ABSTRACT
Glass transition of pomegranate skin was measured by thermal, mechanical, and magnetic resonance techniques. Differential scanning calorimetry thermogram showed a shift (i.e., onset glass transition at 20°C) followed by an endothermic peak (i.e., solids-melting peak at 165°C and enthalpy 140 kJ/kg). Overlapping of the glass transition and melting was observed in the differential scanning calorimetry thermogram; however, more sensitive modulated differential scanning calorimetry allowed to separate two transitions (i.e., glass transition from reversible and melting from non-reversible thermograms). The onset of mechanical glass-rubber transition from differential mechanical thermal analysis was observed at 122°C with a shift in the storage modulus (E′); however, the onset of liquid-like or entangled-reaction dominating transition was observed at 70°C (i.e., onset peak in loss modulus, E′′) and peak at 184°C. In addition onset peak in tan δ was observed at 113°C and peak at 201°C. Spin–spin (T2 relaxation) and spin-lattice (T1 relaxation) relaxations in time domain nuclear magnetic resonance was modeled by two-exponential relaxation curve (i.e., rigid and flexible domains). T2 relaxation showed maximum peak with an onset at 40°C with maximum peak at 150°C. Rigid domain of T1 relaxation showed a minimum peak onset at 40°C and a minimum peak at 120°C, whereas flexible component showed an onset at 20°C and a minimum peak at 160°C.
Introduction
Thermal characteristics of foods and biomaterials, such as glass transition, stickiness, caking, and melting are important to determine the processing conditions, as well as its stability during storage. The glass transition greatly influences food stability, since matrix becomes kinetically immobilized, therefore, it restricts reactions.[Citation1,Citation2] It assists in identifying biomaterial’s stability during storage as well as selecting suitable conditions of temperature and moisture content for processing.[Citation3,Citation4] Measurement of glass transition is not straightforward, especially for non-homogeneous complex biomaterials.[Citation5] Thermal analysis, such as differential scanning calorimetry (DSC) is commonly used to measure glass transition, and this technique is most suitable for sugar based and other simple biomaterials. Recent studies showed the difficulty in using DSC for complex biomaterials.[Citation5,Citation6] In this case modulated DSC and differential mechanical thermal analysis (DMTA) are commonly used.[Citation5,Citation7] DMTA measures the effect of longitudinal and sinusoidal varying stress on dynamic moduli and modulated DSC measures the reversible and non-reversible components of total heat flow. In some instances, the glass transition measured by DSC are related with the measured values from DMTA[Citation5,Citation8,Citation9] and time domain nuclear magnetic resonance (TD-NMR) relaxation.[Citation6] In order to understand different relaxation processes in the biomaterials, the use of combinations of instruments, such as DMTA and DSC could be necessary.[Citation10] However, there are negligible work has been conducted to compare the glass transition measurement by thermal, mechanical, and magnetic relaxation techniques.
Recently interests are growing to use the wastes or byproducts, such as seeds, skins, and waste from fruit pulp to convert these into value added products. In order to convert the skin into different value added functional products, it is important to understand its physico-chemical and structural characteristics. Gambhir et al.[Citation11] measured the T2 and T1 relaxations of chilled and frozen-thawed peel and flesh of a naval orange, which could be used to determine the damage during frozen-thawed or chilled injury of orange during storage. However, negligible work of TD-NMR signal characteristics as a function of temperature was presented in the literature. The objectives of this work were to measure the glass transition of pomegranate skin by thermal, mechanical, and nuclear magnetic resonance techniques, and to determine their relationships. The information on the glass transition of pomegranate skin could assist in determining its stability during processing and storage (i.e., retaining its functional components or to develop new functional biomaterials).
Materials and Methods
Materials
Fresh pomegranate fruits (variety: Helow, literally sweet) were purchased from a local farm in Al-Jabal Al-Akhdar, Oman and transported to the Laboratory in an electric cooler box maintained at 9°C. Fruits were evaluated for defects visually and were cut into four portions. The arils were separated manually from peels and these were packaged in polyethylene bags (500 g) and stored frozen at –40°C until used for experiments.
Dried Skin Preparation
Peels were cut into 1 cm × 1 cm squares and then placed into different plastic containers (50 mL). It was kept in a freezer at –40°C for at least 16 h. The frozen peels were freeze-dried at 20°C (i.e., drying started at –40°C and ended at 20°C) for 96 h using Edwards K4 Freeze Dryer (Corawky, Crawley, England). Freeze-dried pomegranate peels were placed in an open weighing bottles and stored in an air-tight container maintained at specific relative humidity environments. The humidity was created and maintained at relative humidity 11.1%. This humidity in the chamber was created by saturated lithium chloride salt solution in a beaker placed in the chamber. A layer of salt crystal was maintained in the slurries during the equilibration period, which ensured saturated condition. The samples were equilibrated until constant mass was achieved. Intact pieces were used for mechanical analysis. Equilibrated freeze-dried peels were also ground into powder using a KMF grinder (KIKA Werke, Wilmington, USA) running at 6000 rpm, and used for thermal and magnetic relaxation analyses.
DSC
DSC (Q10, TA Instruments, New Castle, DE, USA) with a mechanical refrigerated cooling system (i.e., capable to cool up to –90°C) was used to measure thermal characteristics. The instrument was calibrated for heat flow and temperature using distilled water (melting point, mp: 0°C; ΔHm: 334 J/g) and indium (melting point, mp: 156.5°C; ΔHm: 28.5 J/g). An aluminum pan of 30 μL, which could be sealed with a lid, was used in all experiments. An empty sealed pan was used as reference and nitrogen at a flow rate of 50 mL/min was used as the carrier gas. Sample (10 mg) was placed into a hermetically sealed aluminium pan and cooled to –90°C at a rate of 5°C/min, and maintained for 10 min. Subsequently they were scanned from –90 to 300°C at a rate of 10°C/min. The glass transition was identified from a shift (i.e., onset, mid, and end temperatures; specific heat change) in the thermogram line (i.e., plot of heat flow versus temperature) and solids-melting was identified from an endothermic peak characterized as onset, maximum slope, peak, and end temperatures; and area of the peak as the solids-melting enthalpy. Measurement was replicated four times and presented as average with standard deviation.
Modulated DSC (MDSC)
In the case of MDSC (Q1000, TA Instruments, New Castle, DE), samples were scanned from –90 to 200°C at a constant rate 10°C/min with a modulation of 0.5°C amplitude and 40 s period of modulation. The sample preparation and calibration procedures were same as described in the DSC. Thermograms were analyzed from its total, reversible, and non-reversible heat flow. The glass transition was determined from a shift in the reversible heat flow thermogram and solids-melting was determined from an endothermic peak in the non-reversible thermogram. The shift and peak were characterized similarly as discussed in the earlier section of DSC. The average values and standard deviations of three replicates were obtained.
Dynamic Mechanical Thermal Analysis (DMTA)
The DMTA V (Rheometrics, Piscataway, NJ) in compression and parallel-plate geometry was used to determine the E′ (storage modulus), E′′ (loss modulus), and tan δ. Instrument was operated as a compression in vertical orientation (i.e., a motor driven shaft moved upward and downward direction) and it was cooled using a cryogenic accessory with liquid nitrogen from –80°C and then controlled heating was performed up to 300°C. Sample (1 cm × 1 cm) was placed on the round stud attached with drive-shaft and it was sandwiched with another fixed round stud fixed on the top. At 30°C, linear viscoelastic region was determined at a 0.5% compression within the frequency range 0.1–100 Hz. The frequency of 0.1 Hz was selected based on the viscoelastic region as determined during initial experiment. To conduct temperature sweeps, samples were heated at a heating rate of 10°C/min from –80 to 300°C at 0.5% compression with frequency of 0.1 Hz. Different characteristic temperatures were determined from the mechanical spectrum as suggested by Rahman and Al-Saidi.[Citation5] From the storage modulus, a shift or change in slope of E′ was defined as the mechanical-glass transition (onset, glass-leathery transition, Tri and end, onset of rubbery region, Tru). From the loss modulus, an onset of liquid-like or entangled-reaction dominating transition (Trz) at onset of maximum peak and a peak (Trs) indicating the start of softening region were determined. In addition, a sub-glass relaxation was also detected with a small peak at low temperature. The tan δ plot showed an onset peak (Tdi) and peak (Tdp), respectively.
TD-NMR
TD-NMR measurements were performed by means of a Bruker Minispec 20 MHz RF (mq one with modular or pulse generator, 0.47 T, P20, Karlsruhe, Germany) equipped with NMR data station analysis software. The equipment was calibrated with three standards provided by the manufacturer. The protocol was first optimized with gain, number of scans, and duration of relaxation. Proton spin-spin T2 relaxation was measured with Carr-Purcell Plus sequence procedure and signal after first 90° pulse was acquired (i.e., fid-cp-mb procedure). Proton spin-lattice T1 relaxation was measured by inversion recovery pulse sequence (i.e., sat-mb procedure). shows the conditions of T2 and T1 relaxation used in this study. Free induction decay (FID) signal was recorded automatically and analyzed by bi-exponential function as:[Citation12]
Table 1. TD-NMR conditions for T2 and T1 relaxation.
where S1 and S2 are the intensities at any time for T1 and T2 relaxations, I11, I12, I21, and I22 are the first and second onset intensities for first and second linear regions in the cases of T1 and T2 relaxations (i.e., pre-exponential factors) and T11, T12, T21, and T22 are the relaxation times for rigid and flexible domains, respectively, K1 and K2 are the offsets for T1 and T2 relaxations. The pre-exponent factors and relaxation times were plotted as a function of temperature in order to identify characteristic relaxation and molecular order in the peels. Powder sample was filled in the NMR tube (inside diameter: 8 mm), placed in an aluminum block and then stored at –80°C for 24 h in a small ultra-low temperature freezer capable to cool up to –120°C. After equilibration, sample tube was quickly placed inside the NMR measurement hole and measured its magnetic relaxation. After measurement, the tube was placed again in the freezer at different preset equilibrium temperatures for 30 min and then measured its relaxation. The sample equilibration time was kept for 30 min until 20°C. The sample tube was heated in a special designed heater supplied by Bruker for the measurement above 20°C. The sample equilibration time was then kept for 5 min.
Results and Discussion
shows the DSC and MDSC thermograms of freeze-dried pomegranate skin powder (moisture content: 7.7 g/100 g peel). The DSC thermogram shows an endothermic shift (marked as G), an endothermic peak for solids-melting (marked as M) and an exothermic shift (marked as C) at high temperature after solids-melting (). The endothermic shift was due to the glass-rubber transition followed by solids-melting peak. It was difficult to separate the onset of glass transition and solids-melting due to the overlapping of the two thermal processes. Thus two enthalpy values were determined one onset at point “a” and end at e2, and another one onset at point “b” and end at e1. The onset glass transition was observed at 20°C (specific heat change at transition: 1058 J/kg K), while onset and peak of the melting endotherm were observed at 47 and 165°C (enthalpy: 140 kJ/kg), respectively (). However, glass transition shift and endothermic peak for solids-melting can be separated using MDSC, as discussed later. The exothermic shift (marked as C in ), after solids-melting, was due to the interactions of different melted components in the matrix. Similarly exothermic peak after solids-melting was observed in the case of dried milk[Citation13] and date-pits.[Citation7]
Table 2. Thermal characteristics measured by differential scanning calorimeter (DSC) and modulated DSC (MDSC).
Figure 1. Heating themogram line of pomegranate peel powder as a function of temperature. A: DSC heat flow (G: glass transition endothermic shift, M: solids-melting, C: exothermic shift), B: Total MDSC heat flow (G: glass transition shift, N: wax melting, M: solids-melting), C: MDSC reversible heat flow (G: glass transition, S: structure formation), D: MDSC non-reversible heat flow (N: wax-melting, M: solids-melting).
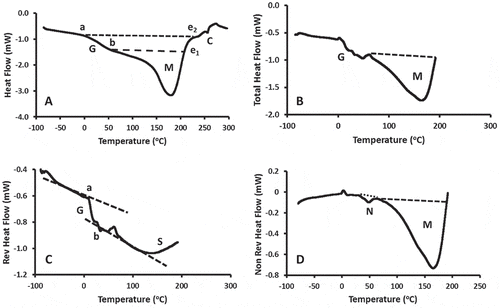
The MDSC thermogram (i.e., total heat flow) shows a shift (marked as G) and an endothermic peak (). The total heat flow thermogram can be separated as reversible (i.e., processes for the specific heat changes) and non-reversible heat flow (i.e., processes involved latent heat). The non-reversible heat flow indicated glass transition through a shift (marked as G) and increase in specific heat (marked as S) due to the structure formation in the rubbery state (). The structure formation was also observed by Lu et al.[Citation14] after the end of glass transition if two amorphous and/or crystalline components were present. The non-reversible heat flow thermogram showed two main endothermic peaks, one for melting of wax in the skin (marked as N) and another one for solids-melting (marked as M; ). The data of glass transition, wax-melting, and solids-melting are presented in . The onset glass transition from the reversible heat flow was observed at 11°C (specific heat change: 247 J/kg K). The lower value was due to the relaxed sample in MDSC as compared to the relatively stiff sample in DSC during heating. Modulated heating in MDSC caused relaxation while heating. The lower specific heat change (220 J/kg) as compared to DSC (i.e., 1058 J/kg K) also justified more relaxed molecules. However, another possibility of the difference in specific heat changes could be due to the interference of the melting process to the state change. The reversible heat flow also showed structure building at 119°C after glass transition (). Considering non-reversible heat flow, the wax-melting peak at 46°C (enthalpy change: 0.5 J/kg K) and solids-melting peak at 152°C (i.e., enthalpy change: 167 kJ/kg). The melting point of candelilla wax was varied from 67.0–79.0°C,[Citation15] whereas sugarcane wax varied from 77.6–80.0°C.[Citation16] However, the melting temperature was reduced to 40°C when these wax were used in preparing organogels,[Citation17] which was similar to the results observed in this study.
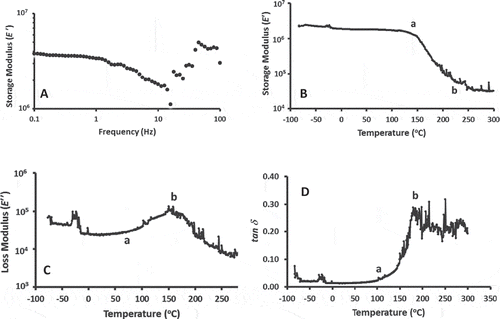
In the case of DMTA, initial trial indicated that 0.5% compression provided good contact and reproducibility during complete temperature scan. shows the storage modulus (E′) measured at 0.5% compression as function of frequency. This figure indicates that the peel at 0.5% compression showed the viscoelastic region up to frequency of 1 Hz, thus 0.1 Hz was used for DMTA measurement and characteristic temperatures are shown in . The storage modulus (E′) shows an onset shift (Tri) at 122°C considered as mechanical-glass transition and end (Tru) at 216°C considered as onset of rubbery or end of leathery transition (). The loss modulus (E′′) showed an onset peak (Trz) at 70°C and peak (Trs) at 184°C (), whereas tan δ showed an onset peak (Tdi) at 113°C, and peak (Tdp) at 201°C, respectively (). The lower value of Trz as compared to Tri could be related with the structural characteristics of pomegranate peel considering cellulosic (i.e., fibers) network (back-bone of the structure), which was filled with the cementing materials (mainly polysaccharides and wax). The mobility or softening or weakening started first on the cementing components at 70°C without affecting the back-bone; however, the softening of the back-bone started at 122°C as evidence from the storage modulus spectra. Other characteristic temperatures at higher temperature indicated the mechanical softening of the complete cellular matrix, which was similar to the end of the solids-melting determined from DSC and MDSC. Earlier literature showed that mechanical-glass transition of biological materials observed more at higher temperature as compared to the thermal-glass transition.[Citation9] In the case of organogel (i.e., soft fat gel), the behaviors of modulus and tan δ was observed similar but the shift of modulus and onset of tan δ peak were observed at the same temperature.[Citation17] In the case of date fruit, Tgi was observed at –50°C by DSC (heating rate: 10°C/min) whereas Tri and Trp were observed at –20 (i.e., 30°C higher) and 20°C, respectively as measured by MDSC.[Citation5] Jankowsky et al.[Citation18] observed increased glass transition due to the inclusion of fibers in polymer composites as compared to the composites without fibers. In addition fibers orientation played a role in increasing glass transition. They pointed that fibers restrict the polymer molecular motion, and modified packing density, conformation, and orientation of polymer chain segments due to the strong interfacial adhesion. Kasapis et al.[Citation19] identified 17°C decreased in the mechanical glass transition of freeze-dried apple (moisture: 81 g/100 g sample) when porosity increased from 0.4 to 0.8, while there was no significant change of DSC (i.e., thermal) glass transition as a function of porosity. However, thermal DSC was close to the high porosity values of the mechanical glass transition. Homer et al.[Citation20] observed good agreement between DSC and DMTA for the higher moisture content, while much higher values were observed in the case of DMTA at lower moisture contents (<13%). Therefore, glass transition measured by mechanical method was more complicated as compared to the thermal glass transition, and mechanical process is strongly related with composition as well as macro-structure.
Table 3. Characteristic temperatures from mechanical spectrum determined by DMTA.
Figure 2. Storage and loss modulus, and tan δ measured by DMTA. A: Frequency swift at 30°C. B: Storage modulus (E′, Pa) as a function of temperature, C: Loss modulus (E′′, Pa) as a function of temperature, D: Phase angle (tan δ) as a function of temperature.
presents the relaxation times of TD-NMR for rigid and flexible domains for T2 and T1 relaxations. The characteristic temperatures are shown and . Both relaxation times (T21 and T22) plots as shown in and show nearly constant values below 40°C (marked as r) and a sharp increase followed by a peak at 150°C (marked as j). Similar behavior of nearly constant value below critical temperature and sharp increase with another constant region were reported in the literature.[Citation21,Citation22] Hills and Pardoe[Citation23] observed similar trends in the case of T21 and T22 relaxation times for 10% water-maltose. In the case of frozen fish fillet (T21 from –50 to 30°C), Pitombo and Lima[Citation24] also observed similar curve with a critical temperature at –22.4°C with slow increase up to –6.3°C followed by a sharp increase and then nearly constant region. They pointed that these critical temperatures are related to the melting of ice during heating. In the case of starch gel, this critical temperature was related to the Tm′ (i.e., end of melting or onset of glass transition after maximal-freeze-concentration condition).[Citation25] Chung et al.[Citation26] also observed similar behavior for T21 in the cases of different food powders and determined that the peak temperature was related to the sticky point of power. In the case of maize, Borompichaichartkul et al.[Citation27] observed similar curve for T22, while a sharp increase in the T21 was also observed at low temperature.
Table 4. Characteristic temperature of TD-NMR relaxation (relaxation time).
Table 5. Characteristic temperature of TD-NMR relaxation (pre-exponent intensities).
Figure 3. Relaxation time for spin-spin relaxation (T2 relaxation) and spin-lattice (T1 relaxation). A: T21, relaxation time for first portion of T2 relaxation (rigid domain), B: T22, relaxation time for second relaxation portion of T2 relaxation (flexible domain), C: T11, relaxation time for first portion of T1 relaxation (rigid domain), B: T12, relaxation time for second relaxation portion of T1 relaxation (flexible domain).
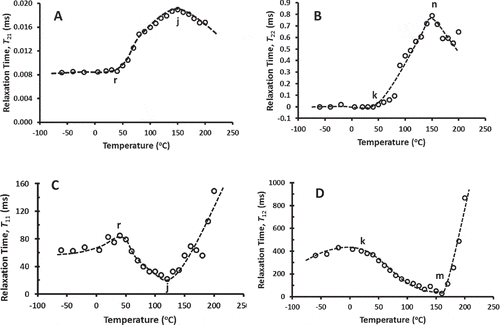
In the case of T1 relaxation, T11 showed little rise in relaxation up to a critical temperature 40°C (i.e., onset of minimum peak, marked as r) and then decreased to a minimum peak at 120°C (marked as j; ). Kruk et al.[Citation28] also observed similar behavior in the cases of organic compounds and they identified that the onset peak temperature was related to the glass transition. Similarly T12 showed an onset of minimum peak at 20°C (marked as k) and minimum peak at 160°C (marked an n; ). This indicated that onset peak (i.e., 20 or 40°C) could be related to the glass transition (11 or 20°C) as measured by thermal DSC or MDSC. However, it was clear that low frequency TD-NMR determined relaxation at little higher temperature (i.e., 20–29°C higher than thermal transition). The T2 relaxation (i.e., direction to the magnetic field) was generally associated with the thermal motion (i.e., mainly vibration) of the molecules. In the glassy state, the relaxation times of the rigid and flexible domains were 8.6 and 20 μs, respectively. However the onset of softening for the rigid and flexible domains started with relaxation times at 20 and 788 μs, respectively. The matrix acted as a solid-like (i.e., consolidate mass) in the glassy state since it lost their segmental mobility. However, in the rubbery state (i.e., above its glass transition) such motion was intensified due to the increased the pendants and segments mobility of the molecules. This caused steeper slope in the T2 relaxation in the rubbery state with increasing temperature.[Citation6]
In the case of T1 relaxation (i.e., perpendicular to the magnetic field), there was little molecular rotation in the glassy state. In the glassy state, the relaxation times of the rigid and flexible domains were 85 and 404 ms, respectively. However, the onset of softening for the rigid and flexible domains started with relaxation times at 22 and 30 ms, respectively. The comparisons of the T2 and T1 relaxation indicated that molecular rotation (i.e., observed from T1, perpendicular to the magnetic field) in the glassy state was two orders low as compared to the vibrational motion (i.e., observed from T2, parallel to the magnetic field). Thus, reactions dominated by molecular vibration (segmental motion) could be initiated much early in the glassy state. The low T1 relaxation was due to the proton rotation at a frequency much lower than the resonance frequency. The number of low protons rotating at the resonance frequency caused lower dynamic contribution to the T1 relaxation process. This inefficient released spin energy to the lattice took a long equilibrium time. However, T1 relaxation decreased above glass transition due to the increased number of protons rotating at the resonance frequency as the sample transformed to rubbery state (i.e., structure was transformed from rigid to soft-flexible). The T1 relaxation was expected to increase again due to the thermal motion of the molecules in the rubbery state.[Citation6] Comparing the viscoelastic relaxation, de la Batie et al.[Citation29] showed that local motion observed by T1 relaxation belong to the glass transition phenomena. Using T21, Ries et al.[Citation30] used Vogel-Tamman-Fulcher equation to predict the glass transition which was similar to DSC glass transition. Ruan et al.[Citation6,Citation31] measured low field NMR of food polymer by T2 and T1 relaxations; and they observed turning point in the T21 and T11 plots as a function of temperature. The turning point was related with the DSC glass transition. However, the trends and shapes of the curves depended on the type of materials[Citation22] The first maximum peak of T11 at 40°C (marked r in ) could be related to the glass transition (11 or 20°C) as measured by thermal DSC or MDSC. The maximum or minimum peak (i.e., 120, 150, or 160°C) was related to the softening or sticking point similar to solids-melting (152 or 165°C) as measured by thermal analysis. Chung et. al.[Citation26] compared the glass transition of caramel (i.e., sugar-based syrup) measured by DMTA and NMR techniques. They observed that the glass transition measured by DMTA (i.e., maximum peak in the storage modulus) was 0.5–11.5°C lower as compared to the measured from T2 relaxation, whereas it was 5.8–8.1°C higher as compared to the T1 relaxation. However, these differences decreased with the increase of polydextrose content in the case of T2 relaxation, while opposite trend was observed in the case of T1 relaxation.
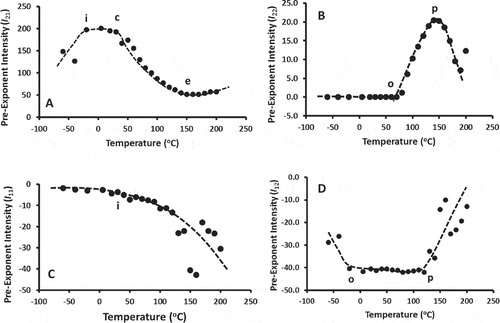
shows the onset intensity (i.e., pre-exponent factor) of T1 and T2 relaxation and characteristic temperatures are shown in . Onset rise in I21 reached a plateau (marked as I in ) at –20°C, followed by a sharp exponential decay (onset marked as c, 30°C and constant value reached marked as e at 150°C). The slow relaxation component, I22 remained constant up to 70°C (marked as o in ) followed by a maximum peak marked as p (140°C). The pre-exponent for T1 relaxation showed different nature as shown in and . The characteristic temperatures are shown in . In the literature negligible efforts were made to relate these transitions with the physico-chemical characteristics of the materials.
Figure 4. Initial intensity (i.e., pre-exponent) for spin-spin relaxation (T2 relaxation) and spin-lattice (T1 relaxation). A: I21, initial intensity for first portion of T2 relaxation (rigid domain), B: I22, initial intensity for second relaxation portion of T2 relaxation (flexible domain), C: I11, initial intensity for first portion of T1 relaxation (rigid domain), B: I12, initial intensity for second relaxation portion of T1 relaxation (flexible domain).
Conclusion
The onset peak temperature of T2 and T1 relaxation times (i.e., 20 or 40°C) could be related to the glass transition as measured by thermal DSC or MDSC (i.e., 11 or 20°C) indicating that the change in magnetic TD-NMR relaxation (i.e., change in slope) at little higher temperature (i.e., 20–29°C higher than thermal transition). However, mechanical glass transition (i.e., mechanical relaxation) from the onset shift of storage modulus (i.e., 122°C) and onset peak of loss modulus (i.e., 70°C) were observed at much higher than thermal and magnetic relaxation. This could be explained by the structural characteristics of the pomegranate peel. Thermal and magnetic relaxations observed at their molecular level, whereas mechanical relaxation occurred at the macro-structural levels considering cellulosic backbone with cementing components (i.e., sugars, pectin, and wax). In addition, TD-NMR could identify many other characteristic temperatures varied from –20 to 193°C and these could be related to other physico-chemical process in the peels during heating. Future works need to be targeted to correlate the TD-NMR characteristics temperatures with physico-chemical changes of the peels and this could expand wide applications of TD-NMR.
Nomenclature
| RT | = | Relaxation time (ms or μs) |
| Tdi | = | Onset temperature of tan δ (°C) |
| Tdp | = | Peak temperature of tan δ (°C) |
| Tgi | = | Onset glass transition temperature (°C) |
| Tgp | = | Mid glass transition temperature (°C) |
| Tge | = | End glass transition temperature (°C) |
| Tmi | = | Onset of solids-melting temperature (°C) |
| Tmm | = | Maximum slope of solids-melting temperature (°C) |
| Tmp | = | Peak of solids-melting temperature (°C) |
| Tme | = | End of solids-melting temperature (°C) |
| Tms | = | Structure forming temperature after glass transition (°C) |
| Tri | = | Onset of shift temperature for storage modulus (E′; °C) |
| Trp | = | Mid of shift temperature for storage modulus (E′; °C) |
| Tru | = | End of shift temperature for storage modulus (E′; °C) |
| Trz | = | Onset temperature for loss modulus (E′′; °C) |
| Trs | = | Peak temperature for loss modulus (E′′; °C) |
| Txp | = | Wax melting peak temperature (°C) |
| T2r | = | Onset of maximum peak for T21 (i.e. relaxation for the first part, i.e., rigid domain) |
| T2j | = | Maximum peak for T21 (i.e., relaxation for the first part, i.e., rigid domain) |
| T2k | = | Onset of maximum peak for T21 (i.e., relaxation for the second part, i.e., flexible domain) |
| T2n | = | Maximum peak for T21 (i.e., relaxation for the second part, i.e., flexible domain) |
| T1r | = | Onset of maximum peak for T11 (i.e., relaxation for the first part, i.e., rigid domain) |
| T1j | = | Minimum peak for T12 (i.e., relaxation for the first part, i.e., rigid domain) |
| T1k | = | Onset of minimum peak for T12 (i.e., relaxation for the second part, i.e., flexible domain) |
| T1n | = | Minimum peak for T12 (i.e., relaxation for the second part, i.e., flexible domain) |
| ΔCp | = | Specific heat change at glass transition (J/kg K) |
| (ΔHm)x | = | Wax-melting enthalpy change (kJ/kg) |
| T2r | = | Onset of maximum peak for T21 (i.e., relaxation for the first part, i.e., rigid domain) |
| T2j | = | Maximum peak for T21 (i.e., relaxation for the first part, i.e., rigid domain) |
| T2k | = | Onset of maximum peak for T21 (i.e., relaxation for the second part, i.e., flexible domain) |
| T2n | = | Maximum peak for T21 (i.e., relaxation for the second part, i.e., flexible domain) |
| T1r | = | Onset of maximum peak for T11 (i.e., relaxation for the first part, i.e., rigid domain) |
| T1j | = | Minimum peak for T12 (i.e., relaxation for the first part, i.e., rigid domain) |
| T1k | = | Onset of minimum peak for T12 (i.e., relaxation for the second part, i.e., flexible domain) |
| T1n | = | Minimum peak for T12 (i.e., relaxation for the second part, i.e., flexible domain) |
| T2i | = | Onset of plateau for I21 (i.e., initial intensity for the first part, i.e., rigid domain) |
| T2c | = | End of plateau for I21 (i.e., initial intensity for the first part, i.e., rigid domain) |
| T2e | = | Onset of nearly constant intensity for I21 (i.e., initial intensity for the first part, i.e., rigid domain) |
| T2o | = | Onset of maximum peak for I22 (i.e., initial intensity for the second part, i.e., flexible domain) |
| T2p | = | Maximum peak for I22 (i.e., initial intensity for the second part, i.e., flexible domain) |
| T1i | = | Onset of slope change for I11 (i.e., initial intensity for the first part, i.e., rigid domain) |
| T1o | = | Onset of plateau region for I11 (i.e., initial intensity for the first part, i.e., rigid domain) |
| T1p | = | End of plateau region or onset of sharp increase for T11 (i.e., initial intensity for the second part, i.e., flexible domain) |
Funding
This study was funded by His Majesty’s Research Trust Fund SR/AGR/FOOD/11/01 and Ms. Anami Al-Rawahi was received a Ph.D. scholarship from the Sultan Qaboos University. Authors would like to acknowledge the support of the Sultan Qaboos University toward this research in the area of food biophysics.
Additional information
Funding
References
- Levine, H.; Slade, L. A Polymer Physico-Chemical Approach to the Study of Commercial Starch Hydrolysis Products (SHPs). Carbohydrate Polymer 1986, 6, 213–244.
- Roos, Y.; Karel, M. Applying State Diagrams to Food Processing and Development. Food Technology 1991, 44(12), 66–71.
- Rahman, M.S. State Diagram of Foods: Its Potential Use in Food Processing and Product Stability. Trends in Food Science and Technology 2006, 17, 129–141.
- Rahman, M.S. Food Stability Beyond Water Activity and Glass Transition: Macro-Micro Region Concept in the State Diagram. International Journal of Food Properties 2009, 12(4), 726–740.
- Rahman, M.S.; Al-Saidi, G. Thermal Relaxation of Gelatin and Date Flesh Measured by Isothermal Condition in Differential Scanning Calorimetry (DSC) and Its Relation to the Structural and Mechanical Glass Transition. International Journal of Food Properties 2010, 13(4), 931–944.
- Ruan, R.R.; Long, Z.; Song, A.; Chen, P.L. Determination of the Glass Transition Temperature of Food Polymers Using Low Field NMR. Food Science and Technology 1998, 31(6), 516–521.
- Suresh, S.; Guizani, N.; Al-Ruzeiki, M.; Al-Hadhrami, A.; Al-Dohani, H.; Al-Kindi, I.; Rahman, M.S. Thermal Characteristics, Chemical Composition, and Polyphenol Contents of Date-Pits Powder. Journal of Food Engineering 2013, 119, 668–679.
- Hashimoto, T.; Hagiwara, T.; Suzuki, T.; Takai, R. Study on the Glass Transition of Katsuobushi (Boiled and Dried Bonito Fish Stick) by Differential Scanning Calorimetry and Dynamic Mechanical Analysis. Fisheries Science 2003, 69, 1290–1297.
- Rahman, M.S.; Al-Marhubi, I.M.; Al-Mahrouqi, A. Measurement of Glass Transition Temperature by Mechanical (DMTA), Thermal (DSC and MDSC), Water Diffusion, and Density Methods: A Comparison Study. Chemical Physics Letters 2007, 440, 372–377.
- Vodovotz, Y.; Chinachoti, P. Thermal Transitions in Gelatimination Wheat Starch at Different Moisture Contents by Dynamic Mechanical Analysis. Journal of Food Science 1996, 61(5), 932–941.
- Gambhir, P.N.; Choi, Y.J.; Slaughter, D.C.; Thompson, J.F.; McCarthy, M.J. Proton Spin–Spin Relaxation Time of Peel and Flesh of Navel Orange Varieties Exposed to Freezing Temperature. Journal of the Science of Food and Agriculture 2005, 85(14), 2482–2486.
- Herrera, M.L.; M’Cann, J.I.; Ferrero, C.; Hagiwara, T.; Zaritzky, N.; Hartel, R.W. Thermal, Mechanical, and Molecular Relaxation Properties of Frozen Sucrose and Fructose Solutions Containing Hydrocolloids. Food Biophysics 2007, 2, 20–28.
- Rahman, M.S.; Al-Hakmani, H.; Al-Alawi, A.; Al-Marhubi, I. Thermal Characteristics of Freeze-Dried Camel Milk and Its Major Components. Thermochimica Acta 2012, 549, 116–123.
- Lu, Z.P.; Li, Y.; Ng, S.C.; Feng, Y.P. Study of Glass Transition of Metallic Glasses by Temperature-Modulated Differential Scanning Calorimetry (MDSC). Thermochimica Acta 2000, 357–358, 65–69.
- Tulloch, A.P. Comparison of Some Commercial Waxes by Liquid Gas Chromatography. Journal of American Oil Chemists’ Society 1973, 50, 367–371.
- Lopes, J.D. (2010). Simplified Process to Production of Concentrated Long Chain Fatty Acids from Sugar Cane Wax (Saccharum Officinarum L.). Master Thesis, Universidade Estadual de Campinas, Brazil.
- Rocha, J.C.B.; Lopes, J.D.; Mascarenhas, M.C.N.; Arellano, D.B.; Guerreiro, L.M.R.; da Cunha, R.L. Thermal and Rheological Properties of Organogels Formed by Sugarcane Or Candelilla Wax in Soybean Oil. Food Research International 2013, 50, 318–323.
- Jankowsky, J.L.; Wong, D.G.; Di Berarrdino, M.F.; Cochran, R.C. Evaluation of Upper Use Temperature of Toughened Epoxy Composites. In Assignment of the Glass Transition, ASTM STP 1249, Seyler, R.; Ed.; American Society for Testing and Materials: Philadelphia, PA, 1994; 277–292.
- Kasapis, S.; Sablani, S.S.; Rahman, M.S.; Al-Marhoobi, I.M.; Al-Amri, I.S. Porosity and the Effect of Structural Changes on the Mechanical Glass Transition Temperature. Journal of Agricultural and Food Chemistry 2007, 55, 2459–2466.
- Homer, S.; Kelly, M.; Day, L. Determination of the Thermo-Mechanical Properties in Starch and Starch/Gluten Systems at Low Moisture Content—A Comparison of DSC and TMA. Carbohydrate Polymers 2014, 108, 1–9.
- Chung, M.; Ruan, R.; Chen, P.; Kim, J.; Ahn, T.; Baik, C. Predicting Caking Behaviors in Powdered Foods Using a Low-Field Nuclear Magnetic Resonance (NMR) Technique. Food Science and Technology 2003, 36(8), 751–761.
- Lin, X.; Ruan, R.; Chen, P.; Chung, M.; Ye, X.; Yang, T.; Doona, C.; Wagner, T. NMR State Diagram Concept. Journal of Food Science 2006, 71(9), R136–R144.
- Hills, B.P.; Pardoe, K. Proton and Deuterium NMR Studies of the Glass Transition in a 10% Water-Maltose Solution. Journal of Molecular Liquids 1995, 63, 229–237.
- Pitombo, R.N.M.; Lima, G.A.M.R. Nuclear Magnetic Resonance and Water Activity in Measuring the Water Mobility in Pintado (Pseudoplatystoma Corruscans) Fish. Journal of Food Engineering 2003, 58(1), 59–66.
- Kim, Y.R.; Yoo, B.S.; Cornillon, P.; Lim, S.T. Effect of Sugars and Sugar Alcohols on Freezing Behavior of Corn Starch Gel as Monitored by Time Domain 1H NMR Spectroscopy. Carbohydrate Polymers 2004, 55, 27–36.
- Chung, M.; Ruan, R.R.; Chen, P.L.; Wang, X. Physical and Chemical Properties of Caramel Systems. Food Science and Technology 1999, 32, 162–166.
- Borompichaichartkul, C.; Moran, G.; Srzednicki, G. S.; Driscoll, R. H. Studies of Physical State of Water in Maize from Northeast China (cv. Huangmo 417) During Drying at Subzero Temperatures. Drying Technology 2004, 22(1–2), 295–305.
- Kruk, D.; Herrmann, A.; Rossler, E.A. Field-Cycling NMR Relaxometry of Viscous Liquids and Polymers. Progress in Nuclear Magnetic Resonance Spectroscopy 2012, 63, 33–64.
- de la Batie, R.D.; Laupretre, F.; Monnerie, L. Carbon-13 NMR Investigation of Local Dynamics in Bulk Polymers at Temperature Well Above Glass Transition Temperature 1. Poly(Vinyl Methyl Ether). Macromolecules 1988, 21, 2045–2052.
- Ries, M.E.; Klein, P.G.; Brereton, M.G.; Ward, I.M. Proton NMR Study of Rouse Dynamics and Ideal Glass Transition Temperature of Poly(Ethylene Oxide) LiCF3SO3 Complexes. Macromolecules 1998, 31, 4950–4956.
- Ruan, R.; Long, Z.; Chen, P.; Huang, V.; Almaer, S.; Taub, I. Pulse NMR Study of Glass Transition in Maltodextrin. Journal of Food Science 1999, 64(1), 6–9.
