ABSTRACT
A study was conducted to investigate the distribution of volatile organic compounds in the juice and seed of the most popular five pomegranate cultivars (“Ekşi,” “Devedişi,” “Hicaz,” “Katırbaşı,” and “Keben”) in Turkey. The volatile organic compounds were analyzed using solid phase micro-extraction and gas chromatography–mass spectrometry. A total of 60 volatile organic compounds, belonging to six chemical groups including aldehydes, alcohols, esters, terpenes, ketones, acids, and phenol were identified. There were 11 volatile organic compounds (1-hexanol, [Z]-3-hexen-1-ol, 1-octanol, α-terpineol, β-myrcene, limonene, [E]-α-bergamotene, β-caryophyllene, hexanal, [E]-2-hexenal, and guaiacol) common to all five pomegranate juices and seeds. Hexalin, phenylacetaldehyde, 3-methyl butanal, and methyl-(1-methylethenyl) benzene were found in seeds only. According to discriminant analysis based on Eigenvalues, volatile organic compounds recovered in the juices could be used to discriminate and classify the pomegranate cultivars. “Devedişi” and “Hicaz” were the most promising cultivars with respect to the largest volatile organic compounds, high total soluble solid and deep red color.
Introduction
Pomegranate (Punica granatum L.) originated in Central Asia has been widely grown in Mediterranean countries (Egypt, Morocco, Spain, Turkey, and Tunisia), Afghanistan, Iran, India, China, Japan, and the United States (California)[Citation1] due to easy and good adaption to different weather conditions, and also its nutritional and health benefits.[Citation2,Citation3] Turkey is one of the major producers of pomegranates in the world, with 383,085 tonnes grown annually.[Citation4] Most of this production takes place in the Mediterranean area.[Citation5] Pomegranate is consumed as a fresh fruit and also processed into various products such as juice, jams, and sauce, since pomegranate cultivars display a distinct flavor profile and aril color. For example, “Hicaz” cultivar is the most preferred variety by the Turkish fruit juice industry due to its deep violet-red color and sweet–sour taste. “Eksi” (sour) cultivar is generally used for sauce-making, especially in the Mediterranean region. “Katırbaşı,” “Keben,” and “Devedişi” cultivars are mostly consumed as fresh fruit.
Pomegranate fruit is generally characterized by its low aromatic intensity.[Citation6] Thus, the isolation and identification of aromatic volatile organic compounds (VOCs) may be difficult. The profiles and concentrations of VOCs in pomegranate juice can vary depending on cultivar used, growing conditions, volatile extraction technique used, fruit pressing technique such as arils or whole fruit.[Citation7,Citation8] Accordingly, there are conflicting reports on VOCs in pomegranate fruit juice. While Andreu-Sevilla, Mena, Marti, Garcia Viguera, Carbonell-Barrachina[Citation9] reported terpenes as predominant VOCs, the others[Citation7,Citation10] were found to be alcohols such as 1-hexanol and (Z)&(E)-3-hexenol. Mayuoni-Kirshinbaum, Tietel, Porat, and Ulrich[Citation11] indicated that the majority of aroma-active compounds in ‘Wonderful’ pomegranates were terpenes and aldehydes. Hexanal, limonene, (E)-2-hexenal, and (Z)-3-hexenol were the most abundant compounds in fresh pomegranate juices from nine Spanish cultivars.[Citation12] In the consensus listing emanated from several cultivars of worldwide commercial importance, the 21 compounds including hexanol, (Z)-3-hexenol, 2-ethylhexanol, hexanal, (E)-2-hexenal, heptanal, octanal, nonanal, (Z)-3-hexenal, 6-methyl-5-heptene-2-one, α-pinene, β-pinene, α-terpinene, p-cymene, limonene, γ-terpinene, 4-terpineol, α-terpineol, α-bergamotene, β-caryophylene, β-bisabolene were considered to have aroma/flavor impact importance in pomegranates.[Citation13] Hexanol with a “resin,” “floral,” and “green” flavor; (Z)-3-hexenol with a “moss” and “fresh” flavor; hexanal with a “green,” “tallow,” and “fat” flavor; (E)-2-hexenal or (Z)-3-hexenal with a “fruity,” “green,” “leafy,” and “vegetable” limonene with a “lemon,” and “orange” flavor; and α-terpineol with an “oil,” “anise,” and “mint” flavor were the most abundant VOCs in most pomegranate cultivar juices.[Citation7,Citation8,Citation10–Citation12] It can be seen that hexanal and its derivates (E)-2-hexenal, hexanol and (Z)-3-hexanol, and terpenes limonene and α-terpineol are key aroma volatiles in pomegranate fruits. Additionally, Bealieu and Stein-Chisholm[Citation8] isolated ethanol, β-myrcene, 1,4-cineole, 2-nonanone, linalool, 2-methyl-3-buten-2-ol, and α-terpinolene in freshly pressed whole fruit “Wonderful” juice samples. They believed that these compounds might confer desirable sensory attributes and might be listed to confer potential aroma in pomegranate.
Several studies have been reported on the chemical and antioxidant properties of pomegranate accessions in Turkey.[Citation14–Citation19] However, no studies on the VOCs present in the seed and freshly pressed arils juices of selected pomegranate cultivars grown in Turkey have been published. Thus, the specific objectives of the present study were: (1) to identify major VOCs in the juice and seed of selected pomegranate accessions from the Mediterranean region of Turkey; and (2) if “Eksi,” “Devedisi,” “Hicaz,” “Katırbası,” and “Keben” designated as sour, sweet, and sour–sweet can be grouped based on their VOCs.
Materials and Methods
Plant Material
The samples (three fruit per tree 9× trees for each accession) of uniform size of five pomegranate accessions (“Devedişi,” “Eksi,” “Hicaz,” “Katırbaşı,” and “Keben”) were randomly collected from orchards in Adana, Hatay, and Mersin provinces in 2013, which are located in the Eastern Mediterranean Region of Turkey and are mainly pomegranate-growing locations. Fruits were harvested at horticulture maturity as determined by customary local practices. After harvest, fruits were quickly transported in cold chain to the research laboratory at the Food Engineering Department, Mustafa Kemal University, Hatay, Turkey. Phytochemical identifications of the pomegranate fruits were conducted on samples of 27 fruits per accession in nine replicates, each consisting of three fruits. All analyses were carried out within one week after harvest.
Fruit Juice Extraction
Many commercial pomegranate juices in Turkey and other Mediterranean regions (Spain, Israil, Iran, Tunisia) are produced from arils of only locally grown important regional cultivars. Therefore, juices are produced from arils without crushing their kernel. The sampled fruits were cut into halves, and arils were carefully, manually separated from the mesocarp (known as the albedo). In order to prevent a probably metal contamination from using a kitchen juicer (spinning cutter/strainer), the juice of each accession was obtained from pomegranate arils inside a nylon mesh bag by hand press. After squeezing, juice and seed (kernel) were separated and were carried out analyzes.
Quality Parameters of Juices (Total Soluble Solids [TSS], Titratable Acidity [TA], pH, Brima Index, and Color Measurements)
The TSS and pH of pomegranate juices were measured at 20°C using a hand-held refractometer (Fisher Scientific, Pittsburgh, PA, USA) and a pH meter (370, Orion, Beverly, Mass., USA), respectively. The TA was determined by titrating one mL of each sample diluted to 20 mL final volume with deionized water) with 0.1 N NaOH and expressed as percentage of citric acid equivalents. Maturity index (MI) is a ratio of TSS to TA. BrimA is a variant of TSS/TA ratio used to determine acceptability of fruit juice, which was calculated using the formula (BrimA = TSS – k × TA) reported by Jordan, Seelye, and Mc Glone[Citation20] where k is the tongue’s sensitivity index normally ranging from 2–10.[Citation21] In order to avoid negative BrimA, k-value of 2 is used.[Citation22]
The L*, a*, and b*-values were determined on fruit juices using a Hunter MiniScans XE Plus, portable color spectrophotometer (Hunter Associates Laboratory Inc. Reston, VA, USA). The instrument was calibrated using the black and white standard tiles that came along with the instrument. The operating conditions were 10º observer, D65 illuminant and 45/0 sensor. Measurements were recorded in L* (lightness), +a* (redness) and +b* (yellowness) International Commission on Illumination (CIE) color coordinates. Finally, Chroma (C = is the radial component. Hue (h° = arctan b*/a*) is the angular component of the polar representation of the product color. Chroma defines color saturation and the hue angle is color shadiness, that is, 0◦ = red–purple, 90◦ = yellow, 180◦ = bluish–green and 270◦ = blue. Quality parameter reads and measurements were made triplicate from each replication.
VOC Analysis
After juice extraction, juices from three fruit were filled into a beaker and used to VOC analysis. The seeds (without crushing the kernels) were washed with deionized water to remove all extraneous membranes and juice, and then gently dried in cloth towels. After that, seeds were placed in a chilled mortar and ground with a pestle. After mixing the ground samples from three fruit, samples for VOC analyses were prepared in duplicate from each replication (n = 9). Then ten g of fruit juice and seed were separately transferred to a 20 mL head-space vial (Agilent, Palo Alto, CA, USA), containing three g NaCl, to inhibit any enzyme reaction. The vials were sealed using crimp-top caps with Tetrafluoroethylene-silicone headspace septa (Agilent) and immediately frozen at –20ºC until use. Prior to each analysis, the frozen samples were thawed at 4ºC overnight. The extraction and identification of VOCs were carried out using SHS-gas chromatography (GC)-mass spectrometry (MS) according to the procedure reported by Guler, Karaca, and Yetişir.[Citation23]
Statistical Analysis
All data were subjected to analysis of variance (ANOVA), and means were compared using Duncan’s multiple range test (SPSS Version 17.0; SPSS Inc., Chicago, IL, USA) to determine the statistically significant differences at p ≤ 0.05. All the data on VOCs obtained from the juices were used for discriminant function analysis based on Eigenvalues.
Result and Discussion
Quality Parameters of Juices
The quality characteristics such as TSS, TA, pH, TSS/TA ratio, BrimA, and color parameters of the aril juice samples are shown in . “Devedişi” pomegranate categorized as sweet had the highest TSS content of 17.40 ± 0.07% whereas “Hicaz” classified as sweet–sour had the second highest TSS (17.27 ± 0.83%). Cultivars “Eksi,” “Katırbaşı,” and “Keben” had significantly lower TSS contents ranging from 14.78 ± 0.10 to 15.72 ± 0.74% than “Devedişi” and “Hicaz.” The lowest TSS observed in “Eksi” corresponded with a fruit designated as sour (). TA and pH varied from 0.42 to 1.62% (as citric acid) and from 2.97 ± 0.13 to 3.31 ± 0.17, respectively. Interestingly, “Hicaz” with the high TSS had the highest TA, and it is designated as sweet–sour. This could be attributed to high organic acid, in particular citric acid content, since high citric acid may mask the perception of sweet taste.[Citation24] However, cultivars such as “Katırbaşı” and “Keben” considered sour–sweet had moderate and identical TA (1.06 ± 0.22 and 1.01 ± 0.03%, respectively), and pH values (3.01 ± 0.15 and 3.16 ± 0.03). Sour cultivar “Eksi” had the second highest TA (1.47%) and lowest pH. Sweet cultivar “Devedişi” had lowest TA, significantly differing from the other cultivars.
Table 1. Chemical and physical characterization1 in the juice of five pomegranate cultivars grown in Turkey.
MI or TSS/TA ratio is an accepted main flavor quality in most fruit species. “Devedişi” with the lowest TA had the highest TSS/TA ratio (41.43 ± 0.04). Aside from “Hicaz,” cultivars considered sour–sweet (Katırbaşı and Keben) had moderate TSS/TA ratio values (14.83 ± 2.23 and 14.65 ± 0.05). The ratio value (10.70 ± 0.84) of “Hicaz” was similar to that of “Eksi” (). A similar result for “Hicaz” has been previously reported by Türkyılmaz.[Citation19] Actually, a tight relationship between TA, TSS, and sourness or sweetness was not found, as reported by Beaulieu et al. [Citation7] For example, “Hicaz” (sour–sweet) and “Eksi” (sour) had almost identical TSS/TA ratio values; that is, TSS/TA ratio did not correlate well with taste in cultivar “Hicaz.” Perhaps this cultivar would be better designated as “sour.” However, more sampling would be required to make this assertion. To further explore the relationship between TSS and TA, BrimA was calculated. While “Eksi” (sour) had the lowest BrimA value (11.84 ± 1.40), “Devedişi” (sweet) had the highest (16.56 ± 0.11). BrimA index of “Hicaz” with value of 14.03 was similar to those in sour–sweet cultivars (.) According to this finding, BrimA may be more sensitive for the classification of pomegranate cultivars with respect to their sweetness or sourness, than TSS/TA ratio which is generally used to track the flavor change of a given cultivar through growth and development into maturity.[Citation22] TSS, TA, pH, and TSS/TA ratio values were within ranges (14.31–17.70% for TSS; 0.22–1.89% for TA; 2.76–4.26 for pH; and 7.76–72.93 for TSS/TA ratio) previously reported for the various pomegranate cultivars.[Citation6,Citation7,Citation12,Citation25] The color of pomegranate juices is very attractive to consumers; that is, pomegranate fruits having low values of the L* (lightness) are recommended. There were no significant differences in L* values of juices among cultivars. Regarding the other color coordinates, while “Devedişi” and “Hicaz” had the highest a* (deep red color), “Keben” had lowest a*, b* and hue (less yellowness) values (). Therefore, this cultivar had extremely low C (duller), significantly lower than the other cultivars. “Devedişi” and “Hicaz” had the highest C (chroma) values, which indicate that the purity or saturation of color is higher compared to the other cultivars. Aside from “Keben,” the other cultivars had almost identical h° value which denotes the subtle distinction or variation in color. The color values of juices, with exception b* and hue for “Keben,” were within ranges reported for cultivars “Mollar de Elche,” “Valenciana,” and “Wonderful.”[Citation25] However, lightness (L*) values observed in this study were lower than those reported by the others.[Citation6,Citation7,Citation12] This could be related to the method of fruit pressing and sample preparation, and also cultivar used. In this study, juice was obtained from aril pulp only without crushing seed, whereas in most other juice extraction methods juice is prepared from the seeds/arils using a blender or from whole fruit with a pressing machine which may affect lightness (L*). According to the physic-chemical analysis results, cultivars “Devedişi” and “Hicaz” have a dark red juice color, and high TSS may be a good choice for fresh fruit and juice markets.
VOCs
Although pomegranate cultivars have previously been considered to have low aromatic intensities (Calin-Sanchez et al.),[Citation6] a total of 60 and 31 VOCs were identified in experimental pomegranate juices and seeds, respectively. More VOCs were identified in the present study than those in previous studies.[Citation7,Citation8,Citation26] The differences in our data might be related to the method pressing of fruit and sample preparation, and also the cultivars used. As shown in , VOCs were grouped according to their chemical classes and the profile was composed of 22 alcohols, ten terpenes, eight esters, seven aldehydes, four ketones, three acids, and six benzene derivative compounds. Chemical groups found in five pomegranate cultivars were similar to those reported by others.[Citation7,Citation8,Citation27] Most of VOCs identified are commonly presented in pomegranate juices. Whereas some VOCs were newly identified in freshly squeezed aril juice, which are dihydromyrcenol, 1-decanol, β-citronellol, ethyl salisylate, iron, monocarbonyl-(1,3-butadiene-1,4-dicarbonic acid, diethyl ester) a,a’-dipyridyl, hexyl isobutyrate (hexyl-2-methylpropionate), methyl dihydrojasmonate, p-menth-1-en-3-one, semicarbazone, 6-methyl-γ-ionone, styrene (cinnamane), 2,3-dimethyl oxirane, 6-methyl-2-phenylindole, and decanoic acid, it would be very difficult to compare them now. Some VOCs such as hexalin, phenylacetaldehyde, 3-methyl butanal, and methyl-(1-methylethenyl) benzene were found in the pomegranate seeds only. To our knowledge, this study is the first report on VOCs in pomegranate seeds (kernels).
Table 2. Mean contents (relative percentages) of the volatile organic compounds (VOCs) identified in the headspace of the arils-only juices and seeds (kernel without pulp) from selected five pomegranate cultivars.
As shown in and in and , alcohol chemical group was the major VOC in all five pomegranate juices and seeds, in terms of their number and their percentage composition. The main alcohol and also VOC was hexanol, which represented approximately 33 and 52% of the total of VOCs identified in the headspace of the juices and seeds, respectively. The finding was in agreement with the results obtained by others.[Citation7,Citation8,Citation10,Citation28] While (Z)-3-hexenol was the second most abundant alcohol in seeds of four cultivar (“Katırbaşı,” “Eksi,” “Keben,” “Devedişi”) and in juices of “Eksi” and “Devedişi”, 3-methyl-2-butanol was in “Katırbaşı” and “Keben” juices, and ethanol was in “Hicaz” juice and seed. Cultivar “Hicaz” was different from the other cultivars, with respect to high ethanol (16%) percentage. Similarly, Beaulieu and Stein-Chisholm[Citation8] have found ethanol in “Wonderful” juice at a considerable level. On the other hand, “Katırbaşı” and “Keben” juices had the highest percentages (12 and 15%, respectively) of 3-methyl-2-buten-1-ol (prenol) which has fruity flavor. Prenol, one of the simplest natural terpene alcohols known as hemiterpenoid, has previously been found in raspberry fruit[Citation29] and in “Wonderful” pomegranate concentrate juice.[Citation26] From the other terpenoids, α-terpineol (with “floral,” “lilac,” and “fragrant” odor) and its isomer 4-terpineol (with “mold,” “rotten,” and “citrus” odor) were routinely identified in all the pomegranate juices at relatively low percentages, with some significant cultivar differences (). These terpenoids have been previously found in the various pomegranate cultivars.[Citation6,Citation7,Citation9]
Figure 1. Percentage compositions of the main classes of volatile organic compounds (VOCs) in the juice (Panel A) and seed (Panel B) of “Devedişi,” “Ekşi,” “Hicaz,” “Katırbaşı,” and “Keben” pomegranate accessions.
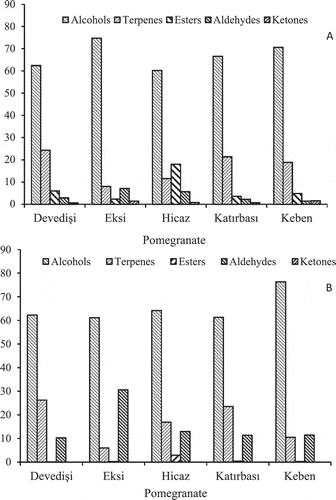
Terpenes were listed as the second largest volatile group () and included α-pinene, β-myrcene, limonene, (E)-α-bergamotene, β-caryophyllene, which were present in all the pomegranate juices, with the exception of “Hicaz,” in which was esters, and seeds with the exception of “Eksi” in which was aldehydes (). Terpenes have a very diverse flavor, ranging from turpentine and resinous impressions to citrus and flowery notes. β-Myrcene, an acyclic monoterpene, was the main terpene in “Eksi,” “Katırbaşı,” and “Keben” juices and all the seeds, ranging from 5 to 15%. β-Caryophyllene, bicyclic sesquiterpene, and limonene, monocyclic monoterpene, were found in “Devedişi” and “Hicaz” juices as main terpene, respectively. “Devedişi” juice had the highest percentages of terpenes, followed by “Katırbaşı,” “Keben,” “Hicaz,” and “Eksi” (). This might be related to low TA and high MI since the highest TSS/TA ratio value and the lowest TA were observed in “Devedişi” pomegranate. In the previous studies, limonene, although, was described to be a dominant VOC or terpen, β-myrcene was detected mostly at trace levels.[Citation7,Citation11,Citation12] However, myrcene was the second most abundant VOC in “Wonderful” pomegranate juice in Spain.[Citation9] Conversely, some researchers reported that β-caryophyllene, α-farnesene, and limonene were the most abundant VOCs in fresh-squeezed juice from “Wonderful” pomegranate grown in the United States.[Citation26,Citation30] This could be due to different growing conditions or weather and differences in extraction procedures used.
Ester VOCs were the second most abundant chemical group in juice of “Hicaz” cultivar only, in terms of their percentage (18%). Ethyl acetate (13.74%), known to have sweet–fruity odor, was main ester in “Hicaz” juice. Methyl- and ethyl-salisylate, methyl dihydrojasmonate and hexyl isobutyrate were routinely identified in all the pomegranate juices at relatively low percentages. Esters were sporadically detected in pomegranate seeds (.). Ethyl acetate, 3-methylbutyl acetate (isoamyl acetate), methyl salicylate, and hexyl butanoate identified in juices has been earlier reported by others,[Citation7,Citation10,Citation30] but ethyl salicylate and methyl dihydrojasmonate were identified for the first time in pomegranate fruit. While methyl- and ethyl-salicylates possibly originates from trans-cinnamic acid,[Citation31] jasmonates are synthesized in fruits via α-linolenic acid (18:3) pathway.[Citation32]
Aldehydes were the second most abundant chemical group (30.59%) in the seed of “Eksi” (sour) cultivar only () due to its high hexanal and (E)-2-hexenal percentages (16 and 6%, respectively). The experimental pomegranate seeds had higher levels of aldehydes than juices. The majority of aldehydes were composed of hexanal, (E)-2-hexenal, 3-methyl butanal and phenylacetaldehyde. The last two aldehydes identified in the pomegranate seeds only () have previously been found in tomatoes.[Citation33] The origin of aldehydes dominating in seeds are different, that is, hexanal, (E)-2-hexenal, phenylacetaldehyde and 3-methyl butanal are derived mainly from linoleic, linolenic unsaturated fatty acids, phenyl alanine and leucine amino acids, respectively.[Citation34] It probably seems to be high of essential amino acids and unsaturated C18 fatty acids in seeds of pomegranate. Interestingly, as the percentages of aldehydes increased in “Eksi” (sour) cultivar, the percentages of terpenes and esters decreased.
Ketones were detected in juices only at minor levels. 2-Nonanone and p-menth-1-en-3-one semicarbazone were detected in all the pomegranate juices. Acids were sporadically found in pomegranate juices and seeds. Volatile phenols such as guaiacol (2-methoxy phenol) and methyl isoeugenol give off sweet and honey-like scents and are produced in the Shikimate.[Citation35] They were routinely found in all the juices and seeds. Overall, a total of C6 compounds such as hexanal, (Z)-3-hexenal or (E)-2-hexenal and their corresponding alcohols hexanol and (Z)-3-hexenol was the highest in all five pomegranate cultivar juices and seeds, ranging from 34 to 68% and from 59 to 81%, respectively. Carbon-6 compounds known as green-leaf aldehydes are the main products of lipid oxidation and are derived from the 13-hydroperoxides of linoleic acid and linolenic acid.[Citation33] This indicates that pomegranate juice and especially seed may contain predominantly linoleic and linolenic acids or isomers. The presence of C6 alcohols and aldehydes as the main components has been previously reported for the various pomegranate cultivars in Spain, India, and the United States.[Citation7,Citation10,Citation12]
On the other hand, most of the previously mentioned VOCs have displayed consensus in aroma/flavor impact importance list in pomegranate accessions.[Citation13] 15 of consensus compounds were routinely recovered in juice from cultivars “Devedişi” and “Hicaz.” However, only 12 consensus compounds occurred in all the juice samples. These included: 1-hexanol, (Z)-3-hexenol, 2-ethyl hexenol, 4-terpineol, α-terpineol, α-pinene, limonene, γ-terpinene, (E)-α-bergamotene, (E)-β-caryophyllene, hexanal, and (E)-2-hexenal (). The other consensus compounds such as β-pinene, α-terpinolene, nonanal, and 6-methyl-5-hepten-2-one were detected in at least two out of the five cultivars. However, octanal, (Z)-3-hexenal, heptanal, α-terpinene and β-bisabolene were either not recovered or not confidently recovered. There were seven consensus compounds (1-hexanol, (Z)-3-hexenol, α-terpineol, limonene, (E)-β-caryophyllene, hexanal, and (E)-2-hexenal) common to juices and seeds. In this study, 3-methyl-2-buten-1-ol and β-myrcene with high relative percentages and also menthol, octanol, nonanol, dihydromyrcenol, methyl- and ethyl-salisylate, hexylisobutyrate, methyl dihydrojasmonate, 2-nonanone, guaiacol, and methyl isoeugenol with low relative percentages were routinely isolated in all the pomegranate juices. However, they have not been listed as the important volatiles for pomegranate flavor.[Citation13] Additionally, ethanol and ethyl acetate were isolated in “Hicaz” cultivar at high levels, but they were formerly not considered important aroma consensus compounds. These compounds might confer desirable sensory attributes, if above threshold concentrations. For example, ethanol may contribute to apple and sweet notes[Citation27,Citation36] and ethyl acetate has been described as sweet, fruity, ethereal flavor.[Citation10,Citation26] Myrcene has balsamic, fruity, grape, musty, sweet, wine-like, and woody flavor notes.[Citation9,Citation11,Citation37] Three-methyl-2-buten-1-ol has a fruity flavor.[Citation36] The other minor VOCs such as octanol, nonanol, dihydromyrcenol, jasmanate, ethyl- and methyl-salisylate, hexyl isobutyrate, 2-nonanone, p-menth-1-en, semicarbazon, guaiacol, and methyl isoeugenol have citrus-like, soapy, sweet, fresh lime-like, waxy, fruity, floral, jasmine, green, wintergreen, spicy, balsamic, peppermint, apple- and pear-like, minty, phenolic, smook, clove blossom, and carnation woody flavor notes.[Citation6,Citation9,Citation11,Citation35,Citation36,Citation38]
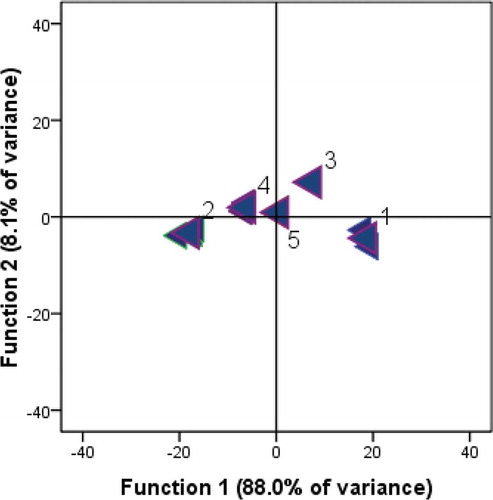
Discriminant analysis was applied to data on VOCs obtained from the juices only since there were more VOCs identified in juices compared with seeds. According to discriminant analysis based on Eigenvalues, VOCs could be used to discriminate and classify the pomegranate cultivars (). “Devedişi” (1) cultivar was located in the negative region for Function 1 and the positive region for Function 2, and was clearly differentiated from the other pomegranate cultivars. The volatile profile for “Devedişi” contained a higher proportion of 4-terpineol, β-caryophyllene, €-α-bergamoten€ (E)-β-farnesene 1-nonanol, and hexyl isobutyrate (). The “Eksi” (No. 2) cultivar was located in the negative regions of Function 1 and Function 2, which related to hexanol, (Z)-3-hexanol and hexanal compounds. This suggests that these C6 compounds may be important for differentiating “Eksi” cultivar from the other cultivars. “Hicaz” (No. 3) with the highest ethanol, ethyl acetate and limonene, and the lowest hexanol was separated from (1) “Devedişi” and (2) “Eksi,” but was close to (4) “Katırbaşı” and (5) “Keben” which were closest to each other because of their content of hexanol, (Z)-3-hexanol, 3-methyl-2-buten-2-ol, β-myrcene and β-caryophyllene (). These findings suggest that VOCs such as 1-hexanol, (Z)-3-hexanol, hexanal, etanol, ethyl acetate, 3-methyl-2-buten-1-ol, 4-terpineol, limonene, β-myrcene, β-caryopyl€e, (E)-α-berg€tene, (E)-β-farnesene, nonanol, and hexyl isobutyrate could be the most important VOCs that could be used for the classification of pomegranate fruit cultivars or for the assessment of pomegranate origin as varietal markers. However, the number of pomegranate cultivars analyzed in this study was relatively small for accurate generalization. Future studies should increase the number of cultivars.
Conclusions
This is one of the first reports for the VOC profiles in freshly squeezed aril juices and in seeds of the various pomegranate cultivars grown in Turkey with a large number of VOCs using solid phase micro-extraction (SPME) coupled with GC-MS. Results from this research support published relationships regarding generalized attributes such as TA, TSS and TSS/TA ratio value in both sweet and sour fruit. However, there was an anomaly in attempting to classify cultivar “Hicaz” designated as sweet–sour. In general, there were significant differences in the pattern of TSS, pH, TA, juice color, and VOCs of aril juices of pomegranate accessions. Likewise, the number of VOCs identified in seeds was less than those in juices andsignificantly varied from cultivar to cultivar. The majority of VOCs identified in pomegranate seeds were constituted hexanol, (Z)-3-hexen-1-ol, ethanol (for cv. Hicaz seed), β-myrcene, 3-methyl butanal, (E)-2-hexenal, and hexanal (for cv. “Eksi” seed). According to discriminant analysis applied to data on VOCs such as 1-hexanol, (Z)-3-hexanol, hexanal, ethanol, ethyl acetate, 3-methyl-2-buten-1-ol, 4-terpineol, limonene, β-myrcene, β-caryophyllene, (E)-α-bergamotene, (E)-β-farnesene, nonanol, and hexyl isobutyrate can be used to discriminate and classify the pomegranate cultivars. Furthermore, a long-term goal of this research is to determine the correlations between individual aroma compound and flavor attributes in more pomegranate cultivars grown in Turkey using commercial cultivar “Hicaz” as a control.
References
- Stover, E.; Mercure, E.W. The Pomegranate: A New Look at the Fruit of Paradise. HortScience 2007, 42(5), 1088–1092.
- Lansky, E.P.; Newman, R.A. Punica Granatum (Pomegranate) and Its Potential for Prevention and Treatment of Inflammation and Cancer. Journal of Ethnopharmacology 2007, 109(2), 177–206.
- Teixeira da Silva, J.A.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate Biology and Biotechnology: A Review. Scientia Horticulturae 2013, 160, 85–107.
- TUIK Turkish Statistical Institute. Crop production statistics. http://www.tuik.gov.tr/bitkiselapp/bitkisel.zul (2014).
- Ercisli, S.; Agar, G.; Orhan, E.; Yildirim, A.; Hizarci, Y. Interspecific Variability of RAPD and Fatty Acid Composition of Some Pomegranate Cultivars (Punica Granatum L.) Growing in Southern Anatolia Region in Turkey. Biochemical Systematics and Ecology 2007, 35(11), 764–769.
- Calin-Sanchez, A.; Martinez, J.J.; Vazquez-Araujo, L.; Burlo, F.; Melgarejo, P.; Carbonell-Barrachina, A.A. Volatile Composition and Sensory Quality of Spanish Pomegranates (Punica Granatum L.). Journal of the Science of Food and Agriculture 2011, 91(3), 586–592.
- Beaulieu, J.C.; Lloyd, S.W.; Preece, J.E.; Moersfelder, J.W.; Stein-Chisholm, R.E.; Obando-Ulloa, J.M. Physicochemical Properties and Aroma Volatile Profiles in a Diverse Collection of California-Grown Pomegranate (Punica Granatum L.) Germplasm. Food Chemistry 2015, 181, 354–364.
- Beaulieu, J.C.; Stein-Chisholm, R.E. HS-GC-MS Volatile Compounds Recovered in Freshly Pressed and Commercial “Wonderful” Pomegranate Juices. Food Chemistry 2016, 190, 643–656.
- Andreu-Sevilla, A.J.; Mena, P.; Marti, N.; Garcia Viguera, C.; Carbonell-Barrachina, T.A. Volatile Composition and Descriptive Sensory Analysis of Pomegranate Juice and Wine. Food Research International 2013, 54(1), 246–254.
- Tripathi, J.; Chatterjee, S.; Gamre, S.; Chattopadhyay, S.; Variyar, P.S.; Sharma, A. Analysis of Free and Bound Aroma Compounds of Pomegranate (Punica Granatum L.). LWT–Food Science and Technology 2014, 59(1), 461–466.
- Mayuoni-Kirshinbaum, L.; Tietel, Z.; Porat, R.; Ulrich, D. Identification of Aroma-Active Compounds in “Wonderful” Pomegranate Fruit Using Solvent-Assisted Flavour Evaporation and Headspace Solid-Phase Micro-Extraction Methods. European Food Research Technology 2012, 235(2), 277–283.
- Melgarejo, P.; Calin-Sanchez, A.; Vazquez-Araujo, L.; Hernandez, F.; Martinez, J.J.; Legua, P.; Carbonell-Barrachina, Á.A. Volatile Composition of Pomegranates from 9 Spanish Cultivars Using Headspace Solid Phase Microextraction. Journal of Food Science 2011, 76(1), S114–S120.
- Mayuoni-Kirshinbaum, L.; Porat, R. The Flavor of Pomegranate Fruit: A Review. Journal of the Science of Food and Agriculture 2014, 94(1), 21–27.
- Caliskan, O.; Bayazit, S. Phytochemical and Antioxidant Attributes of Autochthonous Turkish Pomegranates. Scientia Horticulturae 2012, 147, 81–88.
- Cam, M.; Hisil, Y.; Durmaz, D. Characterization of Pomegranate Juices from Ten Cultivars Grown in Turkey. International Journal of Food Properties 2009, 12(2), 388–395.
- Ozgen, M.; Coşkun, D.; Serce, S.; Kaya, C. Chemical and Antioxidant Properties of Pomegranate Cultivars Grown in the Mediterranean Region of Turkey. Food Chemistry 2008, 111(3), 703–706.
- Poyrazoglu, E.; Gokmen, V.; Artik, N. Organic Acids and Phenolic Compounds in Pomegranates (Punica Granatum L.) Grown in Turkey. Journal of Food Composition and Analysis 2002, 15(5), 567–575.
- Turfan, O.; Türkyılmaz, M.; Yemiş, O.; Özkan, M. Anthocyanin and Colour Changes During Processing of Pomegranate (Punica Granatum L., Cv. Hicaznar) Juice from Sacs and Whole Fruit. Food Chemistry 2011, 129(4), 1644–1651.
- Türkyılmaz, M. Anthocyanin and Organic Acid Profiles of Pomegranate (Punica Granatum L.) Juices from Registered Varieties in Turkey. International Journal of Food Science and Technology 2013, 48(10), 2086–2095.
- Jordan, R.B.; Seelye, R.J.; McGlone, V.A. A Sensory-Based Alternative to Brix-Acid Ratio. Food Technology 2001, 55(6), 36–44.
- Jaya, S.; Das, H. Sensory Evaluation of Mango Drinks Using Fuzzy Logic. Journal of Sensory Studies 2003, 18 (2), 163–176.
- Fawole, O.A.; Opara, U.L. Changes in Physical Properties, Chemical, and Elemental Composition and Antioxidant Capacity of Pomegranate (Cv. Ruby) Fruit at Five Maturity Stages. Science Horticulturea 2013, 150, 37–46.
- Guler, Z.; Karaca, F.; Yetişir, H. Volatile Compounds in the Peel and Flesh of Cucumber (Cucumis Sativus L.) Grafted onto Bottle Gourd (Lagenaria Siceraria) Rootstocks. Journal of Horticultural Science & Biotechnology 2013, 88(2), 123–128.
- Hasnaoui, N.; Mars, M.; Ghaffari, S.; Trifi, M.; Melgarejo, P.; Hernandez, F. Seed and Juice Characterization of Pomegranate Fruits Grown in Tunisia: Comparison Between Sour and Sweet Cultivars Revealed Interesting Properties for Prospective Industrial Applications. Industrial Crops and Products 2011, 33(2), 374–381.
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Marti, N. Phytochemical Characterisation for Industrial Use of Pomegranate (Punica Granatum L.) Cultivars Grown in Spain. Journal of the Science of Food and Agriculture 2011, 91(10), 1893–1906.
- Vazquez-Araujo, L.; Koppel, K.; Chambers, E.; IV, Adhikari, K.; Carbonell-Barrachina, A.A. Instrumental and Sensory Aroma Profile of Pomegranate Juices from the USA: Differences Between Fresh and Commercial Juice. Flavour and Fragrance Journal 2011a, 26(2), 129–138.
- Fawole, O.A.; Opara, U.L. Physicomechanical, Phytochemical, Volatile Compounds, and Free Radical Scavenging Properties of Eight Pomegranate Cultivars and Classification by Principal Component and Cluster Analyses. British Food Journal 2014, 116(3), 544–567.
- Raisi, A.; Aroujalian, A.; Kaghazchi, T. Multicomponent Pervaporation for Volatile Aroma Compounds Recovery from Pomegranate Juice. Journal Membrane Science 2008, 322(2), 339–348.
- Honkanen, E.; Pyysalo, T.; Hirvi, T. The Aroma of Finnish Wild Raspberries, Rubus Idaeus. Zeitschrift für Lebensmittel-Untersuchung und—Forschung 1980, 171(3), 180–182.
- Vazquez-Araujo, L.; Chambers, E.; IV, Adhikari, K.; Carbonell-Barrachina, A.A. Physico-Chemical and Sensory Properties of Pomegranate Juices with Pomegranate Albedo and Carpellar Membranes Homogenate. LWT–Food Science and Technology 2011b, 44(10), 2119–2125.
- Osorio, S.; Mu˜noz, C.; Valpuesta, V. Physiology and Biochemistry of Fruit Flavors. In The Handbook of Fruit and Vegetable Flavors, Hui, Y.H.; Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2010; 25–43.
- Cheong, J.J.; Do Choi, Y. Methyl Jasmonate as a Vital Substance in Plants. TRENDS in Genetics 2003, 19(7), 409–413.
- Xu, Y.; Barringer, S.; Comparison of Tomatillo and Tomato Volatile Compounds in the Headspace by Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). Journal of Food Science 2010, 75(3), 268–273.
- Eskin, N.A.M. Biochemistry of Foods, 2nd Ed; Academic Press: New York, NY, 1990; 530.
- Chen, L.; Zhang, X.Z.; Jin, Q.; Yang, L.; Li, J.; Chen, F. Free and Bound Volatile Chemicals in Mulberry (Morus Atropurpurea Roxb.). Journal of Food Science 2015, 80(5), 975–982.
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 Varietal Virgin Olive Oils by Their Volatile Compositions. Food Chemistry 2006, 98(2), 243–252.
- Carbonell-Barrachina, A.A.; Calin-Sanchez, A.; Bagatar, B., Hernandez, F.; Legua, P.; Martinez-Font, R.; et al. Potential of Spanish Sour-Sweet Pomegranates (Cultivar C25) for the Juice Industry. Food Science and Technology International 2012, 18(2), 129–138.
- Cheng, H.; Chen, J.; Chen, S.; Wu, D.; Liu, D.; Ye, X. Characterization of Aroma-Active Volatiles in Three Chinese Bayberry (Myrica Rubra) Cultivars Using GC–MS–Olfactometry and an Electronic Nose Combined with Principal Component Analysis. Food Research International 2015, 72, 8–15.