ABSTRACT
Like tea, the leaves of Turkish thyme (Thymus vulgaris) can be boiled in water to produce an extract. This is widely used as syrup for the treatment of coughs and bronchitis at alternative medicine clinics in many parts of the world. In the current study, we assessed the phenolic content and antioxidant activity of thyme. The antioxidant activities of both ethanol and aqueous extracts of thyme were determined using various in vitro methods. The total phenolic and total flavonoid contents were determined to be a gallic acid equivalent and a quercetin equivalent, respectively. Finally, the quantities of the phenolic compounds were detected using high-performance liquid chromatography and tandem mass spectrometry. The total phenolic compounds in the aqueous extract and ethanol extracts of Turkish thyme were 256.0 μg gallic acid equivalent/mg dried extract and 158.0 μg gallic acid equivalent/mg dried extract, respectively. Conversely, the total flavonoid compounds in both extracts were 44.2 μg and 36.6 μg quercetin equivalent/mg dried extract, respectively. For the first time, we determined phenolic contents and investigated the antioxidant potential of thyme. The results indicate that Turkish thyme is a good dietary source with phenolic properties.
Introduction
Free radicals are atomic or molecular structures with unpaired electrons. Free radicals have an important role in food, pharmacological, and biological processes, as well as in a variety of pathophysiological conditions.[Citation1,Citation2] However, they can also oxidize biomolecules that contain amino acids, proteins, carbohydrates, lipids, and nucleic acids.[Citation3,Citation4] Free radicals are implicated in the progression of a variety of disorders in humans, including tissue damage, cell death, cancer, central nervous system injury, ageing, arthritis, atherosclerosis, cardiovascular diseases, ischemic heart diseases, obesity, neural disorders, inflammation, gastritis, and reperfusion injuries of many tissues.[Citation5,Citation6] Conversely, antioxidants protect living organisms against the oxidative damage of free radicals. They can inhibit the effect of oxidants by donating hydrogen atoms or by chelating metals.[Citation7,Citation8] Antioxidant compounds may be endogenous or exogenous. Exogenous antioxidants must be taken through dietary means to supplement the endogenous ones. Exogenous antioxidants are classified as either deriving from a natural or a synthetic group. There is an increasing interest in the development of natural antioxidants because of concerns related to synthetic antioxidant effectiveness. Many plants, such as vegetables, fruits, and herbs, are the main sources of natural antioxidants.[Citation9,Citation10]
Thyme is widely cultivated and used in cooking throughout the world. This plant is well known for its antiseptic, antimicrobial, aromatic, and antioxidant properties.[Citation11] The leaves of thyme can be used either fresh or dried in foods. Like tea, the leaves of thyme can be boiled in water to produce an extract that is widely used as syrup for the treatment of coughs and bronchitis at alternative medicine clinics in many parts of the world. The leaves of thyme contain medicinal essential oils. The essential oils, as an additional biological activity of thyme, have been previously determined.[Citation12,Citation13] The essential oil of thyme has been used in both folk and modern medicine as an inhalant for the treatment of respiratory disorders, a topical treatment for skin disorders, and in dental care.[Citation14]
Phenolic compounds have attracted particular interest because these compounds demonstrate effective antioxidant potential.[Citation15,Citation16] Phenolic compounds in plant materials protect cells against the oxidative damage that is caused by reactive oxygen species or free radicals.[Citation17] Additionally, few reports have been produced regarding the identification of the phenolic compounds of thyme and the comparison of the antioxidant activities of ethanol extract of Turkish thyme (EETT) and aqueous extract of Turkish thyme (WETT). Therefore, this study sought to determine the antiradical and antioxidant activities of both WETT and EETT. To do so, the hydrophilic and lipophilic phytochemicals of Turkish thyme were extracted by water and ethanol. Additionally, we determined the amount of total phenolic and flavonoid compounds, in addition to identifying the phenolic compounds in WETT and EETT by liquid chromatography and tandem mass spectrometry (LC-MS/MS).
Materials and methods
Plant material
The plant material, Turkish thyme, was gathered from Erzurum, Turkey. The leaves of the thyme were air-dried. The air-dried leaves were then crumbled and stored until use.
Chemicals and stock solutions
The high-performance liquid chromatography (HPLC)-grade compounds that were used as standards for analysis by LC-MS/MS were obtained from Sigma-Aldrich. We freshly prepared curcumin solutions (1 mg/L) and used 0.1 mL as an internal standard (IS) in all LC-MS/MS experiments. Curcumin (97%) and HPLC-grade methanol were purchased from Merck (Darmstadt, Germany). The stock solutions were prepared at 5 mg/L in ethanol with either catechol or ascorbic acid, which were prepared at 50 and 25 mg/L, respectively, in the same solvent. Calibration solutions were prepared in ethanol:water (50:50, v/v) in a linear range. Dilutions were performed using automatic pipettes and glass volumetric flasks (A class), which were stored at –20°C in glass containers. The other chemicals used for bioanalytical purposes were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany) and Merck (Darmstadt, Germany).
Chemicals and solutions
Stock solutions were prepared at 5 mg/L in ethanol. Catechol and ascorbic acid were prepared as 50 and 25 mg/L, respectively, in the same solvent. Curcumin (97%) and HPLC-grade methanol were purchased from Merck (Darmstadt, Germany). Calibration solutions were prepared in ethanol:water (50:50, v/v) in a linear range (). Dilutions were performed using automatic pipettes and glass volumetric flasks (A class), which were stored at –20°C in glass containers. A curcumin solution of 1000 µg/L was freshly prepared. From this solution, 100 μL was used as an IS in all LC-MS/MS experiments.
Table 1. Total phenolic and total flavonoid content of WETT and EETT (WETT: lyophilized water extract of thyme [Thymus vulgaris]; EETT: ethanol extract of thyme [Thymus vulgaris]).
Preparation of WETT and EETT
The preparation of water and ethanol exactions was described in detail previously.[Citation18–Citation20] Briefly, we prepared WET by mixing 50 g of air-dried Turkish thyme leaves with 400 mL of distilled water. This mixture was boiled and stirred for 10 min, and the extract was filtered, frozen, and lyophilized in a lyophilizer (Labconco, Freezone 1 L, 5 mm Hg, –50°C). The lyophilized powder was stored at –30°C until analysis. We prepared EETT by mixing 50 g of air-dried Turkish thyme leaves with 400 mL of ethanol solution. This mixture was stirred for 10 min, and the extract was filtered through filter paper. The filtrates were evaporated with a rotary evaporator. The evaporated sample was stored at –30°C until use.
Determination of total phenolic content by the Folin–Ciocalteu assay
The total phenolic contents of WETT and EETT were assessed using the Folin–Ciocalteu method,[Citation21] as previously described.[Citation22] Initial stock solutions of WETT and EETT at 1 mg/mL were prepared.
Absorbance (λ760) = 0.0021x Total phenols (gallic acid equivalent [GAE; μg])
The content of total phenolics in WETT and EETT was calculated by employing this graph (R2: 0.9706), which was prepared using gallic acid and expressed as micrograms of GAE.
Determination of total flavonoid contents
The total flavonoid contents of WETT and EETT were estimated by a colorimetric assay, which was described in previous studies.[Citation23,Citation24] A quercetin calibration curve was prepared, and the quantity of flavonoid was determined using the linear regression equation that was obtained from the calibration curve. Finally, the results were calculated as quercetin equivalents (QEs) per mg extract.
Absorbance (λ415) = 0.0127x Total phenols (QE[μg]) + 0.0475
The contents of total flavonoid in WETT and EETT were calculated by using the graph, which was prepared using quercetin and calculated as QE micrograms.
LC-MS/MS studies
Preparation of the test solution for LC-MS/MS and of the instruments and chromatographic conditions were performed as previously described.[Citation24] The samples in the auto sampler were stored at 15°C during the experiment. The experiments were performed with a Zivak® HPLC and Zivak® Tandem Gold Triple quadruple mass spectrometer, which was equipped with a Macherey-Nagel Nucleoder C18 gravity column (125 × 2 mm i.d., 5 µm particle size). The mobile phase was composed of methanol (A, 0.5% formic acid) in water (B, 0.5% formic acid). The gradient program was 0–1.00 min at 50% A and 50% B, 1.01–30.00 min at 100% A, and finally 30.01–35.00 min at 50% A and 50% B. The flow rate of the mobile phase was 0.3 mL/min, and the column temperature was set to 30°C. The injection volume was 10 µL.[Citation6] Optimization of the HPLC method and the LC-MS/MS procedure was described previously.[Citation25] The triple quadruple MS system has been widely used due to the stability of its fragmented ions.[Citation26] The optimum electroscopy ionization (ESI) parameters were determined as 2.40 mTorr CID gas pressure, 5000 V ESI needle voltage, 600 V ESI shield voltage, 300°C drying gas temperature, 50°C atmospheric pressure ionization (API) housing temperature, 55 psi nebulizer gas pressure, and 40 psi drying gas pressure.
Validation, linearity, recovery, repeatability, precision
In the validation experiments of all phenolic compounds, curcumin was used as an IS. Linearity, recovery, repeatability, limit of detection (LOD) and limit of quantitation (LOQ) were used as validation parameters of the experiments. The unspiked plant extracts were also analyzed to determine the selectivity of curcumin (IS) in the blank sample, for which no peak was found. The recoveries of the reported compounds were evaluated for each fortification level. We evaluated the precision by repeating the measurements at three concentrations for each compound. The precision was determined to be good, and the results were implemented into the uncertainty budget. The LOD and LOQ of the LC-MS/MS method for the reported compounds were found to be 0.5–50 µg/L. The LOQs were determined to be 10 times higher than the S/N with respect to the indicated concentrations.[Citation27]
Estimation of uncertainty
The estimation of each compound’s concentration in the WETT and EETT solutions was expressed as µg/L within the linear range. The concentrations of the phenolic compounds in the solution that were calculated by the calibration curve were converted to units of mg/kg of crude sample.[Citation6] The sources and the quantification of the uncertainty for the method that was applied were evaluated and calculated using the EURACHEM/CITAC Guide, 2012, and equation three, respectively (EURACHEM/CITAC 2012). It was determined that the sources of uncertainty for the LC-MS/MS experiments were the impurity of the reference standard, the sample weighing, the calibration curve and the dilution of the solutions. For all compounds, the maximum contribution was derived from the calibration.[Citation22]
Biochemistry studies
The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) scavenging capabilities of WETT and EETT were evaluated using the free radical method that was reported by Gülçin.[Citation28,Citation29] This method measures the reaction of the antioxidants with stable DPPH free radicals (0.1 mM). The absorbance was measured at 517 nm against a blank sample. A decreasing absorbance correlates to DPPH free radical scavenging activity.[Citation4,Citation30]
The ABTS•+ radical scavenging activities of the WETT and EETT solutions were evaluated according to the method of Re et al.[Citation30] with slight modifications.[Citation31,Citation32] ABTS•+ has an intrinsic absorbance at 734 nm. The ABTS•+ cation radical developed from its reaction with ABTS (2 mM) in H2O and K2S2O8 (2.45 mM) at room temperature for 12 h. The absorbance at 734 nm was measured for each sample relative to a blank. A decreased absorbance in a sample correlates to ABTS•+ cation radical scavenging activity.[Citation33,Citation34]
The reducing power of the ferric (Fe3+) ions of both extracts was measured using Oyaizu’s method[Citation35] with slight modification.[Citation36] The basis of this method is the reduction of (Fe3+) to ferricyanide in stoichiometric excess relative to the antioxidants. In this method, the absorbance measurements of the samples were obtained at 700 nm using an ultraviolet (UV) spectrophotometer.[Citation37]
The reducing power of the cupric ions (Cu2+) of WETT and EETT were determined using Apak et al.,[Citation38] with slight modifications.[Citation39] To do so, we used an ethanolic neocuproine solution. Then, different concentrations of WETT and EETT were pipetted into test tubes. The final volume was adjusted to 2 mL with distilled water. The tubes were kept at room temperature for 30 min. Absorbance was measured at 450 nm against a reagent blank. Increasing absorbance indicated an elevated Cu2+ reducing power.[Citation40]
The ferric thiocyanate method was used to determine the total antioxidant activity of WETT and EETT, as previously described.[Citation32] We prepared a linoleic acid emulsion system. The mixtures were incubated at 37°C in glass flasks. The peroxide levels were recorded across temporal intervals during the incubation by measuring the absorbance at 500 nm in a spectrophotometer after the reaction with Fe2+ and SCN−. During linoleic acid peroxidation, peroxide was formed, and ferrous ions (Fe2+) were oxidized to ferric ions (Fe3+). Then, Fe3+ formed a complex with SCN− that maximally absorbed at 500 nm.[Citation41]
Statistical analysis
The experimental results were performed in triplicate. The data were recorded as the mean ± standard deviation and analyzed by SPSS (version 17.0 SPSS Inc.). A one-way analysis of variance (ANOVA) was performed. Significant differences between means were determined using Duncan’s multiple range tests; p < 0.05 was considered significant.
Results and discussion
The antioxidant compounds that are found in plant materials have an important role in scavenging and inhibiting free radicals.[Citation2] Radical scavenging, lipid peroxidation inhibition, and reducing power assays were used to determine the antioxidant potential of Turkish thyme. Phenolic properties are strongly related to antioxidant activities. For this reason, we quantified the amount of total phenolic and flavonoid compounds as GAE and QE, respectively. Finally, we used LC-MS/MS analysis to identify the phenolic compounds that are responsible for the antioxidant properties of WETT and EETT.
Phenolics are compounds that contain at least one hydroxyl group (-OH) that is conjugated to an aromatic ring. They exist in the aerial parts of plants, such as in flowers, leaves, seeds, fruits, stems, and roots. Increasing attention has been focused on phenolic compounds due to their attractive biological properties, such as their antioxidant and radical scavenging activities.[Citation6,Citation42] The hydroxyl groups in the meta-positions of carboxyl groups (-COOH) can increase antioxidant activity more than that in the ortho- or para-positions due to the electron pushing effect of carboxyl group (-COOH) promoting H-donating ability of hydroxyl groups (-OH). Of course, ortho-substitution of -OH group with electron-donating groups like methoxy groups (-OCH3) can also increase the antioxidant activity.[Citation36] For example the -CH=CH-COOH groups in cinnamic acid can produce greater H-donating ability and subsequent antioxidant activity than the -COOH groups in benzoic acids through stabilizing the radical by resonance of -C=C-.[Citation43] Phenolic compounds can arrest chain oxidation reactions using various mechanisms, such as via the donation of hydrogen atoms or chelation of metal ions. For this reason, they act as metal chelators, antioxidants, reducing agents, or single oxygen and H2O2 decomposers. Additionally, these important plant metabolites display antioxidant properties.[Citation44,Citation45]
The total phenolic contents of both extracts are presented in . The total phenolic content in EETT and WETT was 158 and 256 μg GAE/mg dried extracts, respectively. As shown in , the total phenolic content of WETT was higher than that of EETT. Our results agree with the literature, which reports the phenolic content of another thyme species (Thymus spathulifolius) to be μg GAE/mg dried extract.[Citation13] The high level of phenolic compounds indicates the elevated antioxidant capacity of thyme. The high phenolic content in both extracts is an important factor in the antioxidant capacities of WETT and EETT.
Table 2. LC-MS/MS parameters of selected compounds and concentrations (mg/kg) of antioxidants in WETT and EETT.
Flavonoids are polyphenol compounds that are widely distributed in plants, and they perform many functions. These polyphenolic compounds may also act as chemical messengers, physiological regulators, and cell cycle inhibitors.[Citation46,Citation47] The amount of total flavonoids in WETT and EETT was found to be 44.2 µg QE/mg dried extract and 36.6 µg QE/mg dried extract, respectively. In addition, the total flavonoid content of WETT was observed to be higher than that of EETT ().
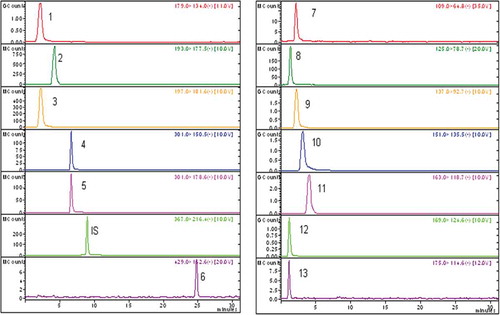
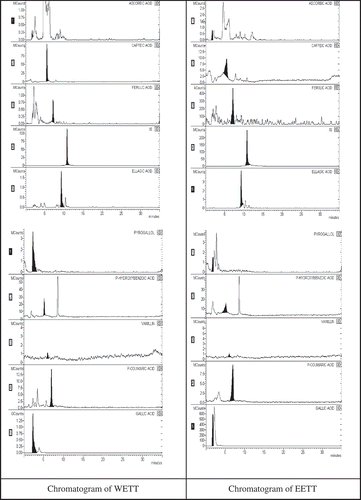
According to recent reports, phenolic and flavonoid compounds have antioxidant, radical scavenging, and metal chelating properties. Several studies have indicated a positive correlation between phenolic contents and the ferric reducing powers of plant extracts.[Citation48,Citation49] We quantified the levels of phenolic compounds in WETT and EETT using LC-MS/MS analysis. We found that gallic acid (5150.2 and 1801.2 mg/kg in WETT and EETT, respectively) was the most abundant phenolic compound in both WET and EET. Validation and uncertainty parameters were determined in a previous study.[Citation6] The effective antioxidant and antiradical activities of WETT and EETT might be due to their elevated levels of phenolic compounds. Additionally, we also observed both pyrogallol and ferulic acids as being the most abundant compounds in WETT and EETT (, and ).
Figure 2. WETT and EETT chromotogram of antioxidants determined by LC-MS/MS. Samples three through eight were diluted for use in the linear range. WETT: lyophilized water extract of thyme (Thymus vulgaris); EETT: ethanol extract of thyme (Thymus vulgaris).
Plant materials include many phenolic compounds that contain hydroxyl groups (-OH) conjugated to aromatic rings.[Citation50] These phenolic compounds block chain oxidation reactions by chelating metals or donating hydrogen atoms. Therefore, these plant metabolites act as reducing agents, metal chelators, singlet oxygen quenchers, and antioxidants. Many studies have shown that the phenolic contents of plants display some antioxidant properties.[Citation44,Citation51] Free radicals or ionic radicals are highly reactive species that are responsible for many cell disorders due to their effects on proteins, lipids, and DNA.[Citation52,Citation53] The radical scavenging activity of a compound indicates its antioxidant activity and ability to inhibit the initiation of an oxidation chain. DPPH and ABTS assays have been widely used to determine the radical scavenging activity of a compound.[Citation25,Citation54]
The DPPH free radical scavenging activities of WETT, EETT, and positive antioxidants, such as buthylated hydroxyansole (BHA), buthylated hydroxytoluene (BHT), and Trolox®, were investigated. Additionally, we determined the IC50 values (i.e., the effective concentration of standard or sample at which DPPH radicals were reduced by 50%) of both extracts and standards. The results are summarized in . The IC50 values of DPPH scavenging of both extracts and standard antioxidants decreased. BHT (31.2 µg/mL; R2: 0.8810) decreased more than α-tocopherol (13.6 µg/mL; R2: 0.8453), which decreased at a similar rate as WETT (13.4 µg/mL; R2: 0.9255), BHA (12.9 µg/mL; R2: 0.9801), and Trolox® (12.7 µg/mL; R2: 0.9343), which decreased more than EETT (12.1 µg/mL; R2: 9656). The lower IC50 values reflect an elevated DPPH radical scavenging effect. It was observed that EETT had the most effective DPPH radical scavenging activity when compared to the other samples. The results indicated that both WETT and EETT have effective DPPH radical scavenging activities ().
Table 3. Concentration required for 50% scavenging (IC50) of DPPH• scavenging activity, ABTS•+ scavenging activity of WETT, EETT and standard compounds, including BHA, BHT, α-tocopherol, and Trolox.
Table 4. The total antioxidant activity, as measured using the thiocyanate method; the reducing ability, as measured using the Fe3+-Fe2+ transformation method; and the Cu2+ reducing ability, as measured using the CUPRAC method, of WETT, EETT, and standard compounds, including BHA, BHT, α-tocopherol, and Trolox at the same concentrations.
We also used an ABTS radical cation decolorization assay to detect radical scavenging activity. In this assay, WETT and EETT demonstrated a similar reduction in free radicals when compared to those obtained in the DPPH reaction. The IC50 values of the ABTS radical scavenging of WETT, EETT, and standard antioxidants decreased such that EETT (54.08 µg/mL; R2: 0.9914) decreased more than WETT (40.03 µg/mL; R2: 0.9246), which decreased more than BHT (7.11 µg/mL; R2: 0.8426), which decreased more than α-Tocopherol (16.6 µg/mL; R2: 0.9546), which decreased more than Trolox® (6.11 µg/mL; R2: 0.8972), which decreased more than BHA (4.95 µg/mL; R2: 0.9990). Our results indicated that the ABTS cation radical scavenging activity of WETT was greater than that of EETT. Nevertheless, the antioxidant activities of these two extracts were lower than that of all the standard antioxidants ().
The reduction capacity of an agent indicates its antioxidant potential. Antioxidant compounds give electrons to reactive species, thereby improving the stability of or reducing the compound.[Citation55] Plants can contain several reducing agents, which can react with free radicals to stabilize and terminate radical chain reactions.[Citation56,Citation57] In this study, we investigated the reducing powers of WETT and EETT by both the FRAP and CUPRAC assays.
Antioxidant compounds induce the reduction of the Fe3+/ferricyanide complex to its ferrous(Fe2+/ferricyanide) form due to their reductive properties.[Citation8,Citation58] As shown in , the ferric reducing powers increased with an increasing concentration of both WETT and EETT, which was similar to the pattern in the standard antioxidants. The reducing powers of the 10 µg/mL extracts and the standard antioxidants were observed to decrease such that BHA (2.081, R2: 0.8593) was similar to WETT (2.021, R2: 0.8780), which was greater than EETT (1.888, R2: 0.9656), which was similar to Trolox® (1.861, R2: 0.9991), which was greater than BHT (1.657, R2: 0.9408), which was greater than α-Tocopherol (1.465, R2: 0.9993).
Additionally, similarly to many researchers,[Citation38,Citation59] we used the CUPRAC method to assess the reducing power of the compounds. This method is based on the reduction of Cu2+-Cu+ by antioxidants in the presence of neocuproine. In this assay, a higher absorbance indicates a higher cupric ion (Cu2+) reducing power. The cupric ion reducing powers of 10 µg/mL of WETT, EETT, and the standard compounds decreased such that BHT (0.668, R2: 0.9085) decreased more than BHA (0. 565, R2: 0.8046), which decreased more than EETT (0.518, R2: 0.9489), which decreased more than Trolox® (0.457, R2: 0.9655), which decreased more than α-Tocopherol (0.422, R2: 0.9666), which decreased more than WETT (0.400, R2: 0.8699; ).
The reduction of ferric ion (Fe3+) and of cupric ion (Cu2+) is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action.[Citation59–Citation61] Therefore, an increasing reduction power of a sample indicates its increasing antioxidant potential. According to our study, the ferric reducing power of WETT was greater than that of EET, while the cupric ion reducing power of WETT was lower than that of EETT. As shown in , the ferric and cupric reducing powers of the extracts increased with their increasing concentrations.
The total antioxidant activities of WETT and EETT were determined by using the ferric thiocyanate method.[Citation61–Citation63] A linoleic acid mixture that did not contain WETT, EETT, or standard antioxidants was used as control. The percentage of inhibition was calculated at 48 h after the greatest level of absorbance of the control.[Citation64] The percentage of inhibition of lipid peroxidation in the linoleic acid emulsion system was calculated by the following equation:[Citation65–Citation67]
The inhibition levels of lipid peroxidation in the linoleic acid emulsions of WETT, EETT, and the standard antioxidants are shown in . The amount of inhibition increased with increasing concentrations of both extracts (10 and 20 µg/mL). The inhibition effect of the linoleic acid emulsion peroxidation of WETT, EETT, and the standard antioxidants after 48 h of maximal control absorbance decreased such that the decrease in BHA (83.3%) was either less than or similar to the decrease in BHT (82.1%), which either decreased or was similar to the decrease in Trolox® (81.3%), which decreased more than α-Tocopherol (68.1%), which decreased more than EETT (64.4), which decreased more than WETT (51.7).
Conclusion
In conclusion, we confirmed that both WETT and EETT exhibit good antioxidant ability on DPPH and ABTS radical scavenging activity, reducing power, inhibition of linoleic acid peroxidation and ferric and cupric ion reducing capacity. The antioxidant and antiradical activities of WETT were observed to be greater than those of EETT in FRAP, DPPH, and ABTS assays. The total phenolic and flavonoid content in WETT was found to be higher than that of EETT, which is consistent with previous reports. Conversely, the inhibition of the lipid peroxidation of EETT in the linoleic acid emulsion system was found to be greater than that of WETT, which is inconsistent with previous reports. Additionally, we used LC-MS/MS to quantify the amount of phenolic compounds in WETT and EETT. We observed that gallic acid (5150.2 and 1801.2 mg/kg in WETT and EETT, respectively) was the most abundant phenolic compound in WETT and EETT. The effective antioxidant and antiradical activities of Turkish thyme might be due to its elevated concentrations of phenolic compounds. Additionally, pyrogallol and ferulic acid appear as the most abundant compounds in WETT and EETT. The quantity of syringic acid, α-tocopherol, and catechol were below the limits of quantification and were not determined by LC-CS/MS.
Funding
This study was partially supported by the Research Fund of Atatürk University. The author is grateful to the Research Fund of Atatürk University for financial support (Project no: 2012/472). Additionally, İlhami Gülçin would like to thank the Distinguished Scientist Fellowship Program, King Saud University, for financial support. The authors declare that they have no competing interests.
Additional information
Funding
References
- Ansari, KN. The Free Radicals—The Hidden Culprits—An Update. Indian Journal of Medical Sciences 1997, 51, 319–336.
- Gülçin, İ. Antioxidant Activity of Food Constituents: An Overview. Archives of Toxicology 2012, 86, 345–391.
- Gülçin, İ.; Elias, R.; Gepdiremen, A.; Taoubi, K.; Köksal, E. Antioxidant Secoiridoids from Fringe Tree (Chionanthus Virginicus L.). Wood Science and Technology 2009, 43, 195–212.
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, HY. Antioxidant Activity of Clove Oil—A Powerful Antioxidant Source. Arabian Journal of Chemistry 2012, 5, 489–499.
- Polat Köse, L.; Gülçin, İ.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS Analysis, Antioxidant and Anticholinergic Properties of Galanga (Alpinia Officinarum Hance) Rhizomes. Industrial Crops and Products 2015, 74, 712–721.
- Gülçin, İ.; Bursal, E.; Şehitoğlu, H.M.; Bilsel, M.; Gören, A.C. Polyphenol Contents and Antioxidant Activity of Lyophilized Aqueous Extract of Propolis from Erzurum, Turkey. Food and Chemical Toxicology 2010, 48, 2227–2238.
- Bursal, E.; Köksal, E. Evaluation of Reducing Power and Radical Scavenging Activities of Water and Ethanol Extracts from Sumac (Rhus coriaria L.). Food Research International 2011, 44, 2217–2221.
- Gülçin, İ.; Beydemir, S. Phenolic Compounds as Antioxidants: Carbonic Anhydrase Isoenzymes Inhibitors. Mini Review in Medicinal Chemistry 2013, 13, 408–430.
- Elmastas, M.; Türkekul, İ.; Öztürk, L.; Gülçin, İ.; Isıldak, Ö.; Aboul-Enein.; H.Y. The Antioxidant Activity of Two Wild Edible Mushrooms (Morchella Vulgaris and Morchella Esculanta). Combinatorial Chemistry and High Throughput Screening 2006, 9, 443–448.
- Gülçin, İ.; Gagua, N.; Beydemir, S.; Bayram, R.; Bakuridze, A.; Gepdiremen, A. Apoptotic, Antioxidant and Antiradical Effects of Majdine and Isomajdine from Vinca Herbacea Waldst. and Kit. Journal of Enzyme Inhibition and Medicinal Chemistry 2012, 27, 587–594.
- Rouatbi, M.; Duquenoy, A.; Giampaoli, P. Extraction of the Essential Oil of Thyme and Black Pepper by Superheated Steam. Journal of Food Enginering 2007, 78, 708–714.
- Kaloustian, J.; El-Moselhy, T.F.; Portugal, H. Chemical and Thermal Analysis of the Biopolymers in Thyme (Thymus Vulgaris). Thermochimica Acta 2003, 401, 77–86.
- Sökmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in Vitro Antimicrobial and Antioxidant Activities of the Essential Oils and Methanol Extracts of Endemic Thymus Spathulifolius. Food Control 2004, 15, 627–634.
- Al-Saleh, I.A.; Billedeo, G.; El-Doush, I.I. Levels of Selenium, DL-α-Tocopherol, DL-γ-Tocopherol, All-Trans-Retinol, Thymoquinone and Thymol in Different Brands of Nigella Sativa Seeds. Journal of Food Composition and Analysis 2006, 19, 167–175.
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Polat Köse, P.; Gülçin, İ.; Çakmak, K.C.; Küçük, M.; Durmaz, L.; Gören, A.C.; Alwasel, S.H. Antioxidant, Antiradical and Anticholinergic Properties of Cynarin Purified from the Illyrian Thistle (Onopordum Illyricum L.). Journal of Enzyme Inhibition and Medicinal Chemistry 2016, 31, 266–275.
- Topal, M.; Gülçin, İ. Rosmarinic Acid: A Potent Carbonic Anhydrase Isoenzymes Inhibitor. Turkish Journal of Chemistry 2014, 38, 894–902.
- Liu, X.; Zhao, M.; Wang, J.; Yang, B.; Jiang, Y. Antioxidant Activity of Methanolic Extract of Emblica Fruit (Phyllanthus Emblica L.) from Six Regions in China. Journal of Food Composition and Analysis 2008, 21, 219–228.
- Gülçin, İ.; Küfrevioğlu, Ö.İ.; Oktay, M.; Büyükokuroğlu, M.E. Antioxidant, Antimicrobial, Antiulcer and Analgesic Activities of Nettle (Urtica Dioica L.). Journal of Ethnopharmacology 2004, 90, 205–215.
- Gülçin, İ.; Büyükokuroğlu, M.E.; Oktay M.; Küfrevioğlu, Ö.İ. Antioxidant and Analgesic Activities of Turpentine of Pinus Nigra Arn. Subsp. Pallsiana (Lamb.) Holmboe. Journal of Ethnopharmacology 2003, 86, 51–58.
- Gülçin, İ.; Şat, İ.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioğlu, Ö.İ. Comparison of Antioxidant Activity of Clove (Eugenia Caryophylata Thunb) Buds and Lavender (Lavandula Stoechas L.). Food Chemistry 2004, 87, 393–400.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods in Enzymology 1999, 299, 152–178.
- Gülçin, İ.; Topal, F.; Oztürk Sarikaya, S.B.; Bursal, E.; Gören, A.C.; Bilsel, M. Polyphenol Contents and Antioxidant Properties of Medlar (Mespilus Germanica L.). Records of Natural Products 2011, 5, 158–175.
- Köksal, E.; Gülçin İ: Antioxidant Activity of Cauliflower (Brassica Oleracea L.). Turkish Journal of Agriculture and Forestry 2008, 32, 65–78.
- Bursal, E.; Köksal, E.; Gülçin, İ.; Bilsel, G.; Gören, A.C. Antioxidant Activity and Polyphenol Content of Cherry Stem (Cerasus Avium L.) Determined by LC-MS/MS. Food Research International 2013, 51, 66–74.
- Bursal, E.; Gülçin, İ. Polyphenol Contents and in Vitro Antioxidant Activities of Lyophilised Aqueousextract of Kiwifruit (Actinidia Deliciosa). Food Research International 2011, 44, 1482–1489.
- Gören, A.C.; Çıkrıkçı, S.; Çergel, M.; Bilsel, G. Rapid Quantitation of Curcumin in Turmeric Via NMR and LC-Tandem Mass Spectrometry. Food Chemistry 2009, 113, 1239–1242.
- Gülçin, İ.; Topal, F.; Çakmakçı, R.; Gören, A.C.; Bilsel, M.; Erdoğan, U. Pomological Features, Nutritional Quality, Polyphenol Content Analysis, and Antioxidant Properties of Domesticated and Three Wild Ecotype Forms of Raspberries (Rubus Idaeus L.). Journal of Food Sciences 2011, 76, C585–C593.
- Gülçin, İ. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220.
- Gülçin, İ. Antioxidant and Antiradical Activities of L-Carnitine. Life Sciences 2006, 78, 803–811.
- Göçer, H.; Akıncıoğlu, A.; Öztaşkın, N.; Göksu, S.; Gülçin, İ. Synthesis, Antioxidant and Antiacetylcholinesterase Activities of Sulfonamide Derivatives of Dopamine Related Compounds. Archiv der Pharmazie 2013, 346, 783–792.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Gülçin, İ. Comparison of in Vitro Antioxidant and Antiradical Activities of L-Tyrosine and L-Dopa. Amino Acids 2007, 32, 431–438.
- Gülçin, İ. Antioxidant Properties of Resveratrol: A Structure-Activity Insight. Innovative Food Sciences and Emerging Technology 2010, 11, 210–218.
- Gülçin, İ. Measurement of Antioxidant Ability of Melatonin and Serotonin by the DMPD and CUPRAC Methods as Trolox Equivalent. Journal of Enzyme Inhibition and Medicinal Chemistry 2008, 23, 871–876.
- Çetinkaya, Y.; Göçer, H.; Menzek, A.; Gülçin, İ. Synthesis and Antioxidant Properties of (3,4-Dihydroxyphenyl)(2,3,4-Trihydroxyphenyl)Methanone and Its Derivatives. Archiv der Pharmazie 2012, 345, 323–334.
- Oyaizu, M. Studies on Product of Browning Reaction Prepared from Glucose Amine. Japanese Journal of Nutrition 1986, 44, 307–315.
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arabian Journal of Chemistry 2010, 3, 43–53.
- Gülçin, İ. Antioxidant Activity of L-Adrenaline: An Activity-Structure Insight. Chemico-Biological Interaction 2009, 179, 71–80.
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. Journal of Agricultural and Food Chemistry 2004, 52, 7970–7981.
- Köksal, E.; Gülçin, I.; Ozturk Sarıkaya, S.B.; Bursal, E. In Vitro Antioxidant Activity of Silymarin. Journal of Enzyme Inhibition and Medicinal Chemistry 2009, 24, 395–405.
- Ak, T.; Gülçin, İ. Antioxidant and Radical Scavenging Properties of Curcumin. Chemico-Biological Interaction 2008, 174, 27–37.
- Göçer, H.; Gülçin, İ. Caffeic Acid Phenethyl Ester (CAPE): Correlation of Structure and Antioxidant Properties. International Journal of Food Sciences and Nutrition 2011, 62, 821–825.
- Chen, H.; Zhou, Y.; Shao, Y.; Chen F. Free Phenolic Acids in Shanxi Aged Vinegar: Changes During Aging and Synergistic Antioxidant Activities. International Journal of Food Properties 2016, 19, 1183–1193.
- Gülçin, İ.; Kirecci, E.; Akkemik, E.; Topal, F.; Hisar, O. Antioxidant and Antimicrobial Activities of an Aquatic Plant: Duckweed (Lemna Minor L.). Turkish Journal of Biology 2010, 34, 175–188.
- Şerbetçi Tohma, H.; Gülçin, İ. Antioxidant and Radical Scavenging Activity of Aerial Parts and Roots of Turkish Liquorice (Glycyrrhiza Glabra L.). International Journal of Food Properties 2010, 13, 657–671.
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Antioxidant Activity of Essential Oils of Five Spice Plants Widely Used in a Mediterranean Diet. Flavour and Fragrance Journal 2010, 25, 13–19.
- Galeotti, F.; Barile, E.; Curir, P.; Dolci, M.; Lanzotti, V. Flavonoids from Carnation (Dianthus Caryophyllus) and Their Antifungal Activity. Phytochemistry Letters 2008, 1, 44–48.
- Gülçin, İ.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant Activity of Lignans from Fringe Tree (Chionanthus Virginicus L.). European Food Research and Technology 2006, 223, 759–767.
- Abu Bakar, M.F.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and Antioxidant Activity of Different Parts of Bambangan (Mangifera Pajang) and Tarap (Artocarpus Odoratissimus). Food Chemistry 2009, 113, 479–483.
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Screening of Antioxidant and Antiradical Activity of Monodesmosides and Crude Extract from Leontice Smirnowii Tuber. Phytomedicine 2006, 13, 343–351.
- Gülçin, İ.; Berashvili, D.; Gepdiremen, A. Antiradical and Antioxidant Activity of Total Anthocyanins from Perilla Pankinensis Decne. Journal of Ethnopharmacology 2005, 101, 287–293.
- Gülçin, İ. The Antioxidant and Radical Scavenging Activities of Black Pepper (Piper Nigrum) Seeds. International Journal of Food Sciences and Nutrition 2005, 56, 491–499.
- Koksal, Z.; Kalın, R.; Gülçin, İ.; Özdemir, H.; Atasever, A. The Impact of Some Avermectins on Lactoperoxidase from Bovine Milk. International Journal of Food Properties 2016, 19, 1207–1216.
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant Activity of a Triterpenoid Glycoside Isolated from the Berries of Hedera Colchica: 3-O-(β-D-Glucopyranosyl)-Hederagenin. Phytotheraphy Research 2006, 20, 130–134.
- Jacob, J.K.; Hakimuddin, F.; Paliyath, G.; Fisher, H. Antioxidant and Antiproliferative Activity of Polyphenols in Novel High-Polyphenol Grape Lines. Food Research International 2008, 41, 419–428.
- Gülçin, İ.; Oktay, M.; Kireçci, E.; Küfrevioğlu, Ö.İ. Screening of Antioxidant and Antimicrobial Activities of Anise (Pimpinella Anisum L.) Seed Extracts. Food Chemistry 2003, 83, 371–382.
- Gülçin, İ.; Beydemir, Ş.; Şat, İ.G.; Küfrevioğlu, Ö.İ. Evaluation of Antioxidant Activity of Cornelian Cherry (Cornus Mas L.). Acta Alimentaria 2005, 34, 193–202.
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential Oil and Phenolic Compounds of Artemisia Herba-Alba (Asso.): Composition, Antioxidant, Antiacetylcholinesterase, and Antibacterial Activities. International Journal of Food Properties 2016, 19, 1425–1438.
- Hu, W.; Yu, L.; Wang, M.H. Antioxidant and Antiproliferative Properties of Water Extract from Mahonia Bealei (Fort.) Carr. Leaves. Food and Chemical Toxicology 2011, 49, 799–806.
- Köksal, E.; Bursal, E.; Dikici, E.; Tozoğlu, F.; Gülçin, İ. Antioxidant Activity of Melissa Officinalis Leaves. Journal of Medicinal Plants Research 2011, 5, 217–222.
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J.; Characterisation of the Antioxidant Properties of De-Odourised Aqueous Extracts from Selected Lamiaceae Herbs. Food Chemistry 2003, 83, 255–262.
- Gülçin, İ.; Alici, H.A.; Cesur, M. Determination of in Vitro Antioxidant and Radical Scavenging Activities of Propofol. Chemical and Pharmaceutical Bulletin 2005, 53, 281–285.
- Çakmakçı, S.; Topdaş, E.F.; Kalın, P.; Han, H.; Şekerci, P.; Polat Kose, L.; Gülçin, İ. Antioxidant Capacity and Functionality of Oleaster (Elaeagnus Angustifolia L.) Flour and Crust in a New Kind of Fruity Ice Cream. International Journal of Food Sciences and Technology 2015, 50, 472–481.
- Mitsuda, H.; Yuasumoto, K.; Iwami, K. Antioxidation Action of Indole Compounds During the Autoxidation of Linoleic Acid. Eiyo to Shokuryo 1996, 19, 210–214.
- Gülçin, İ.; Büyükokuroğlu, M.E.; Oktay, M.; Küfrevioğlu, Ö.İ. On the in Vitro Antioxidant Properties of Melatonin. Journal of Pineal Research 2002, 33, 167–171.
- Kalın, P.; Gülçin, İ.; Gören, A.C. Antioxidant Activity and Polyphenol Content of Vaccinium Macrocarpon. Records of Natural Products 2015, 9, 496–502.
- Sehitoglu, M.H.; Han, H.; Kalin, P.; Gülçin, İ.; Ozkan, A.; Aboul-Enein, H.Y. Pistachio (Pistacia Vera L.) Gum: A Potent Inhibitor of Reactive Oxygen Species. Journal of Enzyme Inhibition and Medicinal Chemistry 2015, 30, 264–269.