ABSTRACT
This article reports the rapid screening and identification of the triacylglycerol content of shea butter fat, palm kernel oil, and peanut oil sold in the local Ghanaian market for their characterization and identification. Samples were dissolved in chloroform with 2,5-dihydroxybenzoic acid as the matrix. After subjecting the samples to matrix-assisted laser desorption ionization-time-of-flight/mass spectrometry, the spectra obtained showed the characteristic triacylglycerols as sodium adducts. Seven major triacylglycerol species were identified as dipalmitoyl olein, palmitoyl diolein, palmitoyl stearoyl olein, linoleoyl diolein, triolein, stearoyl diolein, and distearoyl olein in all three samples. Palmitoyl linoleoyl olein and tristearin were also identified. Oxygenated triacylglycerols and other species from the fragmentation of triacylglycerols were also obtained. The presence of the oxygenated triacylglycerols and the triacylglycerol fragments may be a result of poor handling and production processes.
Introduction
Vegetable oils continue to be in high demand because they are preferred as healthy diets as well as raw materials for industrial products such as cosmetics and biofuel.[Citation1,Citation2] There are many tropical plant species with high yields of fat and oils. Some of the commonly used ones available in the African markets are, shea nut (Vitellaria paradoxa), palm kernel (Elaeis guineensis), and peanut (Arachis hypogaea) plants from whose seeds the fats and oils are extracted. The fat from the shea nuts, palm kernel, and peanut have been used significantly in applications such as soaps and detergents production, cosmetics, and illuminants for the local industries in the tropical regions of Africa. They have also been used widely in many food products.[Citation3–Citation6] Due to their unique properties and economic importance they are perceived to play a significant role in poverty alleviation and food security in tropical Africa, as well as valued export commodities.[Citation7,Citation8] However, one formidable challenge that is mitigating the complete utilization of the vegetable oils is the incidence of adultration.[Citation9] The major chemical constituents (97–98%) of fats and oils are the triacylglycerols (TAGs). The TAGs are made up of fatty acids esterified on a glycerol backbone.[Citation10] The unique physiological and physico-chemical properties of any vegetable fats and oils depend on the fatty acid composition of their TAG structure.[Citation11,Citation12] According to Sanders the consumption of fats and oils have great influence on the human physiology, such as lipid metabolism; depending on the fatty acid composition, it causes the development of chronic diseases or general well-being of the human body.[Citation13] However, the method of extraction of the oil, geographical location and associated climatic conditions of the origin of the plant influences its physico-chemical and physiological properties.[Citation14,Citation15]
It is, therefore, crucial to determine the TAGs in vegetable fats and oils to assess its purity before a choice of utilization can be made. This will ensure whether it is fit for food, industrial applications or for export. For this reason different analytical methods have been used widely for the determination of intact TAGs within the vegetable fats and oils.[Citation16,Citation17] Recently, the application of soft ionization mass spectrometry technique, mainly the matrix-assisted laser desorption ionization-time-of-flight/mass spectrometry (MALDI-TOF/MS), has been found to be a powerful and important tool for fast and accurate method of analysis of TAGs.[Citation18–Citation20]
The significance of using the MALDI-TOF/MS in TAG analysis is that the technique has the ability to elucidate complex structures with little or no analyte fragmentation. Again, fat and oil analysis in both the sample and the matrix used are soluble in organic solvents, thereby forming a homogeneous matrix/analyte mixture to yield good experimental data. In addition, the use of the MALDI-TOF/MS in the TAG analysis does not require any prior chromatographic separations or derivatization, and it gives results in a short time.[Citation18,Citation21] The main aim of this work was to determine the TAG profile of shea butter fat, peanut oil, and palm kernel oil to assess their purity. The study seeks to characterize the TAGs for rapid identification and assessment of the fat and oils. This application will hopefully contribute to choosing the right fat and oils for their purpose.
Materials and Methods
Samples
To extract the oils, usually the producers collect raw nuts, roast, mill, mix with hot water and boil until the oil floats, after which the oil is skimmed. However, the oils and fat samples used in this study were purchased one month after production and stored in glass bottles as –20°C until required for analysis. One liter of refined vegetable oils of palm kernel and peanut as well as ten g of shea butter fat were obtained from a local market in Kumasi (Ghana). All reagents or solvents used were of analytical high-performance liquid chromatography (HPLC) grade and were purchased from Sigma-Aldrich, Michigan, USA.
Sample Preparation
Extraction of the TAGs was done according to the method described by Kaufmann and Wiesman with slight modification.[Citation18] The TAGs from the samples were extracted by dissolving each of the fat and oils in chloroform. Sodium chloride was added to the chloroform solution as the cationization agent prior to subjecting the sample to Maldi-TofMS analysis. This is because the Maldi-Tof typically produces a spectrum of TAG molecules mainly as their metal adduct. 2,5-dihydroxybenzoic acid (DHB) was also added to the working solution as a matrix for high reproducibility and sensitivity. The working solution was prepared by dissolving 10 mg of oil samples in 1 mL of CHCl3 (10 mg/mL concentration). From the stock solution a concentration of 10 uL/mL was prepared and the matrix solution prepared by dissolving 10 mg of crystalline DHB in 1 mL of methanol containing 0.05% trifluoroacetic acid (TFA). The CHCl3 sample solution was then vigorously shaken with 1M NaCl and the aqueous layer removed. For the MALDI-TOF/MS analysis, an aliquot of the CHCl3 layer (10uL) was mixed with the matrix (1:1 v/v) and 1uL of the resulting solution deposited directly onto the sample plate. This was air-dried and following which the MALDI-TOF/MS spectra was obtained.
Structural Analysis of TAGs
The acquisition was done on the Applied Biosystems 4800 Maldi-Tof/Tof Analyzer (MDs Sciex, Toronto Canada Div. of MDs INC). A Maldi mass spectrum in the range of m/z 600–2000 of the characteristic TAG in the various samples was acquired in the reflectron positive mode at 4400 V. The lowest interfering fragments were achieved by averaging 50 shots in 10 different sub-spectra. The extraction delay was optimized to 400 ns to provide a resolving power (full width at half minimum: FWHM) of approximately 50,000 for TAG peaks in MS/MS mode. Helium collision gas was introduced to attenuate the precursor ion abundance to approximately 50% of the initial value during the acquisition of the product-ion mass spectrum. The laser was operated at a repetition rate of 1000 Hz and the spectra acquired at a rate of two spectra per second. In all 500 spectra were accumulated for each product-ion mass spectrum. Each sample was analyzed in three independent replicates to check repeatability. For the external mass calibration, a solution of the standard TAG mixture was prepared from 10 mg each of six TAG standards namely, triolein (OOO; molecular weight 885.40), palmitoyl dilinolein (molecular weight 855.40), trilinolein (molecular weight 879.38), palmitoyl diolein (OOP; molecular weight 859.39), stearoyl diolein (OOS; molecular weight 887.40) and oleoyl dilinolein (molecular weight 881.40) and a solution of 10 uL/mL prepared and used as the standard. Three spots were put on the sample plate for a separate acquisition to obtain the calibration parameters.
Results and Discussions
TAG composition
The TAG region from the MALDI-TOF/MS spectra of the peanut oil, palm kernel oil, and shea butter fat gave a mass profile representing the TAG and other chemical constituents in the fat and oils in a clear and stepwise manner . The TAGs were identified on the basis of calculation of molecular masses after the necessary correction of the background and that of any isotopic contributions. Data obtained from the spectra are summarized in . All TAGs obtained appeared in the spectrum as their corresponding sodium adduct described by Pittenauer and Allmaier.[Citation20] From the fatty acids constituents of samples, the specific compositions of each TAG were identified as described by Vichi and co-workers.[Citation22] Seven major TAG species were identified as dipalmitoyl olein (POP), OOP, palmitoyl stearoyl olein (POS), linoleoyl dilein (OOL), OOO, OOS, and distearoyl olein (SOS) in all three samples. Palmitoyl linoleoyl olein (PLO) and tristearin (SSS) were also identified.
Table 1. Structural assignment of the mass (m/z) in the oil samples under the MALDI-TOF MS spectra and their corresponding carbon number/double bond ratios.
Figure 1. MALDI-TOF MS TAG profile spectrum of A: peanut oils; B: shea butter; and C: palm kernel oil.
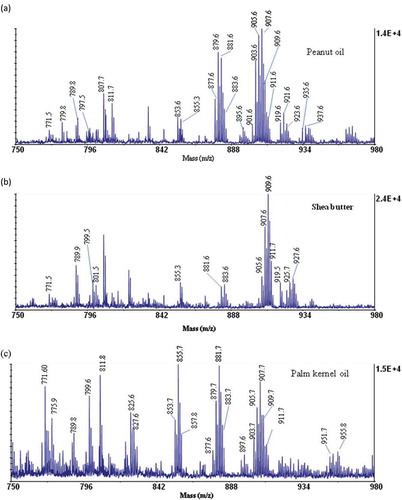
The spectrum obtained for peanut oil () gave a peaks C54:3 (at m/z 907.9), C54:4 (at m/z 905.6) and C52:3 at (at m/z 879.6) representing OOO, OOL, and PLO, respectively. This observation may be a results of the high presence of oleic acid, linoleic acid, and palmitic acid in peanut oils as reported by Chari et al.[Citation23] Shea butter has been reported to generally contain about 45% oleic acid and 42% stearic acid of the total fatty acid constituents.[Citation24] From this study () shea butter gave TAG peaks C54:1 (at m/z 911.7), C54:2 (at m/z 909.6), and C54:0 (at m/z 913.7) corresponding to SOS, OOS, and SSS, respectively. The palm kernel oil () gave C50:1 (at m/z 855.7), C52:2 (at m/z 881.7), and C54:3 (at m/z 907.7) which represents POP, OOP, and OOO, respectively. The TAG composition obtained is accounted for by the high presence of oleic acid and palmitic acid in the palm kernel oils.[Citation25]
Apart from the regular TAG constituents, several oxygenated TAG species were detected within the mass range of the study. These species were obtained as mono-oxygenated groups with their masses at m/z 897.6, 919.6, 927.6, etc., di-oxygenated groups at m/z 933.6, 935.7, and tri-oxygenated groups at m/z 951.8, 955.8. The presence of oxygenated TAG species can be attributed to the susceptibility to attack on unsaturated fatty acids such as linoleic acid and oleic acid by activated oxygen.[Citation21,Citation22] In the presence of activated oxygen, the unsaturated fatty acids, due to the presence of the high electron density at the point of unsaturation tends to have a high affinity for the oxygen, which leads to their oxidation.[Citation28,Citation29] The activated oxygen is likely to be produced through thermal processes during the production and refining of the oils. Again poor handling and poor storage practices at both the production and on the market place may also contribute to oxygenation. It is observed that oxygenated species at progressive increment of 16 Da (at m/z 919.7, 935.7, 951.7) may represent mono-, di-, and tri-oxygenated LLO species and this may be due to the availability of high electron density in such TAG molecules. These species were mostly found in the peanut oil, which contains high amounts of unsaturated fatty acids (oleic and linoleic acid) in their TAG structure. A good number of the oxygenated species were also observed in both the palm kernel oil and the shea butter fat (). The presence of oxygenated TAG compounds in the vegetable oils has also been detected from a MALDI-TOF/MS analysis by other researchers. The presence of 16 Da mass in a MALDI-TOF/MS spectra have thus, been used as a diagnostic method for the presence of an additional oxygen atom in one of the fatty acyl groups of the TAG molecule.[Citation19,Citation22]
Additionally, at low mass regions (m/z 771.4 – 827.6), signals of species corresponding to oxidative cleavage of unsaturated fatty acids were observed. The species obtained may be formed as a result of a homolytic β-scission. The cleavage is more prominent in oleic and linoleic fatty acid constituents in the palm kernel oil. In the palm kernel oil, the homolytic β-scission products may have been generated during production, which is a two-step thermal process, which may lead to the generation of hydroperoxides.[Citation30,Citation31] Once these species are formed they undergo the cleavage, thereby generating short-chain glycerol-bound compounds and aliphatic hydrocarbons.[Citation19,Citation32] Product ions form such cleavage may be harmful to human health upon consumption.[Citation33] Picariello and co-workers[Citation19] reported the homolytic β-scission products in their study of MALDI-TOF/MS profiling of polar and nonpolar fractions in heated vegetable oils. They found that the presence of the homolytic β-scission products is due to thermal stress.
TAG Fragmentations by the MALDI-TOF/TOF-MS
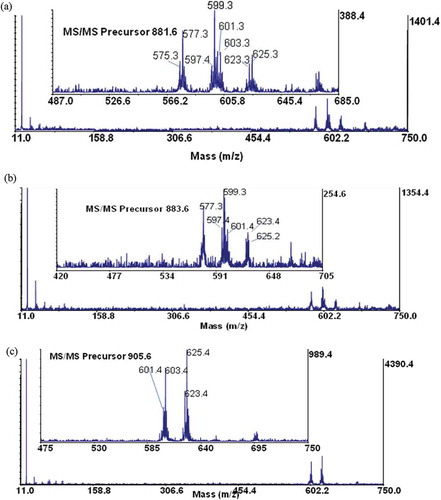
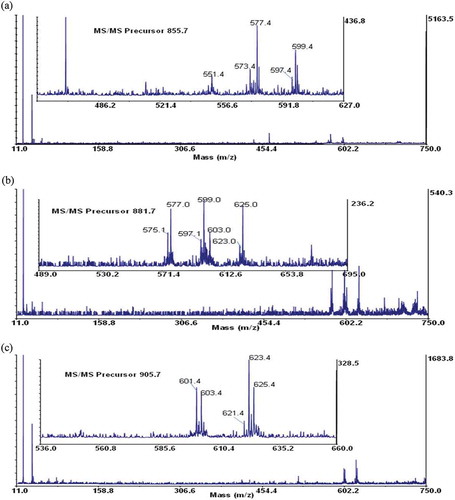
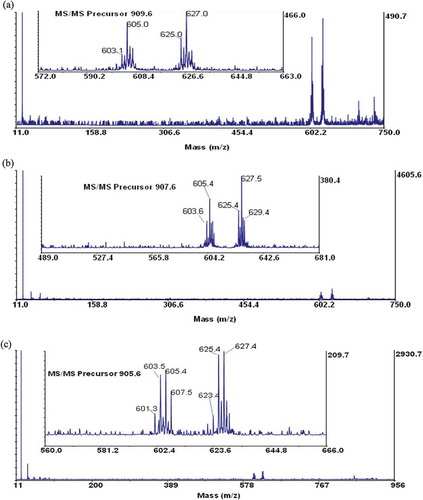
Maldi-TOF/TOF-MS analysis was performed for further confirmation on the structural composition of the fatty acids present in the samples. Three most abundant TAGs were selected from each sample. A special focus was based on the ions detected at the mid mass range of the collision-induced dissociation (CID) spectrum, which predominantly showed the loss of a free fatty acid and a fatty acid sodium carboxylate as indicated by other researchers.[Citation20,Citation34,Citation35] The fragmented TAGs showed a mechanistic path as indicated in . These species give the structural identities of the fatty acid composition of the intact TAG molecule and may be used to confirm the number of atoms in the carbon chain and the unsaturated or saturated bonds present in each fatty acid. From the TOF/TOF spectrum, the peak at m/z 909.7 for shea butter, gave fragment ions at m/z 625.0 and 627.0 (). These represent residue ions after the loss of a free stearic and oleic acid from the TAG molecule and species at m/z 603.1 and 605.0 shows the loss of a sodium stearoyl and sodium oleoyl carboxylate residues, respectively. This pattern of fragmentation shows the strong presence of stearic and oleic acids in the TAG structure. Details of the various product ions are shown in . Considering the spectrum from the peanut oil, the peak at m/z 905.6 gave fragmentations at m/z 601.4, 603.4, 621.3, 623.4, and 625.4 (). The species indicate the possible loss of one free oleic or/and linoleic acid, which accounts for product ions at m/z 623.4 and 625.4, respectively. Product ions at m/z 601.4 and 603.4 indicates the loss of sodium oleoyl and linoleoyl carboxylate residues, respectively, for the TAG structure. Additionally, considering the spectrum of palm kernel oil, the peak at m/z 855.7 gave fragmentations at m/z 573.4, 599.4, 577.4, and 551.4 (). This fragmentation pattern shows the loss of a free oleic and palmitic acid from the TAG structure (at m/z, 573.4 and 599.4, respectively) and the loss of one sodium palmitoyl and sodium oleoyl carboxylate (at m/z 577.4 and 551.4, respectively). Data obtained from the TOF/TOF fragmentation gives information for profiling the TAG composition. This gives a better understanding to the metabolic and physiological effects of the fats and oils.[Citation18]
Table 2. Selected m/z values of [M +Na]+ adduct ions of triacylglycerols and the structurally diagnostic product ions, showing the B- and C-type product ions.
Figure 2. Schematic representation of the dissociation pathways for the sodiated triacylglycerols considered in the study using a high-energy CID.
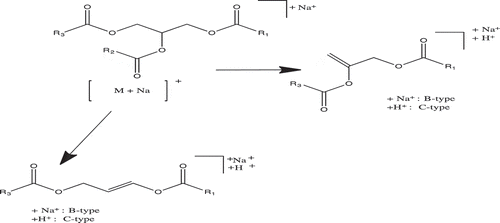
Figure 3. TAG fragments of shea butter, A: Dioleoyl stearoyl glycerol (at m/z 909.6); B: Trioleoyl glycerol (at m/z 907.6); and C: Dioleoyl linoleoyl glycerol (at m/z 905.6).
Conclusions
The structural composition of the TAGs of the shea butter fat, peanut oil, and the palm kernel oil provides important information about their identity and characteristic profile. Such information can be used to estimate the purity and characteristics of the vegetable oils and the fat, which is crucial, especially for their use as food. From the results obtained, the differences in TAG profiles in the shea butter fat, peanut oil, and palm kernel oil sold on the market were clearly determined. The presence of other species such as oxygenated TAGs and TAG fragments was found in the results. The presence of these species may be a result of the poor handling and production processes of the fat and oils. Since there is evidence on the effects of composition and structure on lipid metabolism and their associated risk of coronary heart disease, it is important for fat and oil producers and retailers to adopt proper production practices, such as maintaining good temperature conditions as well as storing the products under good conditions.
Acknowledgments
All experiments performed at the Philip C. Andrews Lab, Department of Biological Chemistry, and University of Michigan, the University of Michigan African Presidential Scholars (UMAPS) program.
References
- Murphy, J.D. The Status of Industrial Vegetable Oils from Genetically Modified Plants; European Chemicals Agency, University of Glamorgan: Pontypridd, UK, 2012; 5–10.
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil Content and Fatty Acids Composition in Brassica Species. International Journal of Food Properties 2015, 18, 2145–2154.
- Honfo, F.G.; Akissoe, N.; Linnem Ann, A.R.; Soum Anou, M.; Van Boekel, M.A.J.S.; Nutritional Composition of Shea Products and Chemical Properties of Shea Butter: A Review. Critical Review in Food Science and Nutrition 2014, 54, 673–686.
- Lovett, P. The Shea Butter Value Chain: Production, Transformation, and Marketing in West Africa; USAID West Africa Trade and Investment Hub: Accra, Ghana, 2004; 1–40.
- Ofosu-Budu, K.; Sarpong, D. Oil Palm Industry Growth in Africa: A Value Chain and Smallholders’ Study for Ghana. In Rebuilding West Africa’s Food Potential, Elbehri, A.; Ed; FAO/IFAD: Rome, Italy, 2013; 349–389.
- Shumaker, G.; McKissick, J.; Smith, N. Economics of Peanuts for Biodiesel Production; Center for Agribusiness and Economics Development, University of Georgia: Atlanta, GA, 2007; 1–5.
- Kavaarpuo, A. Development Implementations of the Shea Industry as a Lead for Northern Ghana: Case Studies in Bole, Wa-West, and Bongo; Kwame Nkrumah University of Science and Technology: Kumasi, Ghana, 2010; 33–36.
- Li, M.; Baughman, E.; Roth, M.R.; Han, X.; Welti, R.; Wang, X. Quantitative Profiling and Pattern Analysis of Triacylglycerol Species in Arabidopsis Seeds by Electrospray Ionization Mass Spectrometry. Plant Journal 2014, 77, 160–172.
- Che Man, Y.B.; Marina, A.M.; Rohman, A.; Al-Kahtani, H.A.; Norazura, O. A Fourier Transform Infrared Spectroscopy Method for Analysis of Palm Oil Adulterated with Lard in Pre-Fried French Fries. International Journal of Food Properties 2013, 17, 354–362.
- Martini, S.; Herrera, M.L.; Hartel, R.W. Effect of Cooling Rate on Crystallization Behavior of Milk Fat Fraction/Sunflower Oil Blends. Journal of American Oil Chemists Society 2002, 79, 1055–1062.
- Obeidat, S.; Khanfar, M.; Obeidat, W. Classification of Edible Oils and Uncovering Adulteration of Virgin Olive Oil Using FTIR with the Aid of Chemometrics. Australian Journal of Basic and Applied Sciences 2009, 3, 2048–2053.
- Abu-Taha, M.; Halasa, M.; Abu-Samreh, M. On the Usage of the Faraday Effect as an Authentication Technique for Vegetable Oils. Journal of Modern Physics 2013, 4, 230–235.
- Sanders, T.A. Polyunsaturated Fatty Acids in the Food Chain in Europe. American Journal of Clinical Nutrition 2000, 71(1), 176S–178S.
- Cozzolino, R.; De Giulio, B. Application of ESI and MALDI-TOF MS for Triacylglycerols Analysis in Edible Oils. European Journal of Lipid Science and Technology 2011, 113, 160–167.
- Marangoni, A.G.; Acevedo, N.; Maleky, F.; Co, E.; Peyronel, F.; Mazzanti, G.; Quinn, B.; Pink, D. Structure and Functionality of Edible Fats. Soft Matter 2012, 8, 1275–1300.
- Fadzlillah, N.A.; Che Man, Y.B.; Rohman, A. FTIR Spectroscopy Combined with Chemometric for Analysis of Sesame Oil Adulterated with Corn Oil. International Journal Food Properties 2014, 17, 1275–1282.
- Kelebek, H.; Kesen, S.; Selli, S. Comparative Study of Bioactive Constituents in Turkish Olive Oils by LC-ESI/MS/MS. International Journal of Food Properties 2015, 18, 2231–2245.
- Kaufman, M.; Wiesman, Z. Pomegranate Oil Analysis with Emphasis on MALDI-TOF/MS Triacylglycerol Fingerprinting. Journal of Agricultural and Food Chemistry 2007, 55, 10405–10413.
- Picariello, G.; Paduano, A.; Sacchi, R.; Addeo, F. Maldi-tof Mass Spectrometry Profiling of Polar and Nonpolar Fractions in Heated Vegetable Oils. Journal of Agricultural and Food Chemstry 2009, 57, 5391–5400.
- Pittenauer, E.; Allmaier, G. The Renaissance of High-Energy CID for Structural Elucidation of Complex Lipids: MALDI-TOF/RTOF-MS of Alkali Cationized Triacylglycerols. Journal American Society Mass Spectrometry 2009, 20, 1037–1047.
- Chapagain, B.P.; Wiesman, Z. MALDI-TOF/MS Fingerprinting of Triacylglycerols (TAGs) in Olive Oils Produced in the Israeli Negev Desert. Journal of Agricultural and Food Chemistry 2009, 57, 1135–1142.
- Vichi, S.; Lazzez, A.; Grati-Kamoun, N.; Caixach, J. Modifications in Virgin Olive Oil Glycerolipid Fingerprint During Olive Ripening by MALDI-TOF MS Analysis. LWT–Food Science and Technology 2012, 48, 24–29.
- Chari, V.; Ronald, A.; Christopher, L.; William, R. Determination of in-Shell Peanut Oil and Fatty Acid Composition Using Near-Infrared Reflectance Spectroscopy. Journal of American Oil Chemists Society 2010, 87, 1103–1114.
- Adubofuor, J.; Sefah, W.; Oldham, J.H. Nutrient Composition of Allanblackia Paviflora Seed Kernels and Oil Compared with some Plant Fats and Oils and Application of the Oil in Soap Preparation. Journal of Cereal Oil Seeds 2013, 4, 1–9.
- Benjapornkulaphong, S.; Ngamcharussrivichai, C.; Bunyakiat, K. Al2O3-Supported Alkali and Alkali Earth Metal Oxides for Transesterification of Palm Kernel Oil and Coconut Oil. Chemical Engineering Journal 2009, 145, 468–474.
- Ayyildiz, H.F.; Topkafa, M.; Kara, H.; Sherazi, S.T.H. Evaluation of Fatty Acid Composition, Tocols Profile, and Oxidative Stability of Some Fully Refined Edible Oils. International Journal Food Properties 2015, 18, 2064–2076.
- Mohamed, K.M.; Elsanhoty, R.M.; Hassanien, M.F.R. Improving Thermal Stability of High Linoleic Corn Oil by Blending with Black Cumin and Coriander Oils. International Journal of Food Properties 2013, 17, 500–510.
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial Effects and Oxidative Stability of Omega-3 Long-Chain Polyunsaturated Fatty Acids. Trends in Food Science and Technology 2012, 25, 24–33.
- Makni, M.; Haddar, A.; Fraj, A.B.; Zeghal, N. Physico-Chemical Properties, Composition, and Oxidative Stability of Olive and Soybean Oils Under Different Conditions. International Journal of Food Properties 2014, 18, 194–204.
- Berdeaux, O.; Velasco, J.; Márquez-Ruiz, G.; Dobarganes, C. Evolution of Short-Chain Glycerol-bound Compounds During Thermoxidation of FAME and Monoacid TAG. Journal of American Oil Chemists Society 2002, 79, 279–285.
- Márquez‐Ruiz, G.; Dobarganes, C. Short‐Chain Fatty Acid Formation During Thermoxidation and Frying. Journal of the Science of Food Agriculture 1996, 70, 120–126.
- Sjövali, O.; Kuksis, A.; Kallio, H. Formation of Triacylglycerol Core Aldehydes During Rapid Oxidation of Corn and Sunflower Oils with Tert-butyl Hydroperoxide/Fe2+. Lipids 2002, 37, 81–94.
- Kubow, S.; Routes of Formation and Toxic Consequences of Lipid Oxidation Products in Foods. Free Radical Biology and Medine 1992, 12, 63–81.
- Asbury, G.R.; Al-saad, K.; Siems, W.F.; Hannan, R.M.; Hill, H.H. Analysis of Triacylglycerols and Whole Oils by Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry. Journal of American Society of Mass Spectrometry 1999, 10, 983–991.
- Kubo, A.; Satoh, T.; Itoh, Y.; Hashimoto, M.; Tamura, J.; Cody, R.B. Structural Analysis of Triacylglycerols by Using a MALDI-TOF/TOF System with Monoisotopic Precursor Selection. Journal of American Society Mass Spectrometry 2013, 24, 684–689.