ABSTRACT
This study investigated the effect of chitosan coating (1 and 2%) on the quality of grass carp fillets stored at 4°C for 20 days. During storage, the physicochemical properties (pH, thiobarbituric acid value, total volatile basic nitrogen value, trimethylamine nitrogen value, K-value, water loss, and instrumental texture), microbiology (total viable count and psychrotrophic count), and sensory properties were evaluated. The results showed that chitosan coating could effectively inhibit bacterial growth, improve physicochemical and sensory qualities, and it reduced the deterioration of the quality of grass carp fillets. Compared with fillets without chitosan coating, the shelf life of fillets with 1 and 2% chitosan coating was extended by ~3 and 6–7 days, respectively.
Introduction
Grass carp (Ctenopharyngodon idellus) is one of most important commercial freshwater fish species in China. The production of cultured grass carp in China is estimated to 5.07 million tons and ranked first among domestic cultured freshwater fish production in 2013.[Citation1] Palatability, nutritional characteristics, and low price of grass carp contribute to its popularity in the market. Traditionally, grass carp fish have been sold live. Undoubtedly, this method of sale reduces the transport efficiency and needs a higher cost to keep fish alive during transportation and distribution. Today, with the rapid development of cold chain and the changes in consumption patterns, the sale of grass carp fillets is increasing.[Citation2] However, high water activity, abundant nutrition, and neutral pH render the fish more perishable compared to terrestrial animals.[Citation3] Among various preservation techniques, refrigerated storage is one of the methods most widely employed for preservation of fish. However, refrigerated storage cannot entirely inhibit the growth of microorganisms and biochemical reactions during storage. Today, many studies are focused on using natural ingredients,[Citation4–Citation6] such as polyphenols, rosemary extract, and essential oils, to improve the quality of fish and ensure a longer shelf life.
Chitosan [b-(1, 4)-2-amino-2-deoxy-D-glucopyranose] is mainly derived from crustacean shells and is the second most abundant natural polymer, only after cellulose. Chitosan has a number of functional properties, which include film formation, biodegradability, biocompatibility, antibacterial, and anti-oxidative activities.[Citation7] Therefore, it has attracted much attention as a natural food additive. Chitosan has been studied for use as edible coatings or film material for marine fish. Fernandez-Saiz et al.[Citation8] reported that the chitosan film could significantly reduce microbial population of sole and hake fillets and lengthen their shelf lives. Mohan et al.[Citation9] showed that chitosan, as an edible coating, could enhance the quality of sardines during storage. These studies indicated the efficiency of chitosan coating for preserving the quality of marine fish. However, the internal microflora and endogenous enzymes for freshwater and seawater fish are different. Therefore, research in the application of chitosan for freshwater fish should be investigated separately. At present, very little work has been carried out to study the effect of chitosan coating on the quality of grass carp. Therefore, this research aimed at evaluating the effect of chitosan coating on the quality and shelf life of refrigerated grass carp fillets by testing the physicochemical, microbiological, and sensory parameters, which may promote the further processing of grass fish.
Materials and Methods
Preparation of Fish Sample
Live grass carp (weight: 2.5 ± 0.2 kg; length: 60.3 ± 5.2 cm) were purchased from China resource Vanguard Supermarket (Wuxi, Jiangsu Province, China) and transported to the Food Processing Technology Lab of Jiangnan University within 30 min. The fish were killed by slurry ice and slaughtered, filleted, and washed under tap water immediately. Each fillet from the fish dorsal muscle was about 18 ± 2 g (4 × 3 × 1.5 cm3).
Preparation of Chitosan Solutions and Treatment of Fillet Samples
Chitosan powder was purchased from Jinan Haidebei Marine Bioeeing Co. (Shandong Province, China), and the molecular weight and deacetylation of chitosan were 300~400 KDa and 85%, respectively. Chitosan solution was prepared in beakers by dissolving chitosan in a 1% (v/v) acetic acid solution to obtain the final concentration of 1 or 2% (w/v) of chitosan solution. To achieve complete dispersion of chitosan, the beakers were placed on a hotplate/magnetic stirrer and stirred for 2 h at room temperature. After, 1% (v/v) glycerol was added to chitosan solution as a plasticizer and stirred for 30 min. The treated fillets were randomly divided into three batches, which also included a control group (control). Control was treated with a solution mixture containing 1% glacial acetic acid and 1% glycerol. From the other two experimental groups, one group was dipped in 1% and the other in 2% cold chitosan solution and they were represented as CH1% and CH2%, respectively. Fillets were immersed in their respective solution under chilled condition (3–5°C) for 5 min, then removed and drained well for 60 min on a pre-sterilized metal net in a cool and dry place (4°C and 50% relative humidity). Thereafter, each sample was individually packed with air-proofed sterile polyethylene bag and subsequently stored up to 20 days in a refrigerator with the temperature of 4 ± 1°C. The samples from each group were randomly withdrawn for analysis according to the predetermined time intervals.
Proximate Composition Analysis
Proximate analysis, which included moisture, ash, crude protein, and crude fat content, were determined by the Association of Official Analytical Chemists (AOAC) methods with fresh fish muscle.[Citation10]
Physicochemical Analysis
The pH values of samples were determined according to the Chinese standard (GB/T 5009.45-2003). Total volatile basic nitrogen (TVB-N; mg N/100 g sample) and trimethylamine nitrogen (TMA-N; mg N/100 g sample) were determined using the AOAC methods. Thiobarbituric acid (TBA; mg malondialdehyde (MDA)/kg sample) values were measured colorimetrically as described by Siu and Draper.[Citation11] Water loss (%) was measured by comparing the difference in weights of the fish sample with and without exudate.
Instrumental Texture Analysis
The texture of the grass muscle fillets was analyzed using a TA.XT Plus Texture analyzer (Stable Micro Systems, Ltd., Surrey, U.K.), equipped with a specific cylindrical probe (P/25). Two consecutive cycles at 50% deformation degree were applied to construct texture profile analysis parameters. The trigger force was 5 g and the testing speed was 1 mm s−1. The texture parameters were calculated using its inbuilt software.
Determination of K-Value
K-value was determined by a procedure described by Gao et al.[Citation12] Standard adenosine triphosphate (ATP) and its breakdown products (adenosine diphosphate [ADP], adenosine monophosphate [AMP], inosine monophosphate [IMP], hypoxanthine [Hx], and inosine [HxR]) were purchased from Sigma-Aldrich (Shanghai, China) and evaluated by a reverse phase high-performance liquid chromatography (HPLC; Waters 1525, USA), equipped with ultraviolet visible (UV-Vis) detector (waters 2489, USA) and waters C18 column. K-value was calculated by the following equation:
Microbiological Analysis
Each sample (10 g) was homogenized with 90 mL sterile normal saline (0.85%). The sample was diluted serially with same normal saline. An aliquot (1 mL) of the diluent was poured into a Petri dish and mixed with plate count agar medium. All operations were performed under aseptic conditions. The inoculated plates were incubated at 30°C for 2 days for total viable count (TVC) and 10°C for a week for psychrotrophic count (PTC). All counts were expressed as log10 colony forming units (CFU) g−1.
Sensory Evaluation
Sensory analysis of raw grass carp fillets was carried out using the quality index method (QIM), as shown in , by 13 panelists, who were trained by professional laboratory staff (six women and seven men). The panelists recorded their scores from 1 to 5 for color, odor, and overall acceptability, as prescribed by Fan et al.[Citation13] with some modification, in which five presented the best quality in terms of freshness and the scores decreased according to gradual deterioration of fillets’ quality. The fillets with corresponding three-digit numbers were used for sensory evaluation, without any information about storage time and groups. An overall acceptability score of three was viewed as the borderline for acceptable quality.
Table 1. Sensory evaluation criterion of grass carp fillets.
Data Analysis
All parameters were determined in triplicate except for texture and sensory analysis, which were carried out six times. Statistical analysis was done using the Statistical Product and Service Solutions (SPSS) software 19.0 (SPSS Inc., Chicago, USA). The difference between the means was determined by Duncan’s test and significance was defined at p < 0.05.
Results and Discussion
Proximate Composition Analysis
The composition of fish may influence its sensory characteristics, such as color, taste, odor, flavor, and texture, and also its stability during storage.[Citation14] The mean (±SD) proximate composition of moisture, ash, crude protein and crude fat (g/100 g fish muscle) in the fresh grass carp were 78.98 ± 0.61, 1.15 ± 0.09, 18.03 ± 0.35, and 2.12 ± 0.21, respectively. The proximate composition was strongly dependent on the size, age, and gender of the fish, catching season, as well as living circumstance.[Citation15] The results indicated that grass carp muscle had high protein and fat content, reflecting its tendency to microbial spoilage and fat oxidation during storage. Therefore, it is necessary to find out effective methods to inhibit microbial growth and retard the degradation of lipid and protein.
Physiochemical Analysis
Water loss, one of the important physical indicators to evaluate the quality of fish, not only affects the texture, appearance, taste, and shortens its shelf life, but is also of great economic importance, since fish is sold by weight.[Citation16] Water loss in each group of fillets is summarized in . During the first 7 days of storage, there was no significant (p > 0.05) difference between the control and treated samples. The control samples showed a water loss of 7.3% on day 7, whereas, CH1% and CH2% samples were 6.1 and 5.1%, respectively. After 7 days, the water loss was significantly (p < 0.05) higher in control fillets compared to the chitosan coated fillets. The final value for water loss of the control samples was 16.8%, while the corresponding values for CH1% and CH2% samples were only 12.5 and 9.1%, respectively. Water loss in fish fillets is a complicated process, which can be caused by the degeneration of myosin, accompanied by decrease in water-holding capacity.[Citation9] The chitosan coating acts as water vapor barriers[Citation17] or reabsorb the water from the muscle during refrigerated storage,[Citation18] thereby reducing the water loss of fillets and improving their shelf life. Similar effects of chitosan coating were also reported for herring and Atlantic cod.[Citation19]
Figure 1. Changes in a: water loss, b: pH and c: TBA of grass carp fillets during refrigerated storage. Vertical bars represent the standard deviations (n=3).
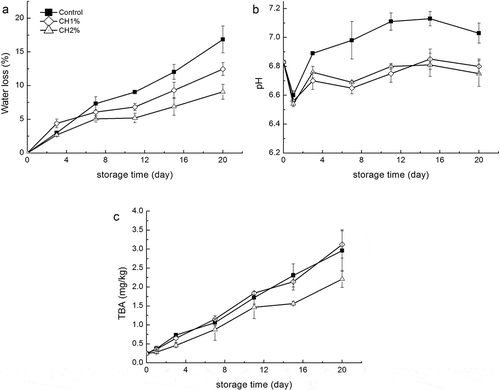
The effects of chitosan coating on pH values of fish fillets during storage are shown in . The changes in pH value for each group were similar, in which the values decreased initially and then increased slowly. Similar results were reported for other species.[Citation20,Citation21] The initial sharp decline in pH values might be due to the acidity of acetic acid solution used for treating fish fillets. In addition, other studies indicated that this phenomenon could be associated with the dissolution of CO2 or accumulated lactic acid during anaerobic glycolysis.[Citation14,Citation15] With prolonged storage time, proteins and other nitrogen-containing substances are decomposed to volatile bases, e.g., amines and trimethylamine by the action of microorganisms and enzymes, which result in an increase in pH.[Citation9] In case of the chitosan treated samples, the pH value was significantly (p < 0.05) lower than the control samples, thus suggesting that chitosan contributed to prevent microbial spoilage which might lead to the formation of alkaline components.
Figure 2. Changes in a: TVB-N value and b: TMA-N value of grass carp fillets during refrigerated storage. Vertical bars represent the standard deviations (n=3).
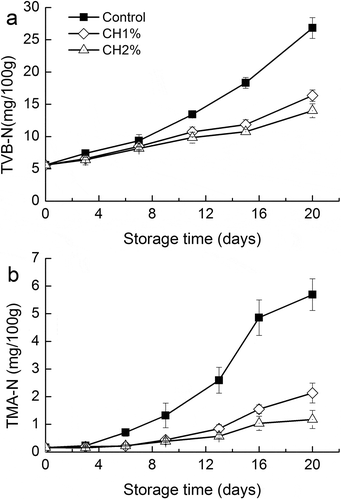
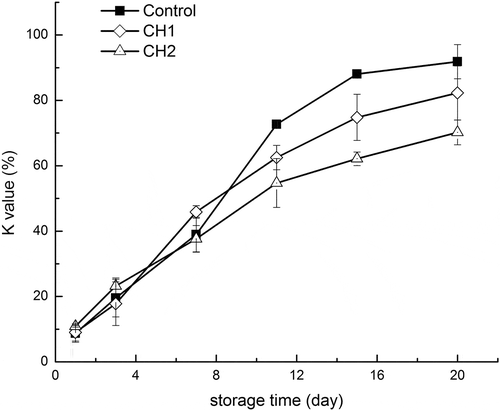
Figure 3. Changes in K value of grass carp fillets during refrigerated storage. Vertical bars represent the standard deviations (n=3).
TBA is widely used as an indicator to evaluate the degree of lipid oxidation. The presence of TBA reactive substances, such as malondialdehyde, are derived from the second stage of auto-oxidation.[Citation22] The changes in TBA values of samples are shown in . The initial TBA value of grass carp fillets was 0.248 mg MDA/kg and it increased during the entire storage period for all treatments. On the 20th day, the TBA values of control, CH1% and CH2% reached 2.96, 3.12, and 2.21 mg MDA/kg, respectively. The lowest value for CH2% showed that 2% chitosan coating could significantly (p < 0.05) delay lipid oxidation. However, the TBA values for control and CH1% showed no significant difference (p > 0.05). This differed from an earlier result,[Citation9] according to which 1% chitosan coating also had the ability to inhibit lipid oxidation of Indian oil sardine. This may be attributed to different fish species having different fat contents. According to Connell,[Citation23] TBA value in the range of 1–2 mg MDA/kg of fish sample is usually regarded as the limit of acceptability, beyond which fish will normally develop an objectionable odor. The time of control and CH1% exceeded this maximum permissible limit nearly 11 days, and more than 15 days for CH2%.
TVB-N and TMA-N produced as a result of bacterial spoilage and endogenous enzymes, which in turn decompose protein and non-protein nitrogenous compounds, are also important indexes of spoilage.[Citation24] Changes in TVB-N values in all groups of fillets during the refrigerated storage are presented in . The initial TVB-N content of 5.59 mg N/100 g was observed for fresh grass carp. TVB-N values for all samples increased with time, accounting for the increases in pH during the later stages of storage. On 20th day, TVB-N values for control, CH1%, and CH2% were 26.82, 16.34, and 14.01 mg N/100 g, respectively, reflecting a 39–47% reduction in the formation of TBV-N in the treated fillets. According to Connell,[Citation23] the limit of acceptability for fresh fish muscle is 35–40 mg N/100 g; however, some researchers also proposed different TVB-N values as the upper acceptable limits, such as 19–20 mg N/100 g for refrigerated sea bass.[Citation25] In this study, based on the results of sensory evaluation, 12–15 mg N/100 g has been considered as the threshold limit for spoilage of grass carp .
TMA is one of the main components responsible for unpleasant fishy taste after fish spoilage.[Citation26] As shown in , a low initial TMA-N value (0.18 mg N/100 g) indicated that fish used in this study were of good quality. TMA-N values between freshwater fish and marine fish showed significant differences,[Citation9,Citation27] mainly due to a far less trimethylamine oxide content in freshwater fish muscle than marine fish. During the first 3 days, TMA-N contents in all groups were at a low level. However, the rate of increase in case of untreated samples was significantly (p < 0.05) higher than the other two groups after 3 days. On the 20th day, TMA-N values of the untreated samples reached 5.69 mg N/100 g, along with a reduction of 62.5 and 79.3% for CH1% and CH2%, respectively. The slow rate of TMA-N production in CH1% and CH2% could be attributed to the antibacterial activity of chitosan. To the best of our knowledge, very little information is available in literature on the effect of chitosan on TMA-N production in freshwater fish. This result indicated that chitosan coating could effectively inhibit the accumulation of TMA-N in grass carp during refrigerated storage.
Texture Measurements
The texture of fish sample was influenced by intrinsic biological factors, such as the microbiological and the endogenous enzyme, causing protein degradation and softening of the muscle tissue.[Citation2] Thus, the texture of fish is an important indicator of freshness. The results of texture analyses of samples are shown in . During the entire storage period, the texture parameters such as hardness, springiness, gumminess, cohesiveness, chewiness, and resilience showed significant reduction (p < 0.05). Hardness, cohesiveness, chewiness, and resilience decreased rapidly during the first 7 days, and the extent of decline exceeded more than 50%. This was probably caused by enzymatic degradation of muscle proteins and loss of its firmness.[Citation28] After 11 days, no further significant (p > 0.05) decrease was observed in all groups of samples. This suggested that the early phase of storage was responsible for the major changes in fillet texture. This was similar to the results obtained by Gao et al.[Citation12] for pompano (Trachinotus ovatus) fillets. The texture properties of chitosan coated fillets showed a lower decreasing trend compared to control. The effect of the 2% chitosan coating was slightly better than that of the 1% chitosan coating. Therefore, texture properties such as hardness, cohesiveness, and chewiness in grass fish can be improved by coating with chitosan during refrigerated storage.
Table 2. Change in texture properties of grass carp fillets during refrigerated storage.
K-Value
During storage of fish postmortem, nucleotides in the fish muscle undergo stages of degradation as a result of endogenous biochemical changes.[Citation17] Calculation of contents of ATP and its related degradation products has been widely used as an effective indicator for monitoring the freshness of fish. Changes in K-value during refrigerated storage are depicted in . The initial K-values of the control, CH1%, and CH2% were 8.69, 9.04, and 10.90%, respectively. K-values of grass carp increased with storage time. After 7 days, the increase in the rate of the K-value in control was significantly higher than that of fillets treated with chitosan. On the 20th day, the mean K-values of control, CH1%, and CH2% were 91.81, 82.28, and 70.22%, respectively. Based on the process of ATP degradation, the main reasons for lower K-values in treated samples were the inhibition of degradation of IMP and the production of Hx. Hence, these results concluded that chitosan had the ability to minimize the activity of 5-nucleotidase.[Citation21] According to previous studies, the rejection level of K-value was close to 60%.[Citation29] Control and CH1% exceeded this limit about on the 11th day (72.7 and 62.5%), while CH2% exceeded this limit about on the 15th day (62.1%).
Microbiological Analysis
Changes in TVC and PTC of grass carp fillets during refrigerated storage are shown in . The initial TVC (log10 CFU/g) of the control, CH1%, and CH2% fillets were 4.90, 4.53, and 4.29, respectively. These values were higher than the values reported by Zhang et al.[Citation30] for grass fish (3.45 log10 CFU/g), which probable could be either due to individual differences or the handling of fish during processing. The lower initial TVC for CH1% and CH2% indicated that chitosan coating reduced the microbial population. The increase was significantly (p < 0.05) higher for untreated samples compared to treated samples. On the 20th day, the microbial population of untreated samples increased to 8.32 log10 CFU/g. Meanwhile, reductions of 1.09 and 1.78 log10 CFU/g were observed for CH1% and CH2% compared to untreated samples. According to ICMSF,[Citation31] 7.0 log10 CFU/g of TVC is regarded as the maximal permissible limit. The shelf life of control and CH1% was less than 11 and nearly 15 days, respectively. For fillets treated with 2% chitosan, TVC below the limit level during the whole storage period.
Psychrotrophic bacteria are mainly responsible for spoilage of fish fillets during refrigerated storage. The changes in PTC of fish fillets are shown in . PTC of grass carp fillets increased progressively from initial values of 3.47, 3.65, and 3.23 log10 CFU/g to final values of 7.66, 6.44, and 6.19 log10 CFU/g for control, CH1%, and CH2%, respectively. Chitosan significantly inhibited the growth of the total psychrotrophic bacteria.
Figure 4. Changes in a: total viable count (TVC) and b: psychrotrophic count (PTC) values of grass carp fillets during refrigerated storage. Vertical bars represent the standard deviations (n=3).
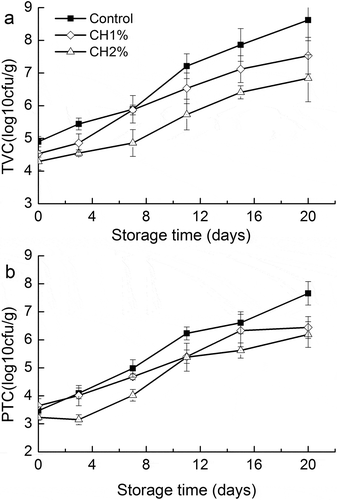
The antimicrobial properties of chitosan coating have been reported in several earlier studies. Fernández-Saiz et al.[Citation8] reported a significant (p < 0.05) increase of the lag phase and a reduction of the final microbial population were detected mainly for total aerobic mesophilic bacteria, H2S-producing bacteria and Pseudomonas of sole and hake fillets after chitosan coating. López-Caballero et al.[Citation32] found that chitosan coating along with gelatin inhibited the growth of gram-negative bacteria in fish patties. Other researchers also reported that the complex chitosan–gelation film reduced the growth of gram-negative bacteria drastically, especially enterobacteriaceae.[Citation33] Numerous intrinsic and extrinsic factors affect the antimicrobial action of chitosan, such as pH, presence or absence of metal cations, molecular weight of chitosan.[Citation34] The mechanism of antimicrobial action of chitosan could be related to interactions between the positively charged chitosan molecules and the negatively charged microbial cell membrane.[Citation7] In the present study, chitosan coating reduced the TVC and PTC of grass carp fillets during refrigerated storage.
Sensory Evaluation
Color, odor, and overall acceptability were chosen to evaluate the freshness of grass carp fillets during storage. The results of the sensory evaluation of samples were presented in . Initially, the fillets were fresh, and all groups of fillets had fresh fish odor and characteristic glossy surface. However, odor scores of control declined rapidly during the whole storage and showed a significantly (p < 0.05) different score compared to both chitosan coated groups. Fishy and putrid odors increased gradually in control after 7 days of storage. These unpleasant odors were released as a result of microbial spoilage which caused the accumulation of metabolites, such as trimethylamine and biogenic amines.[Citation35] Moreover, the surface of fish muscle turned faint yellow as spoilage progressed. The color was not significantly different between control and CH1% from the 3rd day, meanwhile, higher preference scores were obtained for CH2% compared to the other two groups. The results were consistent with results of TBA discussed previously. Considering the overall acceptability, a shelf life of less than 11 days could be observed for control, meanwhile, more than 11 and 15 days for CH1% and CH2%, respectively. Similar results had been reported for lingcod fillets and rainbow trout.[Citation5,Citation36] Therefore, chitosan coating showed a positive effect in the extension of shelf life of refrigerated grass fish.
Table 3. Changes in sensory evaluation of grass carp fillets during refrigerated storage.
Conclusion
An edible coating of chitosan could effectively extend the shelf life of refrigerated grass carp fillets. From the results of physiochemical, microbiological, and sensory analyses, it was proved that chitosan coating could inhibit the degradation of nucleotides and proteins, lower the rate of microbial growth, and maintain good sensory characteristics. In addition, it also contributed to lower water loss and textural changes. Based on comprehensive results of the K-value, TVC, and sensory evaluation, the shelf life of grass carp fillets was extended by about 3 days and 6–7 days for CH1% and CH2%, respectively.
Funding
This research was supported by the earmarked fund for China Agriculture Research System (CARS-46), National Natural Science Foundation of China (NSF31301508), and College Graduate Research and Innovation Project of Jiangsu Province (KYLX-1163).
Additional information
Funding
References
- Anonymous. Fishery Yearbook in China in 2014; Chinese Agriculture Press: Beijing, 2014.
- Liu, D.S.; Liang, L.; Xia, W.S.; Regenstein, J.M.; Zhou, P. Biochemical and Physical Changes of Grass Carp (Ctenopharyngodon Idella) Fillets Stored at –3 and 0 Degrees C. Food Chemistry 2013, 140, 105–114.
- Li, X.; Li, J.; Zhu, J.; Wang, Y.; Fu, L.; Xuan, W. Postmortem Changes in Yellow Grouper (Epinephelus Awoara) Fillets Stored Under Vacuum Packaging at 0°C. Food Chemistry 2011, 126, 896–901.
- Li, T.T.; Li, J.R.; Hu, W.Z.; Zhang, X.G.; Li, X.P.; Zhao, J. Shelf-Life Extension of Crucian Carp (Carassius Auratus) Using Natural Preservatives During Chilled Storage. Food Chemistry 2012, 135, 140–145.
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of Chitosan Coatings Enriched with Cinnamon Oil on the Quality of Refrigerated Rainbow Trout. Food Chemistry 2010, 120, 193–198.
- Yıldız, P.O.; Yangılar, F. Effects of Different Whey Protein Concentrate Coating on Selected Properties of Rainbow Trout (Oncorhynchus Mykiss) During Cold Storage (4°C). International Journal of Food Properties 2015, DOI:151014195705003
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food Applications of Chitin and Chitosans. Trends in Food Science & Technology 1999, 10, 37–51.
- Fernandez-Saiz, P.; Sanchez, G.; Soler, C.; Lagaron, J.M.; Ocio, M.J. Chitosan Films for the Microbiological Preservation of Refrigerated Sole and Hake Fillets. Food Control 2013, 34, 61–68.
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Gopal, T.K.S. Effect of Chitosan Edible Coating on the Quality of Double Filleted Indian Oil Sardine (Sardinella Longiceps) During Chilled Storage. Food Hydrocolloids 2012, 26, 167–174.
- AOAC. Official Methods of Analysis; AOAC: Washington, D.C., 1980.
- Siu, G.; Draper, H. A Survey of the Malonaldehyde Content of Retail Meats and Fish. Journal of Food Science 1978, 43, 1147–1149.
- Gao, M.S.; Feng, L.F.; Jiang, T.J.; Zhu, J.L.; Fu, L.L.; Yuan, D.X.; Li, J.R. The Use of Rosemary Extract in Combination with Nisin to Extend the Shelf Life of Pompano (Trachinotus Ovatus) Fillet During Chilled Storage. Food Control 2014, 37, 1–8.
- Fan, W.J.; Chi, Y.L.; Zhang, S. The Use of a Tea Polyphenol Dip to Extend the Shelf Life of Silver Carp (Hypophthalmicthys Molitrix) During Storage in Ice. Food Chemistry 2008, 108, 148–153.
- Abdollahi, M.; Rezaei, M.; Farzi, G. Influence of Chitosan/Clay Functional Bionanocomposite Activated with Rosemary Essential Oil on the Shelf Life of Fresh Silver Carp. International Journal of Food Science Technology 2014, 49, 811–818.
- Feng, L.; Jiang, T.; Wang, Y.; Li, J. Effects of Tea Polyphenol Coating Combined with Ozone Water Washing on the Storage Quality of Black Sea Bream (Sparus Macrocephalus). Food Chemistry 2012, 135, 2915–2921.
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of Water-Holding Capacity of Meat: The Role of Postmortem Biochemical and Structural Changes. Meat Science 2005, 71, 194–204.
- Song, Y.L.; Liu, L.; Shen, H.X.; You, J.A.; Luo, Y.K. Effect of Sodium Alginate-Based Edible Coating Containing Different Anti-Oxidants on Quality and Shelf Life of Refrigerated Bream (Megalobrama Amblycephala). Food Control 2011, 22, 608–615.
- Duan, J.Y.; Cherian, G.; Zhao, Y.Y. Quality Enhancement in Fresh and Frozen Lingcod (Ophiodon Elongates) Fillets by Employment of Fish Oil Incorporated Chitosan Coatings. Food Chemistry 2010, 119, 524–532.
- Jeon, Y.J.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an Edible Invisible Film for Quality Preservation of Herring and Atlantic Cod. Journal of Agricultural and Food Chemistry 2002, 50, 5167–5178.
- Li, K.; Luo, Y.; Shen, H. Postmortem Changes of Crucian Carp (Carassius Auratus) During Storage in Ice. International Journal of Food Properties 2014, 18, 205–212.
- Fan, W.J.; Sun, J.X.; Chen, Y.C.; Qiu, J.; Zhang, Y.; Chi, Y.L. Effects of Chitosan Coating on Quality and Shelf Life of Silver Carp During Frozen Storage. Food Chemistry 2009, 115, 66–70.
- Lindsay, R. Flavour of Fish. In Seafoods: Chemistry, Processing Technology, and Quality. Springer: New York, 1994; 75–84.
- Connell, J.J. Control of Fish Quality; Fishing News Books Ltd.: London, UK, 1980.
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar Films Containing Green Tea Extract and Probiotic Bacteria for Extending Fish Shelf-Life. LWT–Food Science and Technology 2014, 55, 559–564.
- Grigorakis, K.; Alexis, M.; Gialamas, I.; Nikolopoulou, D. Sensory, Microbiological, and Chemical Spoilage of Cultured Common Sea Bass (Dicentrarchus Labrax) Stored in Ice: A Seasonal Differentiation. European Food Research & Technology. 2004, 219, 584–587.
- Frangos, L.; Pyrgotou, N.; Giatrakou, V.; Ntzimani, A.; Savvaidis, I.N. Combined Effects of Salting, Oregano Oil and Vacuum-Packaging on the Shelf-Life of Refrigerated Trout Fillets. Food Microbiology 2010, 27, 115–121.
- Tsiligianni, M.; Papavergou, E.; Soultos, N.; Magra, T.; Savvaidis, I.N. Effect of Chitosan Treatments on Quality Parameters of Fresh Refrigerated Swordfish (Xiphias Gladius) Steaks Stored in Air and Under Vacuum Conditions. International Journal of Food Microbiology 2012, 159, 101–106.
- Ocaño-Higuera, V.; Maeda-Martínez, A.; Marquez-Ríos, E.; Canizales-Rodríguez, D.; Castillo-Yáñez, F.; Ruíz-Bustos, E.; Graciano-Verdugo, A.; Plascencia-Jatomea, M. Freshness Assessment of Ray Fish Stored in Ice by Biochemical, Chemical, and Physical Methods. Food Chemistry 2011, 125, 49–54.
- Ehira, S. A Biochemical Study on the Freshness of Fish; Bulletin of Tokai Regional Fisheries Research Laboratory, 1976, 88, 130–132
- Zhang, L.N.; Li, X.; Lu, W.; Shen, H.X.; Luo, Y.K. Quality Predictive Models of Grass Carp (Ctenopharyngodon Idellus) at Different Temperatures During Storage. Food Control 2011, 22, 1197–1202.
- ICMSF. Micro-Organisms in Foods: A Publication of the International Commission on Microbiological Specifications for Foods (ICMSF) of the International Association of Microbiological Societies; University of Toronto Press: Toronto, Canada, 1986; 181–196.
- López-Caballero, M.; Gómez-Guillén, M.; Pérez-Mateos, M.; Montero, P. A Chitosan–Gelatin Blend as a Coating for Fish Patties. Food Hydrocolloids 2005, 19, 303–311.
- Gomez-Estaca, J.; Lopez de Lacey, A.; Lopez-Caballero, M.E.; Gomez-Guillen, M.C.; Montero, P. Biodegradable Gelatin-Chitosan Films Incorporated with Essential Oils as Antimicrobial Agents for Fish Preservation. Food Microbiology 2010, 27, 889–896.
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. International Journal of Food Microbiology 2010, 144, 51–63.
- Katikou, P.; Georgantelis, D.; Paleologos, E.K.; Ambrosiadis, I.; Kontominas, M.G. Relation of Biogenic Amines’ Formation with Microbiological and Sensory Attributes in Lactobacillus-Inoculated Vacuum-Packed Rainbow Trout (Oncorhynchus Mykiss) Fillets. Journal of Agricultural Food Chemistry 2006, 54, 4277–4283.
- Duan, J.Y.; Jiang, Y.Y.; Cherian, G.; Zhao, Y.Y. Effect of Combined Chitosan-Krill Oil Coating and Modified Atmosphere Packaging on the Storability of Cold-Stored Lingcod (Ophiodon Elongates) Fillets. Food Chemistry 2010, 122, 1035–1042.
