ABSTRACT
A proper description of the respiration and ethylene generation is important in the development of package systems for the preservation of fresh fruits and vegetables. In this work, a model based on both Michaelis-Menten and chemical kinetics equations was developed and assessed in order to describe the respiration and ethylene generation of avocado (Persea americana cv. Hass) and feijoa fruits (Acca sellowiana Berg) from experimental data obtained at different temperatures by a closed system method. The temperature effect in both processes was described using Arrhenius-type relationships. After this, avocadoes and feijoas were packed in perforated low-density polyethylene (LDPE) and polypropylene (PP) bags for 8 days at 12°C and 80% RH to validate the usefulness of the model to describe the gas evolution in modified atmosphere packaging (MAP). For avocado fruits, respiration rates of 2680-3030 cm3 kg-1 d-1 were obtained at 24 °C and normal atmosphere, and 3650-4230 cm3 kg-1 d-1 for feijoa fruits. As for ethylene production rates, at 24 °C were obtained values of 3.11 cm3 kg-1 d-1 for avocado and 0.50 cm3 kg-1 d-1 for feijoa. At 6 °C, respiration and ethylene production rates were up to 6 times lower. It was possible to describe properly the ethylene generation using a Michaelis-Menten simple equation and the respiration rates using a Michaelis-Menten uncompetitive kinetics for both fruits with coefficients of determination above 0.9 in each case. The overall model was validated in the MAP test being possible to predict successfully the O2, CO2 and C2H4 levels inside the packages.
Introduction
Avocado (Persea americana) and feijoa (Acca sellowiana Berg) are tropical fruits that have had a significant growth in production over recent years. However, once these fruits are harvested, they undergo rapid decay in their quality properties so to the use suitable storage methods is fundamental.
Avocado is a tropical fruit globally distributed.[Citation1] The fruit is recognized for its nutritional characteristics with a high content of unsaturated fatty acids, vitamin E, ascorbic acid, vitamin B6, β-carotene, and potassium.[Citation2] It is a climacteric fruit characterized by an increase in ethylene generation at the onset of ripening.[Citation3] Its postharvest life is limited, ripening after 5–10 days of harvest at 18–22°C.[Citation2] It can be stored at refrigeration temperatures (5–6°C) for 2–4 weeks. The fruit respiration rate ranges between 530 and 1450 cm3 kg−1 d−1 (within climacteric peak), and its ethylene generation rate is between 0.96 and 2.16 cm3 kg−1 d−1 at 20–23°C.[Citation4]
Feijoa or pineapple guava is an evergreen shrub of the Myrtaceae family, native to South America.[Citation5,Citation6] The fruit is recognized for its exceptional nutritional qualities with a high content of iodine and vitamin C, antioxidant compounds such as flavonoids and polyphenols.[Citation7] Feijoa is a climacteric fruit with a limited postharvest life, ripening after 1–2 weeks of harvest at 16–17°C. It has a commercial storage life of four weeks at 4–5°C, with a maximum shelf life of 5 days at 20°C.[Citation8] The fruit respiration rate (rCO2) oscillates between 360 and 700 cm3 kg−1 d−1 at a temperature of 4–5°C; and may change to between 1660 and 1750 (climacteric peak) cm3 kg−1 d−1 at 20–23°C. Its ethylene generation rate is between 0.9–1.2 cm3 kg−1 d−1 at 20–23°C.[Citation8,Citation9]
Given the short shelf life of these products, it is necessary to use proper storage systems such as modified atmosphere packaging (MAP) or controlled atmospheres (CA) to preserve their quality properties longer.[Citation6,Citation10] However, the successful use of these systems depends on the previous consideration of the respiration and ethylene production. As fruits ripen, they will consume oxygen from its surroundings and produce autocatalytic ethylene and CO2, reason for which the desired conditions to be achieved involve a change in the atmosphere surrounding the fresh product reducing O2 and ethylene, and increasing CO2. In this way, it is possible to influence product´s metabolism and/or the activity of decay-causing microorganisms to increase storability.[Citation11,Citation12] Nevertheless, to achieve the wanted gas levels it is necessary to know the rate at which the stored product will consume O2 and will be producing C2H4 and CO2 toward this atmosphere to determine the suitable package permeability in MAP or gas flow required in CA depending on the system conditions.[Citation10,Citation13]
Several authors have developed mathematical models to describe respiration in fruits, many of them based on Michaelis-Menten kinetics (MM).[Citation11,Citation12,Citation14] However, only some of them take into account the combined effect of the concentrations of O2 and CO2 in the atmosphere surrounding the product and the storage temperature. As for the modeling of ethylene production in post-harvest storage only available some works that do not consider all of the above variables and apply to a limited number of produce.[Citation15–Citation17] For avocado and feijoa, some data on respiration and ethylene generation have been published as previously mentioned, but there are no models available yet to describe the behavior of these processes in relation to storage time and temperature. For avocado, Hertog et al.[Citation18] presented a respiration rate equation based on the Michaelis-Menten enzyme kinetics at a single temperature (7°C). Devlegiere et al.[Citation15] also present a model based on simple MM to describe respiration and ethylene production as a function of O2 for avocados and other produce. For feijoa, to the authors’ knowledge, there are no equations available so far. We decided to study these two fruits because they have been considered promising for cultivation in Colombia due their export possibilities.[Citation19]. Therefore, developing models to predict the behavior of respiration and ethylene production with application in MAP will boost these processes.
In this work, a mathematical model to describe the evolution in the respiration and ethylene generation processes for avocado and feijoa fruits is presented, and its application in the modelling of MAP systems for these produce is evaluated. The fruit respiration and ethylene generation rates were estimated at different temperatures by using Michaelis-Menten enzyme kinetics and chemical kinetics (CK) equations together with Arrhenius’ law. The data predicted by these equations were compared with experimental data aiming to select the most suitable ones according to their accuracy and reliability. After this, the usefulness of the respiration and ethylene production equations was validated through an integrated model including gas exchange through the MAP system to predict the evolution of gases in the packaging headspace for both fruits.
Materials and Methods
Fruit Samples
The fruits were obtained from commercial growers located in Rionegro in the department of Antioquia (avocadoes cv. Hass), and Tibasosa, department of Boyacá (feijoas cv. Quimba), in Colombia. The fruits were harvested at physiological maturity stage, 20 weeks from flowering time for feijoa[Citation8] and 35 weeks for avocado,[Citation20] and later transported to the Postharvest Laboratory, Faculty of Agricultural Sciences, Universidad Nacional de Colombia (Bogotá, Colombia) in the following days. After discarding those with evidence of damage, fruits of homogeneous characteristics were selected and stored at 8ºC. The following day, groups of both types of fruits were randomly picked to determine the respiration and ethylene generation rates. The rest of them were packaged in MAP to perform the validation test of the model obtained from the equations used.
Determination of the Respiration Rates
The rates of O2 consumption and CO2 generation at different temperatures by fruits were determined using a closed system method. One avocado fruit (200 ± 20 g) and two feijoa fruits (150 ± 20 g), were placed in open glass containers of 2020 cm3 during an hour of acclimatization at the experiment temperature and then hermetically sealed. O2 and CO2 concentrations in the headspace were measured by taking a gas sample of 5 cm3 through a rubber seal on the top of the container, which was analyzed with an electronic analyzer Oxybaby® 6i (Witt-Gasetechnik GmbH & Co. KG, Witten, Germany) previously calibrated with Gas Chromatography. One cm3 of air was introduced into the container to replace the withdrawn sample. The tests were performed in temperature-controlled cabinets to 6, 10, 17, and 24°C, setting temperature in ±0.2°C and taking measurements at regular intervals for maximum 3 days, avoiding reaching the phase of anaerobic respiration. All measurements were performed in triplicate, reporting the average value of each concentration. Respiration rate to each temperature was calculated using the following equations:
where rO2(t) and rCO2(t) are the respiration rates at time t, Δt is the time elapsing between two successive measurements. yO2t-1, yCO2t-1 are the O2 and CO2 mole fractions determined in the previous measurement than reported at time t and yO2t+1, yCO2t+1 are the O2 and CO2 mole fractions determined in the subsequent measurement than reported at time t, respectively.
To describe the experimental behavior of the respiration process in the fruits (and the effect of atmospheric O2 and CO2 levels), various equations have been proposed. Among these equations stand out the Michaelis-Menten equations based on the enzyme kinetics principle and the CK equations based on the reaction order, which have been used due their suitable representation of this biochemical process and good fit of experimental data.[Citation11,Citation21,Citation22] The simplest Michaelis-Menten Kinetics (MM) is shown in Eq. (3). This equation is based on a limiting enzymatic reaction where the substrate is O2. The respiration rate, in this case the O2 consumption rate rO2, is:[Citation11,Citation23]
A similar equation can be defined for the CO2 production rate, also in function of yO2. In some studies,[Citation21,Citation23,Citation24] the inhibitory effect by CO2 on respiration has been considered using the M with uncompetitive inhibition (MMU) due the CO2.[Citation11,Citation24] The MMU equations for the respiration process in terms of O2 consumption and CO2 generation are respectively:
In CK equations, apparent reaction orders are considered to explain the effect of O2 and CO2 concentrations on the respiration rates:[Citation22]
The respiration rate in the product changes with temperature. In the Michaelis-Menten enzyme kinetics, it can be considered that the parameters outlined above will vary depending on storage temperature.[Citation12,Citation21] Similarly, in the CK equations the rate coefficients are temperature-dependent.[Citation22] The dependence of MM/MMU parameters and CK rate coefficients on storage temperature has been expressed using Arrhenius-type equations as follows (in this case, for the maximum O2 consumption rate for example):
Similar equations can be generated for the other parameters in Eqs. (3–7). In this work, the MMU (Eqs. [4 and [Citation5]) and the CK equations (Eqs. [Citation6] and [Citation7]) were used, evaluating at the end the expression that better fits the experimental data obtained. The experimental values of rO2, rCO2, yO2, and yCO2 generated at each temperature were substituted in the linearized form of the MMU and CK equations to obtain the respective parameters by multiple linear regressions. The temperature dependency was determined by replacing the parameters of each equation in the linearized form of the Arrhenius equation (Eq. [Citation8]) estimating the pre-exponential factors and the activation energies for each parameter.
Determination of the Ethylene Generation
Ethylene generation rate for the avocado and feijoa fruits was determined at the same time as respiration measurements by sampling the hermetically sealed glass containers with the fruits inside. For each temperature, ethylene samples were taken by removing 1 mL of gas with a syringe through the rubber seal, which were then analyzed by injecting the samples into an Agilent 7890A gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA). Calibration was performed, from different mixtures of ethylene (0 to 500 ppm) and making a calibration curve of ethylene concentration (in mole fraction) versus peak area. All measurements were made in triplicate. As described above for respiration, ethylene generation rate to each temperature was calculated as follows:
where rC2H4(t) is the ethylene production rate at time t, Δt is the time interval between two successive measurements, yC2H4t-1 is the ethylene mole fraction determined in the previous measurement than reported at time t and yC2H4t+1 is the ethylene mole fraction determined in the subsequent measurement than reported at time t, respectively.
Ethylene is a phyto-hormone that plays critical roles in many aspects of plant growth and development and environmental responses of plants.[Citation25] Climacteric fruits are characterized by a ripening-related increase in respiration and high ethylene synthesis to coordinate and synchronize ripening.[Citation26] Ethylene is bio-synthesized from methionine in three single steps, involving enzymes S-adenosylmethionine (SAM) synthetase, 1-aminocyclopropane-1-carboxylate (ACC) synthase, and ACC oxidase.[Citation27] ACC oxidase catalyses the formation of ethylene, using ACC and O2 as substrates, with ascorbate as co-substrate, and Fe2+ and CO2 as co-factors.[Citation17] The ethylene production depends on O2 presence, so low O2 levels lead to a decrease in its production rate. In addition, due to CO2 act as inhibitor of ACC oxidase enzyme, high CO2 levels also lead to a decrease in the ethylene production.[Citation28]
There are not many works on the representation of the production of ethylene based on the environmental conditions surrounding a stored produce (O2 and CO2 levels and temperature). De Wild et al.[Citation28] have proposed a simple MM to describe the ethylene production rate as a function of the O2 concentration in the atmosphere, similar to Eq. (3), while Sanders and De Wild[Citation17] considered that a positive cooperativity between substrate and enzyme could exist. The latter means binding of a substrate molecule to one site on an enzyme increases the affinity of other sites on the enzyme. Thus, the ethylene production rate no longer follows a simple MM, so a term relating cooperativity is included becoming the Michaelis-Menten Cooperative (MMC) equation:
In the equation above, h is the term representing the degree of cooperativity, known as Hill coefficient. The highest integer above the estimated h gives the minimum number of binding sites. The effect of temperature on the parameters of the ethylene generation equations also can be represented using Arrhenius’s law.
In similar way as for respiration, to describe the evolution in the ethylene production from the experimental data, the MM simple (similar to Eq. [Citation3]) and MMC kinetics (Eq. [Citation10]) were used, evaluating at the end the expression that better fits the experimental data obtained.. For the MM equation, the experimental values of rC2H4, yO2, and yC2H4 generated at each temperature were substituted in the linearized form of the respective equation and the MM parameters were obtained by multiple linear regressions. For the MMC equation, the corresponding parameters were estimated substituting the experimental data and solving the equation using a non-linear regression. The MM and MMC equations were compared by using an adjusted R2.[Citation16,Citation17] The R2adj allows the comparison between non-linear models and compensates for possible bias due to different number of parameters. As before, the temperature influence was estimated by replacing the parameters of each equation in the linearized form of the Arrhenius’ law and obtaining the pre-exponential factors and the activation energies for each parameter. At this stage, after obtaining the various parameters, the respiration and ethylene production equation that best represented the evolution of gases, were selected to perform the validation experiment in a system of MAP.
Application in MAP and Validation
After finding the parameters of the equations of respiration and ethylene generation and selecting the most suitable in each case, an experiment was conducted packaging avocado and feijoa fruits in two types of perforated bags to validate their capacity to describe the gas evolution in a MAP system. 208.1 ± 11.4 g of avocado and 181.3 ± 9.1 g of feijoa were packed in polypropylene (PP) bags and 181.1 ± 8.9 g of avocado and 189.5 ± 8.3 g of feijoa were packed in low-density polyethylene (LDPE) bags. The initial volume headspace were 427.1 ± 10.9 cm3 for avocado and PP, 359.8 ± 10.6 cm3 feijoa and PP, 477.3 ± 8.8 cm3 for avocado and LDPE, and 503.3 ± 9.8 cm3 for feijoa and LDPE, respectively. In the experiment, cast-PP bags with a thickness of 0.025 ± 0.002 mm and LDPE bags with a thickness of 0.018 ± 0.001 mm (both, Rediplast, Bogotá, Colombia) were used. The bags had a total internal surface area of 1000 cm2 and an effective transfer-area of 800 cm2. In each bag, a single perforation of 0.280 mm effective diameter was made to ensure the O2 level in the headspace did not reach 0% by previously simulating the evolution of the system using the respiration and ethylene production equations and the differential balance equations for each gas (Eqs. [Citation14–Citation16]) described below. The perforation size was measured using an optical microscope (Motic® B1-220A, Speed Fair Co., Ltd., Richmond, Canada). Packages with the fruits inside were stored in a controlled-temperature cabinet to 12 ± 0.2°C for 8 days, keeping relative humidity (RH) constant at 80 ± 1%.
For all tests, measurements of O2, CO2 ethylene concentrations in the packages were performed throughout the storage time in the same way as described above in the respiration and ethylene generation tests. All measurements were conducted in triplicate. The initial headspace gas composition in the packages was similar to that of the atmosphere.
Permeability coefficients of both films to C2H4 were experimentally determined using a static permeation cell with a film transfer-area of 1074 cm2. The test film was placed in the middle of the two compartments. The lower compartment of the permeation cell with a volume of 755 cm3 was flushed with N2 containing 3400 ppm of ethylene and the upper compartment was open to the atmosphere. Once made the flushing, the remaining ethylene concentration in the lower compartment was determined continually at regular intervals by GC as described in the ethylene generation test. The permeability coefficients QC2H4 were calculated as follows:
where V is the lower compartment volume, A is the film transfer-area, yC2H4out is the ethylene concentration in the atmosphere, yC2H4ini is the initial ethylene concentration in the lower compartment and and yC2H4(t) is the ethylene concentration at time t.
O2 and CO2 permeability coefficients of both films were experimentally determined with steady state methods, using a Mocon® Oxtran 2/21 permeation instrument (MOCON, Inc., Minneapolis, MN, USA) for O2 and a chamber specially built with a permeation cell and connected to a gas chromatograph as described in a different article.[Citation29]
As mentioned above, a perforation was made in each package to avoid exhausting O2 in the headspace before the end of the experiment and get an anaerobic phase. It was considered the gas transfer through the perforation in each package follows a modified Fick’s diffusion equation incorporating a term of correction ε for gas diffusion resistance in the perforation.[Citation29,Citation30] The change over time of O2, CO2, and C2H4 in the MAP system can be described from the following differential equations:
In the equations above, it was considered that inside the package volume change is negligible, there is no gas stratification and total pressure is the same as atmospheric. The differential equations were numerically solved using a calculation routine built in Matlab® (MathWorks, Inc., Natick, MA, USA) from the “ode15s” function (an implicit multistep method based on numerical differentiation formulas, NDFs). After the initial gas conditions were defined, in this case atmospheric levels, and the initial values of product weight, package arrangement, temperature, pressure and storage time were set, the change in the concentrations of O2, CO2, and C2H4 were calculated with this routine. In the calculation, respiration and ethylene generation parameters obtained for the equations previously selected were included. The data generated in the calculation routine from the model were then compared with the experimental gas measurements in the packages.
Results and Discussion
Respiration Rates
In and , the respiration rates of the avocado and feijoa fruits as a function of O2 within the hermetic containers are shown. The consumption rate of O2 and the production rate of CO2 were influenced, as shown in the figures, by the O2 concentration in the atmosphere surrounding the product. The rates of O2 consumption and CO2 production were higher to the initial concentrations of O2 (air) and became lower as the O2 was consumed within the package. By decreasing the O2 concentration, the slope of the concentration curves of O2 and CO2 becomes close to zero.
Figure 1. A: O2 consumption and B: CO2 production rates as a function of O2 concentration within the experimental container headspace at 6°C (●), 10°C (■), 17°C (♦), 24°C (▲), and predicted values using the Chemical Kinetics (—) and Michelis-Menten Uncompetitive (―) equations for avocado fruits. Bars represent SD.
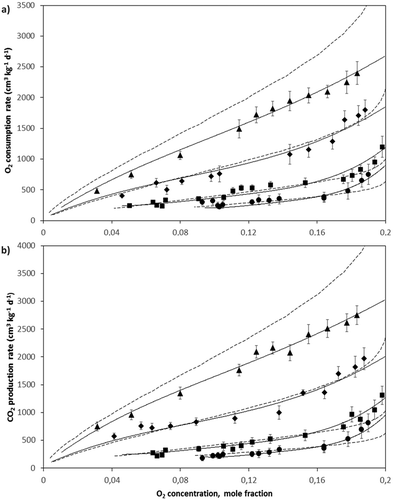
Figure 2. A: O2 consumption and B: CO2 production rates as a function of O2 concentration within the experimental container headspace at 6°C (●), 10°C (■), 17°C (♦), 24°C (▲), and predicted values using the Chemical Kinetics (—) and Michelis-Menten Uncompetitive (―) equations for feijoa fruits. Bars represent SD.
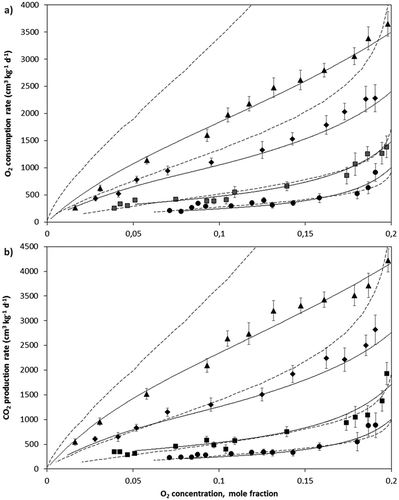
The O2 consumption and CO2 production rates for avocado were somewhat lower compared to feijoa fruits at the different temperatures evaluated. At 24°C and yO2 = 0.2 (pO2 = 15 kPa; beginning of the test), rO2 and rCO2 values of 2680 and 3030 cm3 kg−1 d−1 were obtained for avocado respectively (RQ = 1.13) and of 3650 and 4230 cm3 kg−1 d−1 for feijoa, respectively (RQ = 1.16). López-López and Cajuste-Bontemps[Citation4] obtained a rCO2 value of the same order but 52% smaller, circa 1450 cm3 kg−1 d−1 at 23 °C and pO2 = 17.4 kPa for avocados grown in Michoacan, Mexico. Velho et al.[Citation9] for their part, obtained a rCO2 value of the same order but 40% smaller, circa 1750 cm3 kg−1 d−1 at 23 °C and pO2 = 21 kPa for feijoa grown in Santa Catarina, Brazil. At 6°C and yO2 = 0.2 (pO2 = 15 kPa) the rCO2 obtained for avocado was 960 cm3 kg−1 d−1, larger than the value obtained by Hertog et al.[Citation18] for “Hass” avocadoes freshly harvested and grown in Auckland, New Zealand, 570 cm3 kg−1 d−1 at 7°C and pO2 = 15 kPa. For feijoa, at 6°C pO2 = 15 kPa the rCO2 obtained was 1150 cm3 kg−1 d−1, circa 2.5 times higher than that obtained by East et al.[Citation6] for “Unique” feijoa grown in New Plymouth, New Zealand, 360 cm3 kg−1 d−1 at 5 °C and pO2 = 21 kPa, and circa two times higher than the value obtained by Velho et al.,[Citation9] 700 cm3 kg−1 d−1 at 4°C and pO2 = 21 kPa. These differences can be attributed to the different cultivars and cultivation zones of the fruits used in each particular study.
Respiratory behavior of avocado and feijoa fruits was described using the MMU and CK equations estimating the respective parameters from experimental data. The parameters of these equations are found in . The O2 consumption and CO2 production rates increased directly with the temperature (, , , and ), reaching lower concentrations of O2 (and higher CO2) at higher temperatures. Temperature-dependence of MMU parameters, rmax, Km, and Kmu, and of CK parameter k, was set up obtaining the respective pre-exponential factors and the apparent activation energies by linear regression of the Arrhenius equation (). The positive values of the activation energies for the three parameters indicate a direct relationship between the temperature and respiration rates.
Table 1. Estimated parameters of O2 consumption and CO2 production rates for the Michaelis-Menten Uncompetitive (MMU) and Chemical Kinetics (CK) equations.
Apparent reaction orders a and b in the CK equation were found to be independent of the test temperature. The reaction orders for avocado and feijoa are shown in the . Reaction orders are not integers indicating the complexity in the reaction mechanism of the respiration process. The negative values in the reaction order b in both O2 consumption and CO2 rates is marking the inhibitory effect of CO2 concentration itself. At higher concentrations of CO2, respiration rates will be lower. This inhibitory effect by CO2 is well established considering the good fit obtained with the MMU equation.
Table 2. Estimated parameters of ethylene production rate by Michaelis-Menten (MM) and Michaelis-Menten Cooperative (MMC) equations.
After obtaining the parameters of each equation, the predicted evolution in the O2 consumption and CO2 production rates as a function of O2 concentration at the different test temperatures was calculated for both fruits. Predicted rO2 is shown as lines in and , and predicted rCO2 also as lines in and for the avocado and feijoa fruits respectively. Both equations adequately represent the respiratory behavior of the fruit according to the coefficients of determination R2 of each equation () and considering the fitness between the experimental and predicted data was good.
For the feijoa fruits, the MMU equation (R2 = 0.977 for O2 and 0.974 for CO2) gets closer to the experimental data compared with the CK equation (R2 = 0.917 for O2 and 0.923 for CO2), especially at 17 and 24°C where is clearly shown that the CK equation deviates from the experimental behavior. For avocado fruits both equations adequately represent the experimental behavior although the coefficients of determination for the MMU equation are slightly higher than for the CK equation.
With the CK equation it was possible to describe acceptably the respiratory behavior of the fruits (better for avocado), although the predicted values deviate from the experimental data for the highest temperatures. This is due the temperature influence is only expressed in the k parameter, while in the MMU equation is expressed in three different parameters and confirms the strong influence of the temperature on the respiration. The values predicted by the MMU equation were closer to the experimental data of evolution in the O2 consumption and CO2 production rates for the evaluated fruits and as shown in and do not lose accuracy at higher temperatures. The goodness-of-fit offered by the MMU equation is consistent with the findings for several products in other works.[Citation21,Citation23] The above is not surprising considering the enzymatic nature of this process.
Ethylene Generation Rates
The ethylene production increased progressively with the testing time for the fruits studied in the headspace of the closed system. The ethylene production rates (rC2H4) were much higher for avocado than for feijoa, approximately six times larger, as shown in and . At 24°C and pO2 = 15 kPa, a rC2H4 value of 3.1 cm3 kg−1 d−1 was obtained for avocado and a value of 0.5 cm3 kg−1 d−1 for feijoa fruits. As mentioned in the introduction, López-López and Cajuste-Bontemps[Citation4] report a rC2H4 value of 2.16 cm3 kg−1 d−1 for avocado at 23°C and pO2 = 17.4 kPa, a third smaller than the value obtained in this study. Parra and Fisher[Citation8] in turn, report rC2H4 between 0.9–1.2 cm3 kg−1 d−1 for feijoa at 20°C, around twp=o times higher than the value calculated in this study. For both fruits, rC2H4 was higher at the beginning of the test (atmospheric O2 concentration) and decreased as the O2 in the containers was consumed by the fruits and the O2 concentration came to near zero.
Figure 3. Ethylene production rate as a function of O2 within the experimental container headspace at 6°C (●), 10°C (■), 17°C (♦), 24°C (▲) and predicted values using the Michaelis-Menten simple (—) and Michaelis-Menten Cooperative (―) equations for A: avocado and B: feijoa fruits. Bars represent SD.
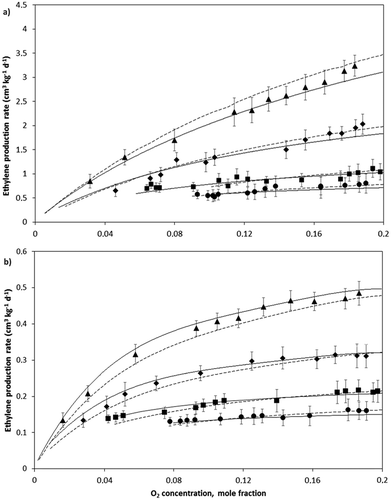
From the experimental data, the respective regression parameters of the MM and MMC equations were calculated (). The equations adequately described ethylene production for both fruits. The predicted values were in good agreement with the experimental data as shown in and . The R2 values obtained for the two were similar: 0.962 for MMC equation and 0.955 for the MM equation in the case of avocado and 0.969 for the MMC equation and 0.961 for the MM equation in the case of feijoa. Further, the cooperative terms h in the MMC equation were close to 1 for the two fruits, 0.95 for avocado and 1.06 for feijoa, respectively, so it can be said the experimental behavior of ethylene production for both fruits could be described acceptably with the simple MM. This is similar to results reported by Sanders et al.[Citation17] who obtained cooperative terms close to one for freshly harvested fruits.
Temperature had an evident effect in the ethylene production for the both fruits. At 24°C and atmospheric O2 concentration (pO2 = 15 kPa), rC2H4 for avocado was approximately 3.11 cm3 kg−1 day−1 while at 6 °C was 0.71 cm3 kg−1 day−1. For feijoa, at 24 °C, rC2H4 was 0.50 cm3 kg−1 day−1, while at 6°C was 0.19 cm3 kg−1 day−1. This temperature-influence was expressed considering the parameters KmC2H4 and rmaxC2H4 in both equations that are temperature-dependent through an Arrhenius-type equation. The pre-exponential values and the activation energies are listed in .
As respiration rate was reduced by decreasing the O2 concentration and increasing the CO2 concentration surrounding the fruits, the ethylene production rate decreased in turn. This relationship between rC2H4 and rO2 is non-linear, which may be inferred because the equations used to describe both processes are also non-linear. Devlieghere et al.[Citation15] found by contrast a linear relationship between rC2H4 and rO2 for several ripe fruits, though they used a simple Michaelis-Menten equation to describe the respiration process without considering the CO2 inhibitory effect and considering ethylene emission as dependent only on the O2 consumption process.
In some studies, it has been assumed that CO2 has an inhibitory effect on the activity of the responsible enzymes of ethylene production in the fruit.[Citation31] For the MM and MMC equations, it has not been considered an effect of CO2 concentration on the ethylene production rate. However, it can be said there is one indirectly, since in both respiration equations evaluated (MMU and CK) the CO2 concentration influences the O2 consumption rate, the O2 concentration in the atmosphere surrounding the fruit and so the rate of ethylene production.
At this point, it is worth mentioning that the parameters of respiration and ethylene production were obtained from experimental data particular to fruits evaluated. This means, from a cultivar, maturity stage and physiological characteristics defined (in this case, fruits of local cultivars and of physiological maturity as outlined in the Materials and Methods section). A more advanced maturity stage, for example, results in higher respiration rates. These variables related to the stored product itself affect the processes of respiration and ethylene production.[Citation11,Citation12,Citation14] Likewise, they also have an impact on the predictive power of the developed model when it is desired to describe the behavior of another set of fruits with different characteristics. In the MM, the effect of these variables will be represented in the pre-exponential factors of the respiration or ethylene production parameters and for avocado or feijoa fruits with a different maturity or ripening stage, or with changes in their structure, for example, these pre-exponential factors will variate compared with the estimated factors for the fruits initially evaluated. For this reason, it is necessary to take into account these considerations in the application of the respiration and ethylene generation equations to predict the produce behavior in the MAP system.
MAP Validation
All the gas permeability parameters for the packaging films used in the MAP experiment are found in . LDPE and PP permeability to C2H4 was determined in the static permeation cell, obtaining the respective permeability coefficients for the test temperature (12°C) with R2 of 0.979 and 0.986 for LDPE and PP, respectively. The permeability coefficients of the two packaging materials to O2 and CO2 were taken from[Citation29] as explained above. Gas exchange through the perforation made in each package was calculated taking a correction factor ε equal to 0.5 times the diameter of the perforation,[Citation29,Citation30] and obtaining the respective diffusion coefficients of O2, CO2 and C2H4 in air at 12°C from data reported in.[Citation32]
Table 3. Parameters for calculating the gas exchange in the packages for the validation in MAP to 12°C.
Table 4. Experimental and predicted equilibrium concentrations of O2, CO2, and C2H4 for the validation in MAP to 12°C.
In all packages, O2 concentration decreased and CO2 and C2H4 concentrations increased to constant levels in the headspace as shown in and . Constant O2 and CO2 levels were reached approximately one and a half days after of the storage beginning ( and ), while constant levels of C2H4 were reached after nearly 2 days (4b and 5b). The achieved equilibrium O2, CO2 and C2H4 concentrations were lower for LDPE bags compared with PP bags given the greater permeability of the former to each gas (). As in tests of closed system, ethylene concentrations in packaging with avocado fruits () were higher than those of feijoa (), given the higher ethylene emission rate of the former.
Figure 4. Evolution in the headspace gas concentration for avocado fruits packed in PP (O2 ○; CO2 □; C2H4 Δ) and LDPE (O2 ●; CO2 ■; C2H4 ▲) bags with one perforation of 280 µm at 12°C and predicted values by the model (PP —; LDPE ―). Bars represent SD.
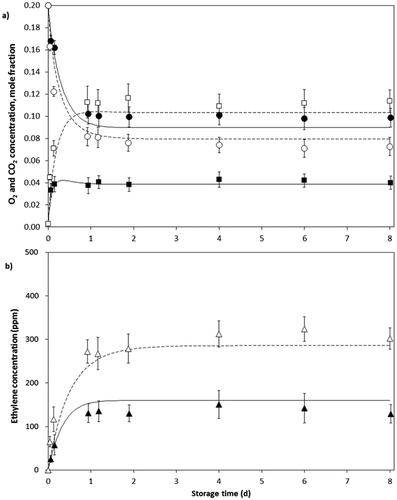
Figure 5. Evolution in the headspace gas concentration for feijoa fruits packed in PP (O2 ○; CO2 □; C2H4 Δ) and LDPE (O2 ●; CO2 ■; C2H4 ▲) bags with one perforation of 280 µm at 12°C and predicted values by the model (PP —; LDPE ―). Bars represent SD.
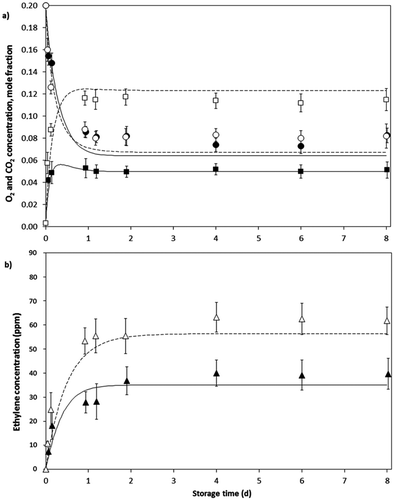
With the respiration and ethylene production equations obtained and chosen above, MM and MMU, respectively, and with the gas exchange parameters in the packaging system, the differential Eqs. (12–14) were numerically solved. From this, predicted gas concentrations were calculated for the MAP system to test conditions.
As can be seen in and , the gas evolution over time was well described by the integrated model. The predicted data suggest an increase and then a slight decrease in CO2 concentration before reaching equilibrium levels inside the LDPE bags, which do not occurs in the PP bags. For O2, a predicted decrease in the concentration occurs and then steady levels are reached in the same way that experimental behavior. The predicted equilibrium values were similar to those obtained experimental data, although seemingly closest for O2 and CO2. The coefficients of determination of the model were above 0.9 for all cases except for ethylene concentration in the LDPE bags where were obtained R2 of 0.893 and 0.879 for feijoa and avocado fruits, respectively. These R2 values and experimental variability shown in C2H4 data ( and ) may be due to the inherent difference between the fruits taken for testing and the small amounts being counted in comparison with the O2 and CO2 concentrations, for example.
Overall, with the integrated model, using the MMU equation for respiration, MM equation for ethylene production and gas exchange parameters through the packages, the gas evolution in the MAP system for the avocado and feijoa fruits was properly described according to the information in – and .
The most important application of the developed model for the evaluated fruits is to setting a specific concentration in the headspace of the MAP system. In designing the MAP system, it is wanted to find the most favorable conditions for the produce preservation. In other words, steady and favorable concentrations of gases in the package headspace and that these equilibrium concentrations to be achieved in the shortest time possible.[Citation29] From the developed model for the avocado and feijoa fruits, the permeability parameters required to achieve a defined equilibrium concentration of O2, CO2, or ethylene can be defined using the differential balance equations (Eqs. [Citation12–Citation14]) and considering that in the period of equilibrium, the change of each gas over time will be zero. The time required to reach the equilibrium concentration depends on the difference between the target concentration and the initial concentration of gas in the MAP system. The less the difference between these concentrations, the equilibrium time will be lower in turn. Once the parameters of respiration and ethylene production are known, the MAP system can be manipulated to balance these processes and achieve the target gas concentration that is more favorable for the fruit. This manipulation of the system is done by altering its capacity to transfer gases according with the growers and dealers possibilities. First, the choice of a packing material to increase or decrease the permeability coefficients. If the packaging material has been defined, it is then possible alter the size of the package and thus increase its surface area and the packaging headspace. If the package size is already defined, then perforations can be made to increase the transfer capacity and achieve the required level of gas.
Conclusions
The MM equation with CO2 MMU adequately describes the respiration process for feijoa and avocado fruits obtaining good fit between the predicted and experimental O2 and CO2 evolution compared to the CK equations. The ethylene production for the fruits was described with a good degree of accuracy using a simple Michaelis-Menten enzyme kinetics considering the likeness in the R2 values obtained for the MM and MMC equations and closeness to unity in the cooperative term h of the MMC equation. The respiration and ethylene parameters were properly correlated with storage temperature using Arrhenius equations. With the developed equations of respiration and ethylene production, an integrated model to describe the evolution of O2, CO2, and C2H4 concentrations in a MAP system was successfully established and validated. In the model, analytical expressions for calculating gas transfer through the package of films and perforations were properly used.
Nomenclature
| a, b | = | Apparent reaction orders |
| A | = | Effective exchange area of the packaging film (m2) |
| Ah | = | Cross-sectional area of the perforations (cm2) |
| CK | = | Chemical kinetics |
| d | = | Diameter of the perforation (cm) |
| de | = | Effective diameter of the perforations (cm) |
| DO2, DCO2, DC2H4 | = | Diffusion coefficient of O2, CO2 and ethylene in air (cm2 d−1) |
| Ea | = | Activation energy (kJ mol−1) |
| H | = | Hill coefficient |
| kO2, kCO2 | = | Rate coefficients (cm3 kg−1 d−1) |
| KmO2, KmCO2, KmC2H4 | = | Dissociation constants of the enzyme-substrate complex |
| KmuCO2, Kmu’CO2 | = | Constants of uncompetitive inhibition for consumption of O2 and generation of CO2, respectively |
| l | = | Film thickness (mm) |
| MM | = | Michaelis-Menten simple kinetics |
| MMC | = | Michaelis-Menten Cooperative kinetics |
| MMU | = | Michaelis-Menten Kinetics with uncompetitive inhibition |
| P | = | System pressure (atm, kPa) |
| pO2 | = | Partial pressure of O2 (atm, kPa) |
| QO2, QCO2, QC2H4 | = | Film permeability coefficient to O2, CO2 and ethylene (cm3 mm m2 d−1 atm−1) |
| R | = | Universal gas constant (0.008314 kJ mol−1 K−1 or 82.057 atm cm3 mol−1 K−1) |
| rO2, rCO2, rC2H4 | = | Consumption of O2 and generation of CO2 and ethylene rates (cm3 kg−1 d−1) |
| rO2max, rCO2max, rC2H4max | = | Maximum consumption of O2 and generation of CO2 and ethylene rates (cm3 kg−1 d−1) |
| RQ | = | Respiratory quotient, rCO2/rO2 |
| t | = | Packaging/storage time (d) |
| Δt | = | Time elapsing between two successive measurements |
| T | = | Temperature (°C, K) |
| V | = | Free package volume - headspace (cm3) |
| W | = | Fruit weight (kg) |
| yO2, yCO2, yC2H4 | = | O2, CO2 and ethylene concentrations inside the package |
| yO2out, yCO2out, yC2H4out | = | O2, CO2 and ethylene concentrations outside the package |
| yC2H4ini | = | Initial ethylene concentration inside the permeation cell |
| ε | = | Correction term for gas transmission through perforations (mm) |
Funding
The authors are grateful for the financial support provided by the Colombian Administrative Department of Science, Technology and Innovation—COLCIENCIAS with its Doctoral Scholarships Program (Convocation 567).
Additional information
Funding
References
- Galindo-Tovar, M.E.; Ogata-Aguilar O.; Arzate-Fernández, A.M. Some aspects of avocado (Persea Americana Mill.) Diversity and Domestication in Mesoamerica. Genetic Resources and Crop Evolution 2008, 55, 441–450.
- Ozdemir, F.; Topuz, A. Changes in Dry Matter, Oil Content, and Fatty Acids Composition of Avocado During Harvesting Time and Post-Harvesting Ripening Period. Food Chemistry 2004, 86(1), 79–83.
- Yousef, A.M.N.; Hassaneine, M.M.M. Influence of Different Harvest Dates and Ripening Periods on Fruit Quality and Oil Characteristics of Fuerte Avocados. Agriculture and Biology Journal of North America 2010, 1(6), 1223–1230.
- López-López, L.; Cajuste-Bontemps, F.J. Comportamiento Poscosecha de Fruta de Aguacate cv. Hass con Base en la Altitud de Producción y Altura de Floración [Postharvest behaviour of avocado fruits cv. Hass based on growing elevation and flowering height]. Revista Chapingo, Serie Horticultura 1999, 5(Sp), 365–371. .
- Dos Santos, K.L.; Peroni, N.; Guries, R.P.; Nodari, R.O. Traditional Knowledge and Management Of Feijoa (Acca Sellowiana) in Southern Brazil. Economic Botany 2009, 63(2), 204–214.
- East, A.R.; Trejo Araya, X.I.; Hertog, M.L.A.T.M.; Nicholson, S.E.; Mawson, A.J. The Effect of Controlled Atmospheres on Respiration and Rate of Quality Change in “Unique” Feijoa Fruit. Postharvest Biology and Technology 2009, 53, 66–71.
- Bontempo, P.; Mita, L.; Miceli, M.; Doto, A.; Nebbioso, A. Feijoa Sellowiana Derived Natural Flavone Exerts Anti-Cancer Action Displaying HDAC Inhibitory Activities. International Journal of Biochemistry & Cell Biology 2007, 39, 1902–1914.
- Parra, A.; Fischer, G. Maduración y Comportamiento Poscosecha de la Feijoa (Acca Sellowiana (O. Berg) Burret) [Ripening and postharvest behaviour of feijoa (Acca sellowiana O. Berg Burret). A review]. Una Revisión. Revista Colombiana de Ciencias Hortícolas 2014, 7(1), 98–110.
- Velho, A.C.; Do Amarante, C.V.T.; Argenta, L.C.; Steffenls, C. Influência da Temperatura de Armazenamento na Qualidade Pós-Colheita de Goiabas Serranas [Storage-temperature effect over the postharvest quality of feijoa fruits]. Revista Brasileira de Fruticultura 2011, 33(1), 14–20.
- Sandhya, K.V.K. Modified Atmosphere Packaging of Fresh Produce: Current Status and Future Needs. LWT–Food Science Technology 2010, 43, 381–392.
- Fonseca, S.C.; Oliveira, F.A.O.; Brecht, J.K. Modelling Respiration Rate of Fresh Fruits and Vegetables for Modified Atmosphere Packages: A Review. Journal of Food Engineering 2002, 52, 99–119.
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Development and Validation of a Comprehensive Model For Map of Fruits Based on Enzyme Kinetics Theory and Arrhenius Relation. Journal of Food Science Technology 2015, 52, 4286–4295.
- Mahajan, P.V.; Oliveira, F.A.R.; Montanez, J.C.; Frias, J. Development of User-Friendly Software For Design of Modified Atmosphere Packaging for Fresh and Fresh-Cut Produce. Innovative Food Science and Emerging Technologies 2007, 8, 84–92.
- Caleb, O.J.; Mahajan, P.V.; Opara, U.L.; Witthuhn, C. Modelling the Respiration Rates of Pomegranate Fruit and Arils. Postharvest Biology and Technology 2012, 64, 49–54.
- Devlieghere, F.; Jacxsens, L.; Serna-Tatay, M.; Debevere, J.; Meirlaen, J. Vanrolleghem, P. Modelling the Relation Between Ethylene Production Rate, Respiration Rate, and Their Influence on Climateric And Non-Climateric Fruits. Acta Horticulturae 2003, 600, 647–651.
- Mendoza, R.; Castellanos, D.A.; García, J.C.; Vargas J.C.; Herrera, A.O. Ethylene Production, Respiration and Gas Exchange Modelling in Modified Atmosphere Packaging for Banana Fruits. International Journal of Food Science and Technology. 2016, 51(3), 777–788.
- Sanders, M.G.; De Wild, H.P.J. The Relation Between in Vivo Ethylene Production and Oxygen Partial Pressure. Postharvest Biology and Technology 2003, 30, 143–151.
- Hertog, M.L.A.T.M.; Nicholson, S.E.; Whitmore, K. The Effect of Modified Atmospheres on the Rate Of Quality Change in Hass Avocado. Postharvest Biology and Technology 2003, 29, 41–53.
- Lasprilla, D. Estado Actual de Fruticultura Colombiana y Perspectivas para su Desarrollo. Rev. Bras. Fruticultura. 2011, 33(Sp. 1), 199–205.
- Bernal, J.A.; Díaz, C.A. Tecnología para el Cultivo del Aguacate; CORPOICA—Centro de Investigación La Selva: Rionegro, Antioquía, 2008; 241.
- Bhande, S.D.; Ravindra, M.R.; Goswami, T.K. Respiration Rate of Banana Fruit Under Aerobic Conditions at Different Storage Temperatures. Journal of Food Engineering 2008, 87, 116–123.
- Wang, Z.W.; Duan H.W.; Hu, C.Y. Modelling the Respiration Rate of Guava (Psidium Guajava L.) Fruit Using Enzyme Kinetics, Chemical Kinetics, and Artificial Neural Network. European Food Research and Technology 2009, 229, 495503.
- Heydari, A.; Shayesteh, K.; Eghbalifam, N.; Bordbar, H.; Falahatpisheh, S. Studies on the Respiration Rate of Banana Fruit Based on Enzyme Kinetics. International Journal of Agriculture and Biology 2010, 12, 145–149.
- Mangaraj, S.; Goswami, T.K. Modeling of Respiration Rate of Litchi Fruit Under Aerobic Conditions. Food and Bioprocess Technology 2011, 4, 272–281.
- Klee, H.J. Ethylene Signal Transduction. Moving Beyond Arabidopsis. Plant Physiology 2004, 135(29), 660–667.
- Cara, B.; Giovannoni, J.J. Molecular Biology of Ethylene During Tomato Fruit Development and Maturation. Plant Science. 2008, 175, 106–113.
- Barry, C; Giovannoni, J. Ethylene and Fruit Ripening. Journal of Plant Growth Regulation 2007, 26, 143–159.
- De Wild, H.P.J.; Woltering, E.J.; Peppelenbos, H.W. Carbon Dioxide and 1-MCP Inhibit Ethylene Production and Respiration of Pear Fruit by Different Mechanisms. Journal of Experimental Botany. 1999, 50, 837–844.
- Castellanos, D.A.; Cerisuelo, J.P.; Hernández-Muñoz, P.; Herrera, A.O.; Gavara, R. Modelling the Evolution of O2 and CO2 Concentrations in MAP of a Fresh Product: Application to Tomato. Journal of Food Engineering 2016, 168, 84–95.
- Chung, D.; Papadakis, S.E.; Yam, K.L. Simple Models For Evaluating Effects of Smalls Leaks on the Gas Barrier Properties of Food Packages. Packaging Technology and Science 2003, 16, 77–86.
- Génard, M.; Gouble, B. Ethy. A Theory of Fruit Climateric Ethylene Emission. Plant Physiology 2005 139, 531–545.
- Xanthopoulos, G.; Koronaki, E.; Boudouvis, A. Mass Transport Analysis in Perforation-Mediated Modified Atmosphere Packaging of Strawberries. Journal of Food Engineering 2012, 111, 326–335.
