit as an Example. Journal of Functional Foods 2013, 5, 715–728.
- Choi, Y.; Lee, S.M.; Chun, J.; Lee H.B.; Lee, J. Influence of Heat Treatment on the Antioxidant Activities and Polyphenolic Compounds of Shiitake (Lentinus Edodes) Mushroom. Food Chemistry 2006, 99, 381–387.
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; Ric De Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, Phenolic Compounds, and Nutritional Quality of Different Strawberry Genotypes. Journal of Agricultural and Food Chemistry 2008, 56, 696–704.
- Escarpa, A.; Gonzalez, M.C. Approach to the Content of Total Extractable Phenolic Compounds from Different Food Samples by Comparison of Chromatographic and Spectrophotometric Methods. Analytica Chimica Acta 2001, 427, 119–127.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin–Ciocalteu Reagent. Methods in Enzymology 1999, 299, 152–178.
- Mohd Nasir, B. The Effect of Extraction Parameters on Antioxidant Activity of Cosmos Caudatus (B.S. thesis in Chemical Engineering) Universiti Malaysia Pahang, Malaysia, 2011.
- Bassani, D.C.; Nunes, D.S.; Granato, D. Optimization of Phenolics and Flavonoids Extraction Conditions and Antioxidant Activity of Roasted Yerba-Mate Leaves (Ilex Paraguariensis A. St.-Hil., Aquifoliaceae) Using Response Surface Methodology. Anais da Academia Brasileira de Ciências [Annals of the Brazilian Academy of Sciences] 2014, 86, 923–933.
- Chen, B.; Zhou, W.; Ning, M.; Wang, Z.; Zou, W.; Zhang, H.; Wang, Q. Evaluation of Antitumour Activity of Tea Carbohydrate Polymers in Hepatocellular Carcinoma Animals. International Journal of Biological Macromolecules 2012, 50, 1103–1108.
- Moskuag, J.O.; Carlson, H.; Myhrstad, M. Molecular Imaging of the Biological Effects of Quercetin and Quercetin-Rich Foods. Mechanisms of Ageing and Development 2004, 125, 315–324.
- Silva, S.F.; Blank, D.E.; Peixoto, C.R.; Moreira, J.J.S.; Moura, N.F. Bioactive Compounds and Antioxidant Activity of Bunchosia Glandulifera. International Journal of Food Properties 2016, 19, 467–473.
- Lee, Y.H.; Choo, C.; Waisundara, V.Y. Determination of the Total Antioxidant Capacity and Quantification of Phenolic Compounds of Different Solvent Extracts of Black Mustard Seeds (Brassica Nigra). International Journal of Food Properties 2015, 18, 2500–2507.
- Kim, H.Y.; Kim, O.H.; Sung, M.K. Effects of Phenol-Depleted and Phenol-Rich Diets on Blood Markers of Oxidative Stress, and Urinary Excretion of Quercetin and Kaempferol in Healthy Volunteers. The Journal of the American College of Nutrition 2003, 22, 217–223.
- Kudolo, G.B. The Effect of 3-Month Ingestion of Ginkgo Biloba Extract (EGb 761) on Pancreatic Beta-Cell Function in Response to Glucose Loading in Individuals with Non-Insulin-Dependent Diabetes Mellitus. The Journal of Clinical Pharmacology 2001, 41, 600–611.
- Hong, C.O.; Lee, H.A.; Rhee, C.H.; Choung, S.Y.; Lee, K.W. Separation of the Antioxidant Compound Quercitrin from Lindera Obtusiloba Blume and Its Antimelanogenic Effect on B16F10 Melanoma Cells. Bioscience, Biotechnology, and Biochemistry 2013, 77, 58–64.
