ABSTRACT
In the present study, the antioxidant activity of sweet potato leaf polyphenols was measured, and the effect of different pH values (3.0, 5.0, 7.0, and 8.0), temperatures (55, 65, 80, and 100°C), and light treatments on sweet potato leaf-polyphenols was investigated. Results showed that the O2− scavenging activity of sweet potato leaf polyphenols from cultivar Jishu No. 04150 was 5.84 and 6.20 times of that of tea polyphenols and grape seed polyphenols, respectively, at the concentration of 20 μg/mL. The oxygen radical absorbance capacity was 1.28 and 1.27 times of that of tea polyphenols and grape seed polyphenols, respectively. Sweet potato leaf polyphenols dissolved in pH 5–7 solutions showed higher retention rates of antioxidant activity. Heat treatment at 50 and 65°C and light treatment had little effects on sweet potato leaf polyphenols. In conclusion, sweet potato leaf polyphenols possessed high antioxidant activity and processing stability, having the potential to be a new type of natural antioxidant.
Introduction
China is the leading country of sweet potato (Ipomoea batatas L.) production all over the world, with the annual production 70,741,161 tonnes in 2013, which was 68.61% of the world’s total production (103,109,367tonnes).[Citation1] Sweet potato roots are mainly used for starch processing, followed by powder, paste, staple food, alcoholic drink, natural colorant, and beverage. During starch processing, a large amount of sweet potato by-products will be generated, e.g., leaves, residues, and waste water. Sweet potato leaves, the above ground parts of sweet potatoes, have been consumed as a green leaf vegetable in most part of the world due to its nutritional and functional values.[Citation2] It has been reported that the content of polyphenols in sweet potato leaves was much higher than that in the whole root, flesh tissue, and peel of sweet potato, as well as other most common commercial vegetables.[Citation3] In the previous study, we already found that the total polyphenol content (TPC) of sweet potato leaves from 40 cultivars was 3–12% dry weight (DW),[Citation4] which was two to three times higher than that of some common vegetables (e.g., spinach, kale, etc.),[Citation5,Citation6] and the leaves of sweet potato cultivar Jishu No. 04150 showed the highest TPC (12.46 ± 0.62 g/100 g DW) and antioxidant activity (0.72 ± 0.00 mg ascorbic acid equivalents [ACE]/mg DW) among the starch type sweet potato cultivars. Pharmaceutical studies already revealed that sweet potato leaf polyphenols (SPLP) possessed various health-promoting biological activities, such as antioxidant, anti-cancer, anti-carcinogenesis, anti-mutagenicity, anti-diabetes, anti-hypertension, anti-inflammation, etc.[Citation2,Citation7,Citation8]
It has been reported that polyphenols are generally sensitive to adverse environmental conditions, including unfavorable temperatures, light, pH, etc., and are, therefore, susceptible to degradative reactions during product processing and storage.[Citation9] However, the study about SPLP was focused on extraction, purification, separation, identification, antioxidant activity, etc. There was no report about the effect of pH, heat, and light treatment on SPLP. The present study aimed to evaluate the antioxidant activity of SPLP from Jishu No. 04150 and another popular starch type sweet potato cultivar Shangshu No. 19 by determining DPPH radical scavenging activity, hydroxyl radical scavenging activity, ferric reducing antioxidant power (FRAP), superoxide anion (O2−) scavenging activity, and oxygen radical absorbance capacity (ORAC), and comparing the antioxidant activity of SPLP with commercial tea polyphenols (TPs) and grape seed polyphenols (GSP). Furthermore, this study also investigated the effect of pH, heat, and light treatments on the TPC and antioxidant activity of SPLP, to preliminarily clear the stability of SPLP, and provide a theoretical basis for the application of SPLP in food, medicine, and other fields.
Materials and Methods
Materials
Fresh sweet potato leaves (cultivar: Jishu No. 04150 and Shangshu No. 19) were obtained from the Academy of Agricultural and Forestry Sciences in Hebei Province, China. All sweet potatoes were planted with standard production practices at the experimental farm of the Academy of Agricultural and Forestry Sciences in Shijiazhuang City, Hebei Province at the beginning of June, 2013. The average temperatures during the growth period of 2013 were as follows: June 26°C, July 26°C, August 31°C, September 23°C, and October 12°C. At the middle of August, the leaves were collected, washed, and freeze-dried. All samples were ground in a commercial grinder and stored at 4°C in sealed aluminum bags.
The AB-8 macroporous resins were purchased from Soledad Technology Ltd. (Beijing, China), and the physicochemical properties are summarized as follows: they are weakly polar, have a surface area of 480–520 mCitation2/g, a moisture content of 60–70% and an average pore diameter of 130–140 A. The resins were pretreated according to the method described by Sun, Guo, Fu, Li, and Li.[Citation10] Briefly, the resins were soaked with four times (w/v) 95% (v/v) ethanol for 24 h, washed thoroughly with distilled water until the water clarified, and then soaked with four times (w/v) 2 mol/L HCl and 2 mol/L NaOH solution successively for 4 h. They were then thoroughly washed in distilled water until the washing fluid became neutral. The resins were then filtered to remove the water before use.
Reagents
Folin–Ciocalteau reagent, 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH), ascorbic acid, 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), chlorogenic acid (CGA), 2,2-diphenyl-1-picryl hydrazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), caffeic acid, and chromatography grade acetonitrile and methanol were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The high-performance liquid chromatography (HPLC) grade caffeoylquinic acids standards (3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, and 3,4,5-tri-O-caffeoylquinic acid) were purchased from AMRESCO Biotechnology Co., Ltd. (Solon, OH, USA). TP and GSP were purchased from Yihe Biotechnology Co., Ltd., Xi’an, China. Sodium fluorescein, sodium hydroxide, phosphate, ethylene diamine tetraacetic acid (EDTA), thiobarbituric acid (TBA), trichloroacetic acid (TCA), and other analytical grade reagents were purchased from Beijing Chemical Reagents Co., Beijing, China.
Extraction of Polyphenols from Sweet Potato Leaves
Sweet potato leaf powder (10 g) was extracted with 70% (v/v) ethanol solution (200 mL) for 30 min at 50°C using ultrasonic (59 KHz) assistance. After the solution was centrifuged at 8711 × g for 10 min, the residue was re-extracted twice with 70% ethanol as previously described. The supernatants were amalgamated and concentrated in a rotary evaporator to obtain crude polyphenol extraction.
TPC
The TPC was measured using the Folin–Ciocalteu method.[Citation11] Briefly, a 0.5 mL sample solution was mixed with 1.0 mL of Folin–Ciocalteu reagent (10× dilution) and allowed to react at 30°C for 30 min. Then 2.0 mL of saturated Na2CO3 (10% w/v) was added and kept at 30°C for 30 min. The absorbance was measured at 736 nm using an ultraviolet (UV)-3010 spectrophotometer (Hitachi, Ltd., Tokyo, Japan). A calibration curve for the CGA standards (at concentrations of 0.02, 0.04, 0.06, 0.08, and 0.10 mg/mL) was prepared. The linear regression equation was y = 8.7671x + 0.0068 and RCitation2 = 0.9994. The TPC was expressed as mg CGA equivalent per milliliter of sample solution (mg CAE/mL).
Purification of SPLP by AB-8 Macroporous Resins
The crude polyphenol solution was diluted by distilled water to 2.0 mg (CAE/mL), and adjusted to pH 3.0 using 2.0 mol/L HCl. The purification process was carried out in a glass column (1 cm × 10 cm) wet-packed with pretreated AB-8 resin. The bed volume (BV) of the resins was 10 mL (equal to 5 g resin).The crude polyphenol solution was allowed to flow through the glass column at a flow rate 1.0 mL/min (the volume ratio between crude polyphenol solution and BV was 5:1). After the adsorption equilibrium had been reached, the column was first washed with distilled water at a flow rate of 1.0 mL/min until the effluent was clear, and then eluted by 70% (v/v) ethanol solution at a flow rate of 1.0 mL/min (the volume ratio between ethanol solution and BV was 3:1). The eluted solution was collected and concentrated in a rotary evaporator at 45°C to remove the ethanol and then freeze dried. The TPCs of purified SPLP from Jishu No. 04150 and Shangshu No. 19 were 84.63 ± 3.07% and 83.15 ± 2.22%, respectively.
Quantification of Individual Phenolic Compounds by Reversed-Phase (RP)-HPLC
Individual phenolic compounds in SPLP were evaluated by RP-HPLC (Agilent Technologies, Palo Alto, CA, USA) according to the method described by Sun, Mu, Xi, and Song.[Citation12] Detection and quantification were carried out with a four-channel gradient pump, a diode array detector, a column heater, a degasser and an auto sampler (Agilent Technologies, Palo Alto, CA, USA). Separations were conducted at 30°C on Agilent ZORBAX EclipsPlus C-18 reversed-phase column (150 mm × 4.6 mm length, 5 μm particle size). The mobile phases were A: 0.1% (W/V) phosphoric acid solution and B: acetonitrile. Flow rate was 1.0 mL/min. For analysis, the purified SPLP from the two cultivars (Jishu No. 04150 and Shangshu No. 19) were accurately weighed and then dissolved in 80% (v/v) methanol to prepare a sample solution (200 μg/mL), and injection volume of the sample solution was 20 μL. The gradient program was as follows: 20–65% B from 0–15 min, 65–80% B from 15–15.1 min, 80% B from 15.1–20 min. Spectral data from 200 to 800 nm were recorded, and the polyphenol chromatograms were monitored at 326 nm. Caffeic acid, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, and 3,4,5-tri-O-caffeoylquinic acid were used as standard. Identification and quantitative analysis were done by comparison with standards. The amount of individual phenolic compound was expressed as gram per 100 g of purified SPLP on DW (g/100 g DW).
Antioxidant Activity Analysis
DPPH radical scavenging activity
The procedure used is essentially as described by Sanchez-Moreno, Larrauri, and Saura-Calixto with some modifications.[Citation13] Briefly, 2.0 mL sample solutions at different concentrations (5.0, 7.0, 10.0, 15.0, and 20.0 μg/mL) were added to 2.0 mL 6 × 10−Citation5 mol/LDPPH solution in ethanol. Then the reactants were incubated at 25°C for 60 min. Instead of sample solutions, in positive control 2.0 mL TP or GSP solutions at different concentrations (5.0, 7.0, 10.0, 15.0, and 20.0 μg/mL) were added, while 2.0 mL distilled water was added into the blank control. After incubation, the absorbance was measured for test (A1), positive control (A1) as well as blank control (A2) at 517 nm. The radical scavenging activity was calculated based on the following equation and expressed on percent basis.
Scavenging activity (%) = (1 − A1 /A2) × 100
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity was determined according to the method of Halliwell, Gutteridge, and Aruoma.[Citation14] The FeCitation3+/ascorbic acid/EDTA/H2O2 system was used to generate hydroxyl radicals. Briefly, the reaction mixture, contained 0.1 mL different concentrations of sample solutions (0.05, 0.10, 0.20, and 0.50 mg/mL), 0.1 mL 28 mmol/L deoxyribose, 0.1 mL 1mmol/L FeCl3, 0.1 mL 1 mmol/L ascorbic acid, 0.1 mL 1 mmol/L EDTA, and 0.1 mL 10 mmol/LH2O2 in KH2PO4-KOH buffer (20 mmol/L pH 7.4), was incubated in a water bath at 37°C for 1 h. The extent of deoxyribose degradation was measured by TBA method. TBA (1 mL, 1% w/v) and TCA (1 mL, 2% w/v) were added to the mixture and heated at 100°C for 20 min. The absorbance was immediately measured at 532 nm. TP and GSP were used as positive control, and the KH2PO4-KOH buffer was used as blank control. The hydroxyl radical scavenging activity was calculated using formula (1) as follows:
where A0 is the absorbance of the blank control, and A1 is the absorbance of samples or positive control.
FRAP
FRAP was assayed according to Benzie and Strain.[Citation15] Stock solutions included 300 mM acetate buffer (pH 3.6), 10 mM TPTZ solution in 40 mM HCl, and 20 mM FeCl3·6H2O solution. A working solution was prepared freshly by mixing 25 mL of acetate buffer, 2.5 mL of TPTZ solution, and 2.5 mL of FeCl3·6H2O solution. The mixed solution was incubated at 37°C for 30 min in a water bath and was referred to as FRAP solution. A sample (150 μL) with the concentration of 10, 50, and 100 μg/mL was mixed with 2850 μL of FRAP solution and kept for 30 min in the dark at room temperature. The ferrous tripyridyltriazine complex (colored product) was measured by reading the absorbance at 593 nm. A sample blank at each concentration was prepared by omitting FeCl3 from the FRAP solution and distilled water was used instead. The standard curve (y = 0.0029x + 0.017, RCitation2 = 0.9918) was prepared using Trolox ranging from 10–200 μg/mL. The activity was expressed as μg Trolox equivalents (TE)/mL of sample solution.
O2− scavenging activity
O2− scavenging activity was determined using an automated photochemiluminescent system (Photochem, Analytik Jena AG, Germany). This system is based on a controlled photochemical generation of radicals, part of which is quenched by antioxidants present in the sample. The remaining radicals in the sample are quantified by a sensitive chemiluminescence-detection method as reported by Cofrades et al.[Citation16] Briefly, a sample (20 μL)with the concentration of 5, 10, and 20 μg/mL was used in a commercial kit for antioxidant capacity determination. Ascorbic acid was used as the standard. The results were expressed as μg ACE/mL of sample solution.
ORAC
ORAC assay was carried out following the procedure established by Prior et al., with slight modification.[Citation17] All samples and reagents in this experiment were dissolved and diluted with phosphate buffer (0.075 M, pH 7.4). Briefly, 20 μL sample solutions at different concentrations (5, 10, and 20 μg/mL) were added to 20 μL phosphate buffer and then mixed with 20 μL 63 nmol/L sodium fluorescein solution in a clear, 96-well microplate and incubated at 37°C for 15 min. Then, 140 μL 18.28 mmol/L AAPH solution was rapidly added to the well. After vigorous shaking, the microplate was placed in the multifunctional microplate reader (Hidex Ltd. Co., Finland). The system was set in the fluorescence mode and the fluorescence intensity of each well was read 60 times at 2 min intervals. The excitation and emission filter wavelengths were set at 485 and 535 nm and the detection temperature was 37°C.
The fluorescence intensity of each sample was determined without the effect of AAPH (i.e., the AAPH solution was replaced by the same amount of phosphate buffer) in order to calculate the relative fluorescence intensity using formula (2). The relative fluorescence intensity was used to calculate the area under the curve (AUC) by the approximate integration method shown in formula (3). The ORAC values were expressed by the net area under the curve (netAUC) between the samples and the blank, as shown in formula (4). A calibration curve for the Trolox standards (at concentrations of 5, 10, 20, 40, and 60 μg/mL) was prepared. The ORAC values of the samples were expressed as μg TE/mL sample solution.
where fi(+AAPH) is the fluorescence intensity of the reaction solution containing the AAPH solution; fi(–AAPH) is the fluorescence intensity of the reaction solution without AAPH; Fi is the relative fluorescence intensity of the reaction solution; AUC is the AUC; Δt is the interval time and the value of Δt in this study was 2; AUCsample and AUCblank are the AUCs of the sample and the blank, respectively, and netAUC is the netAUC between the sample and the blank.
Stability of SPLP
Effect of pH value on the stability of SPLP
The effect of different pH solvent system on the stability of SPLP was carried out by the method reported by Majo et al.[Citation18] Briefly, phosphate buffered solutions of different pH values (3.0, 5.0, 7.0, and 8.0) were prepared with disodium hydrogen phosphate and citric acid. SPLP were dissolved in different pH buffer solution, so that 1.0 mg/mL sample solutions with different pH value were prepared. Then the TPC and antioxidant activity of sample solutions were determined.
Effect of heat treatment on the stability of SPLP
The effect of heat treatment on the stability of SPLP was determined following the method reported by Lee et al.[Citation19] Briefly, 1.0 mg/mL sample solutions were prepared by dissolving SPLP from Jishu No. 04150 and Shangshu No. 19 in distilled water. Then the sample solutions were incubated at 50, 65, 80, and 100°C water bath, respectively. The TPC and antioxidant activity were determined after 0, 10, 30, 60, and 90 min, and the retention rates of TPC and antioxidant activity were calculated according to the following formulas:
where R1 is the retention rate of TPC, C0 is the TPC before heat treatment (mg CAE/mL), and C1 is the TPC after heat treatment (mg CAE/mL).
where R2 is the retention rate of antioxidant activity, A0 is the antioxidant activity before heat treatment (mg TE/mL), and A1 is the antioxidant activity after heat treatment (mg TE/mL).
Effect of light on the stability of SPLP
The effect of light on the stability of SPLP was determined according to the method reported by Wang, Chen, and Wang.[Citation20] Briefly, 1.0 mg/mL sample solutions were prepared by dissolving SPLP from Jishu No. 04150 and Shangshu No. 19 in distilled water. A set of sample solutions were kept in a place with direct sunlight from 10:00 am to 3:00 pm, and another set of sample solutions were covered with tin foil paper and kept in the same place. The TPC and antioxidant activity of sample solutions were determined every hour, and the retention rates of TPC and antioxidant activity were calculated using formulas (5) and (6).
Statistical Analyses
The results were expressed as mean ± standard deviation (SD) of three replicates. Statistical analyses were performed using SAS version 8.1 software (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
Quantification of Individual Phenolic Compounds by RP-HPLC
The quantification result of the individual phenolic compounds was shown in . SPLP was mainly composed of seven caffeoylquinic acids and a certain amount of caffeic acid. The contents of three di-caffeoylquinic acids in Jishu No. 04150 were relatively higher than those in Shangshu No. 19, and the content of 3,5-di-O-caffeoylquinic acid in the purified SPLP from Jishu No. 04150 (27.87 ± 0.34 g/100 g DW) was highest. The content of 3,4,5-tri-O-caffeoylquinic acid was 2.37 ± 0.07 g/100 g DW in the purified SPLP from Jishu No. 04150, which was lower than the contents of di-caffeoylquinic acids, but higher than the contents of mono-caffeoylquinic acids. The content of 4,5-di-O-caffeoylquinic acid (34.04 ± 0.58 g/100 g DW) was highest in Shangshu No. 19, followed by caffeic acid (6.26 ± 0.08 g/100 g DW). The contents of three mono-caffeoylquinic acids in Shangshu No. 19 were higher than those in Jishu No. 04150. The total content of eight individual phenolic compounds in the purified polyphenol product from Jishu No. 04150 was 64.74 ± 0.72 g/100 g DW, which was lower than the TPC of the purified polyphenol product (84.63 ± 3.07%) determined by the Folin–Ciocalteu method. This result also occurred in the sample of Shangshu No. 19. It suggested that some unknown individual phenolic compounds with small absorption peaks in the HPLC chromatograms were not identified by the HPLC method, but were included in the TPC by the Folin–Ciocalteu method.
Table 1. Content of individual phenolic compounds in purified sweet potato leaf polyphenols from Jishu No. 04150 and Shangshu No. 19 (g/100 g DW).
Antioxidant Activity of SPLP
DPPH radical scavenging activity
The effect of antioxidantson DPPH radical scavenging is generally due to their hydrogen-donatingability.[Citation21] DPPH radical scavenging activity of SPLP from Jishu No. 04150 and Shangshu No. 19, TP, and GSP at different concentration was shown in . The activity of all samples increased with increasing concentration (p < 0.05). At the same concentration used, the DPPH radical scavenging activity of SPLP from Jishu No. 04150 was highest, followed by SPLP from Shangshu No. 19, and that of TP was lowest. At the sample concentration of 20 μg/mL, the DPPH radical scavenging ratio of SPLP from Jishu No. 04150 and Shangshu No. 19 reached the highest values, which were 86.12 and 87.63%, respectively. The DPPH radical half maximal scavenging concentration (IC50) of samples from high to low was TP (9.69 μg/mL), GSP (8.12 μg/mL), SPLP from Shangshu No. 19 (7.22μg/mL), and SPLP from Jishu No. 04150 (6.99 μg/mL).
Table 2. DPPH and hydroxyl radical scavenging ratio (%) of sweet potato leaf polyphenols from Jishu No. 04150 and Shangshu No. 19, tea polyphenols, and grape seed polyphenols.
Hydroxyl radical scavenging activity
Hydroxyl radical is an extremely reactive oxygen species, and can modify almost every molecule in the living cells. This radical has the ability to cause strand damages in DNA, which leads to mutagenesis, carcinogenesis, and cytotoxicity. Moreover, hydroxyl radicals are capable of the quick initiation of lipid peroxidation process as by abstracting hydrogen atoms from unsaturated fatty acids.[Citation22,Citation23] Hydroxyl radical scavenging activity of SPLP from Jishu No. 04150 and Shangshu No. 19, TP, and GSP at different concentration was shown in . The activity of all samples showed significant dose-effect relationship (p < 0.05), and at the concentration of 0.50 mg/mL, hydroxyl radical scavenging activity of all samples reached the highest value. The hydroxyl radical IC50 of samples from high to low was TP (0.34 mg/mL), GSP (0.22 mg/mL), SPLP from Shangshu No.19 (0.15 mg/mL), and SPLP from Jishu No. 04150 (0.14 mg/mL).
FRAP
The FRAP of compound may serve as a significant indicator of its potential antioxidant activity.[Citation24] showed that the FRAP of samples (measured at 593 nm) relative to Trolox, a well-known antioxidant. Similar to the antioxidant activity, the FRAP of samples increased in a dose-dependent manner. At the sample concentration of 100 μg/mL, the FRAP of SPLP from Jishu No. 04150 and Shangshu No. 19 was highest, which was 91.55 and 82.75 μg TE/mL, respectively, and significantly higher than TP (64.63 μg TE/mL) and GSP (72.77 μg TE/mL).
Table 3. Ferric reducing antioxidant power (μg TE/mL), O2− scavenging activity, and oxygen radical absorbance capacity of sweet potato leaf polyphenols from Jishu No. 04150 and Shangshu No. 19, tea polyphenols, and grape seed polyphenols.
O2− scavenging activity
O2− scavenging activity of SPLP from Jishu No. 04150 and Shangshu No. 19, TPs, and GSP at different concentration was shown in . The O2− scavenging activity of samples increased in a dose-dependent manner. At all sample concentrations, the O2− scavenging activity of SPLP from Jishu No. 04150 and Shangshu No. 19 was higher than TP and GSP. At the sample concentration of 20 μg/mL, SPLP from Jishu No. 04150 showed the highest activity (61.86 μg ACE/mL), which was 5.84 and 6.20 times of TP and GSP. At the sample concentration of 20 μg/mL, the O2− scavenging activity of SPLP from Shangshu No. 19 was 46.51 μg ACE/mL, which was 4.39 and 4.66 times of TP and GSP.
ORAC
ORAC of samples at different concentration was shown in . At all sample concentrations used, the activity increased with the increasing of concentrations. At the concentration of 5 and 10 μg/mL, the ORAC of SPLP did not show significant difference with TP and GSP. At the concentration of 20 μg/mL, the ORAC of SPLP from Jishu No. 04150 was 54.86 μg TE/mL, significantly higher than other samples, which was 1.28 and 1.27 times of TP and GSP. At the concentration of 20 μg/mL, the ORAC of SPLP from Shangshu No. 19 was 43.12 μg TE/mL, which had no significant difference with TP and GSP.
Effect of pH Value on SPLP
The effect of pH value on the TPC and antioxidant activity of SPLP was shown in . For Jishu No. 04150, there was no significant difference between TPC of SPLP sample solutions in pH 3, 5, and 7 solvent systems, and the TPC of pH 8 sample solution was significantly lower than that of other samples. For Shangshu No. 19, the TPCs of all samples in different pH solvent systems did not show significant difference.
Figure 1. Effect of different pH value on A: the total polyphenol content and B: antioxidant activity of sweet potato leaf polyphenols from Jishu No.04150 and Shangshu No. 19. Values were means ± SD of three determinations. Data on the same broken line that were not significantly different were represented by same letter (p > 0.05).
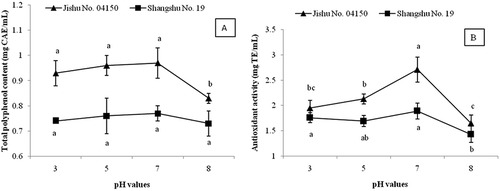
For Jishu No. 04150, the antioxidant activity of SPLP in pH 7 solvent system was 2.71 mg TE/mL, significantly higher than that of other solvent systems, followed by that of pH 5 solvent system (2.13 mg TE/mL), and SPLP in pH 8 solvent system (1.65 mg TE/mL) showed the lowest antioxidant activity. For Shangshu No.19, SPLP in pH 7 solvent system also showed the highest antioxidant activity (1.89 mg TE/mL), and there was no significantly difference between that of pH 3, 5 and 7 solvent systems. SPLP in pH 8 solvent solution showed the lowest antioxidant activity (1.43 mg TE/mL). The above results suggested that the TPC and antioxidant activity of SPLP were higher in neutral and weak acid solvent systems, and the optimum pH range for SPLP was 5.0–7.0.
Effect of Heat Treatment on SPLP
The effect of heat treatment on the TPC and antioxidant activity of SPLP was shown in . For Jishu No. 04150, the retention rates of TPC of samples treated at 50, 65, 80, and 100°C for 90 min were all higher than 91%, which indicated that heat treatment had little effects on TPC of SPLP samples. The antioxidant activity of SPLP samples treated at 50 and 65°C did not show significant difference, and after heat treatment for 90 min, the retention rates of antioxidant activity were 81.33 and 94.55%, respectively. During the heat treatment process at 80 and 100°C, the retention rates of antioxidant activity of SPLP decreased significantly, and after 90 min, the retention rates of antioxidant activity were 62.14 and 61.86%, respectively. For the same treatment time, 100°C heat treatment showed the biggest effect on antioxidant activity of SPLP, and the retention rate of antioxidant activity was significantly lower than other treatment temperatures, indicating that heat treatment at lower temperatures had little effect on antioxidant activity of SPLP, and heat treatment at high temperature could induce sharp decrease of antioxidant activity.
Figure 2. Effect of different heat treatment on the retention rates (%) of total polyphenol content and antioxidant activity of sweet potato leaf polyphenols from Jishu No.04150 at A: 50°C, B: 65°C, C: 80°C, and D: 100°C, and that of Shangshu No.19 at E: 50°C, F: 65°C, G: 80°C, and H: 100°C. Values were means ± SD of three determinations. Data on the same broken line that were not significantly different were represented by same letter (p > 0.05).
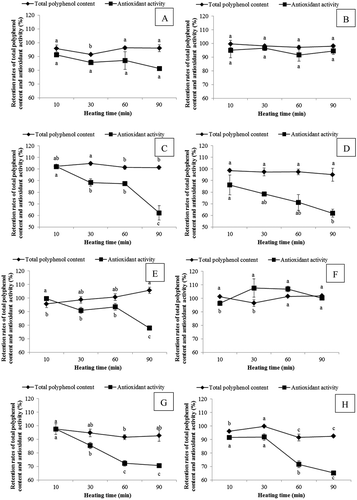
For Shangshu No. 19, the retention rates of TPC of samples at all heat treatment temperatures were higher than 91%. The heat treatment at 65°C showed little effect on antioxidant activity of SPLP, and after treatment for 90 min, the retention rate of antioxidant activity was 100.15%. During the heat treatment process at 80 and 100°C, the retention rates of antioxidant activity of SPLP decreased significantly, and after 90 min, the retention rates of antioxidant activity were 70.65 and 65.25%, respectively. For the same treatment time, 80 and 100°C heat treatment showed bigger effect on antioxidant activity of SPLP, and after 30 min, the retention rate of antioxidant activity was significantly lower than other treatment temperatures. The above results suggested that heat treatment at lower temperatures had little effect on TPC and antioxidant activity of SPLP, and heat treatment at high temperature had bigger effect on antioxidant activity.
Effect of Light on SPLP
The effect of light on the TPC and antioxidant activity of SPLP was shown in . The TPC of SPLP from Jishu No. 04150 and Shangshu No. 19 did not show significant changes under light treatment within 5 h, and there was no significant difference between light treatment group and lucifuge group within same treating time. After 5 h, the retention rates of TPC of Jishu No. 04150 and Shangshu No. 19 with light treatment were 97.55 and 94.87%, respectively.
Figure 3. Effect of different light treatment on the retention rates (%) of total polyphenol content of sweet potato leaf polyphenols from A: Jishu No. 04150 and B: Shangshu No. 19, and effect of different light treatment on the retention rates (%) of antioxidant activity of sweet potato leaf polyphenols from C: Jishu No. 04150 and D: Shangshu No. 19. Values were means ± SD of three determinations. Data on the same broken line that were not significantly different were represented by same letter (p > 0.05).
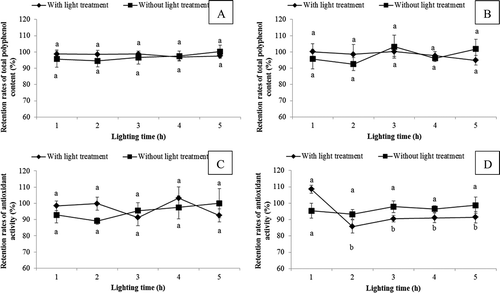
For Jishu No. 04150, the antioxidant activity of SPLP did not change significantly under light treatment within 5 h, and there was no significant difference between light treatment group and lucifuge group. After 5 h, the retention rate of antioxidant activity of SPLP with light treatment was 92.56%. For Shangshu No. 19, though the antioxidant activity of SPLP showed a significant decrease when light treatment for 2 h, there was no significant changes after 2 h, and the light treatment group did not show significant difference compared to lucifuge group. After 5 h, the retention rate of antioxidant activity was 91.43%. The results above suggested that within the testing time, light had a little effect on TPC and antioxidant activity of SPLP.
Discussion
The above results suggested that, SPLP had strong antioxidant activity in vitro, which was closely related to the composition and the molecular structure of SPLP. Some studies have indicated that SPLP were mainly composed of CGAs, where the main CGAs were three disubstituted caffeoylquinic acids: 3,5-di-caffeoylquinic acid, 4,5-di-caffeoylquinic acid, and 3,4-di-caffeoylquinic acid.[Citation25–Citation27] It was reported that the radical scavenging activity of CGAs was positively related to the number of caffeoyl in the molecules, and the radical scavenging activity of disubstituted caffeoylquinic acids was 2.0 times of monosubstituted caffeoylquinic acids, and 1.0 to 1.8 times of ascorbic acid,[Citation28] which were in agreement with the present study. It was found that, the antioxidant activity of phenolic antioxidants was not only related to the number of phenolic hydroxyl, but also related to the electron-donating ability of molecules.[Citation29,Citation30] The electron-donating ability of caffeic acid is higher than that of catechinic acid (the key component of TP), and CGAs especially disubstituted caffeoylquinic acids have two caffeoyls, which is why in the present study, the antioxidant activity of SPLP is higher than that of TP.
The main components of SPLP are chlorogenic acids, which are esterifications of caffeic acid and quinic acid. CGAs can be hydrolyzed into caffeic acid and quinic acid in both acidic and alkaline conditions. Caffeic acid is small molecule phenolic acid, so the hydrolyzation will not induce significant changes of TPC of sample solutions. However, there is a definite positive correlation between antioxidant activity and the number of caffeoyl in the molecular structure of chlorogenic acids.[Citation28]The hydrolyzation of chlorogenic acids under alkaline and strong acidic condition will induce the decrease of the number of caffeoyl, and further decrease the antioxidant activity of molecules. In the present study, we got the same result, that is, SPLP in pH 8 solvent system showed lower antioxidant activity, and in neutral and weak acid system SPLP showed higher antioxidant activity. Some studies reported that the orthophthalic carboxylic structure of chlorogenic acids was very easy to be resolved under high temperature heat treatment, which further decreased the antioxidant activity.[Citation30] In the present study, the antioxidant activity of SPLPs after 100°C heat treatment for 90 min decreased significantly, suggesting that during the process of SPLP, high temperature and long-term heat treatment should be avoided.
Conclusion
In conclusion, the SPLP from Jishu No. 04150 and Shangshu No. 19 both possess high antioxidant activity, that is to say SPLP can eliminate free radicals such as DPPH, OH, and O2− effectively, and have good FRAP and ORAC. SPLP have higher stability in neutral and weakly acidic solutions. There is no significant effect of light and low temperature heat treatment on TPC and antioxidant activity of SPLP. High temperature and long-term heat treatment will cause the significant decrease of the antioxidant activity of SPLP.
Supplement_file.docx
Download MS Word (49.3 KB)Acknowledgments
The authors are grateful to the Academy of Agricultural and Forestry Sciences in Hebei Province for providing the sweet potato leaves used in this study.
Funding
The authors gratefully acknowledge the earmarked fund for the China Agriculture Research System (CARS-11-B-19).
Additional information
Funding
References
- FAOSTAT. Retrieved from http://faostat3.fao.org/browse/Q/QC/E 2015.
- Islam, S. Sweet Potato (Ipomoea Batatas L.) Leaf: Its Potential Effect on Human Health and Nutrition. Journal of Food Science 2006, 71(2), R13–R121.
- Truong, V.D.; McFeeters, R.; Thompson, R.; Dean, L.; Shofran, B. Phenolic Acid Content and Composition in Leaves and Roots of Common Commercial Sweet Potato (Ipomeabatatas L.) Cultivars in the United States. Journal of Food Science 2007, 72(6), C343–C349.
- Sun, H.N.; Mu, T.H.; Xi, L.S.; Zhang, M.; Chen, J.W. Sweet Potato ( Ipomoea Batatas L.) Leaves as Nutritional and Functional Foods. Food Chemistry 2014, 156, 380–389.
- Karna, P.; Gundala, S.R.; Gupta, M.V.; Shamsi, S.A.; Pace, R.D.; Yates, C.; et al. Polyphenol-Rich Sweet Potato Greens Extract Inhibits Proliferation and Induces Apoptosis in Prostate Cancer Cells in Vitro and in Vivo. Carcinogenesis 2011, 32, 1872–1880.
- Xu, W.; Liu, L.; Hu, B.; Sun, Y.; Ye, H.; Ma, D.; Zeng, X. TPC in the Leaves of 116 Sweet Potato (Ipomoea Batatas L.) Varieties and Pushu 53 Leaf Extracts. Journal of Food Composition and Analysis 2010, 23, 599–604.
- Huang, X.; Tu, Z.; Xiao, H.; Li, Z.; Zhang, Q.; Wang, H.; Hu, Y.; Zhang, L. Dynamic High Pressure Microfluidization-Assisted Extraction and Antioxidant Activities of Sweet Potato (Ipomoea Batatas L.) Leaves Flavonoid. Food and Bioproducts Processing 2013, 91(1), 1–6.
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth Suppression of Human Cancer Cells by Polyphenolics from Sweet Potato (Ipomoea Batatas L.) Leaves. Journal of Agricultural and Food Chemistry 2007, 55(1), 185–190.
- Fang, Z.X.; Bhandari, B. Effect of Spray Drying and Storage on the Stability of Bayberry Polyphenols. Food Chemistry 2011, 129, 1139–1147.
- Sun, L.; Guo, Y.; Fu, C.; Li, J.; Li, Z. Simultaneous Separation and Purification of Total Polyphenols, Chlorogenic Acid, and Phlorizin from Thinned Young Apples. Food Chemistry 2013, 136, 1022–1029.
- Yoshimoto, M.; Yahara, S.; Okuno, S.; Islam, M.S.; Ishiguro, K.; Yamakawa, O. Antimutagenicity of Mono-, Di-, and Tricaffeoylquinic Acid Derivatives Isolated from Sweet Potato (Ipomoea Batatas L.) Leaf. Bioscience Biotechnology Biochemistry 2002, 66, 2336–2341.
- Sun, H.N.; Mu, T.H.; Xi, L.S.; Song, Z. Effects of Domestic Cooking Methods on Polyphenols and Antioxidant Activity of Sweet Potato Leaves. Journal of Agricultural and Food Chemistry 2014, 62, 8982–8989.
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F.A. A Procedure to Measure the Antiradical Efficiency of Polyphenols. Journal of the Science of Food and Agriculture 1998, 76, 270–276.
- Aruoma, O.I.; Halliwell, B. Free Radicals and Food Additives. Taylor & Francis Press: London, UK 1991, 59–75.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power the FRAP Assay. Analytical Biochemistry 1996, 239, 70–76.
- Cofrades, S.; Salcedo, L.; Delgado-Pando, G.; López-López, I.; Ruiz-Capillas, C.; Jiménez-Colmenero, F. Antioxidant Activity of Hydroxytyrosol in Frankfurters Enriched with N-3 Polyunsaturated Fatty Acids. Food Chemistry 2011, 129, 429–436.
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; et al. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity [ORAC(FL)]) of Plasma and Other Biological and Food Samples. Journal of Agricultural and Food Chemistry 2003, 51(11), 3273–3279.
- Majo, D.D.; Neve, L.L.; Guardia, M.L.; Casuccio, A.; Giammanco, M. The Influence of Two Different pH Levels on the Antioxidant Properties of Flavonols, Flavan-3-Ols, Phenolic Acids, and Aldehyde Compounds Analysed in Synthetic Wine and in a Phosphate Buffer. Journal of Food Composition and Analysis 2011, 24, 265–269.
- Lee, S.C.; Jeong, S.M.; Kim, S.Y.; Park, H.R.; Nam, K.C.; Ahn, D.U. Effect of Far-Infrared Radiation and Heat Treatment on the Antioxidant Activity of Water Extracts from Peanut Hulls. Food Chemistry 2006, 94, 489–493.
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The Influence of Light and Maturity on Fruit Quality and Flavonoid Content of Red Raspberries. Food Chemistry 2009, 112(3), 676–684.
- Siddhuraju, P.; Becker, K. The Antioxidant and Free Radical Scavenging Activities of Processed Cowpea (Vignaunguiculata (L.) Walp.) Seed Extracts. Food Chemistry 2007, 101(1), 19.
- Aruoma, O.I. Free Radicals, Oxidative Stress, and Antioxidants Inhuman Health and Disease. Journal of American Oil Chemists Society 1998, 75, 199–212.
- Kappus, H. Lipid Peroxidation-Mechanism and Biological Relevance. In O.I. Aruoma & B. Halliwell (Eds.). Free Radicals and Food Additives. London: Taylor & Francis; 1991.
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant Activity of Hot Water Extract from the Fruit of The Doum Palm, Hyphaenethebaica. Food Chemistry 2006, 98, 317–328.
- Islam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamawaka, O. Identification and Characterization of Foliar Polyphenolic Composition in Sweet Potato (Ipomoea Batatas L.) Genotypes. Journal of Agricultural and Food Chemistry 2002, 50(3), 3718–3722.
- Joong-Keun, J.; Seung-Un, L.; Nobuyuki, K.; Carol, E.L.; Mendel, F. Distribution of Phenolic Compounds and Antioxidative Activities in Parts of Sweet Potato (Ipomoea Batata L.) Plants and in Home Processed Roots. Journal of Food Composition and Analysis 2011, 24(1), 29–37.
- Wang, Z.; Michael, N.C. Profiling the Chlorogenic Acids of Sweet Potato (Ipomoea Batatas) from China. Food Chemistry 2008, 106(1), 147–152.
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In Vitro Antioxidative Effects and Tyrosinase Inhibitory Activities of Seven Hydroxycinnamoyl Derivatives in Green Coffee Beans. Journal of Agricultural and Food Chemistry 2004, 52(15), 4893–4898.
- Medina, I.; Gallardo, J.M.; González, M.J.; et al. Effect of Molecular Structure of Phenolic Families as Hydroxycinnamic Acids and Catechins on Their Antioxidant Effectiveness in Minced Fish Muscle. Journal of Agricultural and Food Chemistry 2007, 55(10), 3889–3895.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radical Biology and Medicine 1996, 20(7), 933–956.
