ABSTRACT
Solid-fermentation is a promising way of producing novel surimi-based food. In the present study, changes in physicochemical characteristics, protein hydrolysis, and textual properties of surimi during the fermentation, including pehtze preparation, salting, and ripening stages, were studied. The protease produced by Actinomucor elegans led to continuous hydrolysis of surimi protein, reflected from the high value of protease activity which reached 69.34 U/g after pehtze preparation stage, the decrease of protein bands intensity in sodium dodecyl sulfate polyacrylamide gel electrophoresis image especially for bands of 200, 43, and 36 KDa, and the increasing contents of free amino acids and trichloroacetic acid-soluble nitrogen. Hardness, springiness, and cohesiveness of the surimi all decreased during pehtze preparation stage, increased after salting, and decreased again after ripening, while adhesiveness kept increasing until the ripening stage started. Scanning electron microscopy images showed a dense and soft microstructure of surimi after ripening. The final surimi product showed high overall acceptability and no Enterobacteriaceae was detected.
Introduction
Surimi is one kind of minced fish muscles, and the main component of it is the salt-soluble proteins extracted from minced fish meat.[Citation1] It is available in many shapes, forms, and textures, and often used to mimic the texture and color of the meat of lobster, crab, and other shellfish.[Citation2] However, surimi is highly perishable, with a strong odor and poor taste when spoiled. The deteriorations are due to autolytic spoilage by enzymes, lipid oxidation, microbiological spoilage, or the combination of them.[Citation3,Citation4] Surimi or surimi products should, therefore, be stored in a frozen state.[Citation5] The development of new surimi-based products that can be stored at room temperature without any loss of nutrition would enlarge the application range of low-value fish. Fermentation is promising in developing such a product.
Fermented foods are encountered worldwide, and they are prepared from a wide variety of foods of animal, plant, and micro-organism origins.[Citation6] They are of great significance, providing and preserving vast quantities of nutritious in a wide diversity of flavors, aromas, and textures. Many kinds of fermented fish mince products are now available in the world.[Citation4] Most of them are fermented using bacteria, such as Som-fug, a Thai fermented fish mince using lactic acid bacteria (LAB) starters, fermented fish sausage using LAB, and the fish miso prepared with Aspergillusoryzae-inoculated koji. Up to now, no fermented fish mince was prepared using fungal starter.
Fungal starters including Actinomucor spp., Mucor spp., and Rhizopus spp. have been successfully used for the preparation of sufu, a cheese-like product with a creamy consistency.[Citation7] It is interesting to find that surimi has similar nutritional compositions with fresh soybean curd, which is used for the production of sufu. Moreover, Actinomucor elegans (A. elegans), the traditional fungal starter for sufu, could also produce protease and degrade surimi protein and enrich peptides and amino acids in surimi.[Citation8] So, it’s theoretically feasible to produce fermentation surimi with A. elegans.
The present study aimed to develop a value-added surimi product using a four-step solid-state fermentation technique, which was normally involved in traditional mold-fermented sufu production: (1) preparing surimi curd, (2) preparing surimi pehtze with pure culture mold fermentation, (3) salting, (4) ripening. Physicochemical characteristics, protein hydrolysis, textual properties, and microbiological changes of surimi at the four stages were analyzed, and changes in sensory scores of the fermented surimi during ripening were also evaluated.
Material and methods
Preparation of fungal spore suspension
A. elegans XH-22 strain, kindly provided by Shaoxing Xianheng Foodstuff Co., LTD. (Shaoxing, China), was grown on solid-substrate culture consisting of bran and water (1:1.2~1.4). After incubation in the bran culture medium at 28°C for 3 d, spore suspensions (~105 CFU/mL) were collected through eight layers of cheesecloth for incubation.
Surimi fermentation
Frozen surimi (Grade A) produced from low value marine fish was provided by Zhejiang Industrial Group Co., Ltd. (Zhoushan, China). The initial moisture content of surimi was 74.5%. Food additives in the surimi were 4% sorbitol, 4% sucrose, and 0.3% polyphosphate. Frozen surimi blocks (10 kg each) were shipped in heavily insulated industrial strength boxes filled with ice. Upon arrival surimi blocks were cut into approximately 1 kg units, vacuum-packaged and stored at –80°C until use. To make the surimi more suitable for fungal growth, three food ingredients, including 2% glucose, 5% corn starch, and 10% isolated soy protein, were added to the original surimi following procedure. After addition of 0.5% salt, the mixture was smashed for 15 min and then steam heated for 15 min for the preparation of surimi gel. The surimi product was then cut into rectangular pieces (approximately 3.2 × 3.2 × 1.6 cm) to get surimi curd for fermentation.
For surimi pehtze preparation, 0.2 mL of A. elegans XH-22 spore suspension was sprayed onto the surface of the surimi pieces. The inoculated surimi was evenly spaced in plastic trays and incubated in a ventilating incubator for 36 h at controlled temperature (28ºC) and relative humidity (RH; 93–95%). The surimi pehtze was then transferred into a container and salted (NaCl, 20% of the pehtze, w/w) at 4ºC for 36 h, and then placed into wide-mouthed glass bottles, after which dressing mixture, containing double rice wine (an alcoholic beverage with unique flavor and high nutritional value contained >12% alcohol) and 5% NaCl was added, and the bottles were then closed with food plastic wrap and incubated at 20ºC for 60 d. Samples after pehtze preparation, salting, and ripening were taken for physicochemical characteristics, protein hydrolysis and textual properties analysis. Sensory scores of surimi ripened for 0, 15, 30, 45, and 60 days were also analyzed.
Water content, pH, and total acidity (TA) analysis
For water content determination, samples (2 g) was placed on an aluminum dish, spread evenly across the dish and oven-dried at 105ºC for 24 h.[Citation9] For pH and TA analysis, a total of 10 g of surimi was mixed with 100 mL of CO2-free distilled water and centrifuged at 9000 × g for 10 min. The pH of the supernatant was measured directly with a pH meter, while TA was measured by titrating the supernatant with 0.1 mol/L NaOH solution. pH value was expressed as a percentage of the citric acid.
Protease activity assays
Protease activity was determined according to Keay and Wildi.[Citation10] Ten grams of surimi mixed with 50 mL of 0.3 mol/L NaCl and 0.2 mol/L phosphate buffer (pH 6.8) was homogenized. After equilibrating at room temperature for 60 min with frequent stirring, the mixture was centrifuged at 2100 × g for 5 min. The supernatant was used as crude enzyme extract for protease activity determination. One unit of protease activity was defined as the amount of enzyme that liberates 1 μg of tyrosine per min.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Three grams of surimi samples were homogenized with 27 mL of 5% (w/v) SDS solution and then incubated at 85ºC for 1 h, followed by centrifuging at 10,000 × g for 10 min to remove undissolved debris. Protein concentration was determined by the method of Biuret.[Citation11] The soluble proteins were mixed (1:1, v/v) with sample buffer (0.125 M Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 10% βME, and 0.005% bromophenol blue) and then heated at 100ºC for 6 min before subjected to electrophoresis in a 12% separating gel and 4% stacking gel using a Mini-Protein II system (Bio-rad, Hercules, CA, USA). Aliquots of 20 μL (40 μg protein) were loaded in each well including standard protein marker. Electrophoresis was done at 80–120 V for 1.5 h. The gel was stained with 0.1% (w/v) Coomassie Brilliant Blue R-250 in 45% methanol and 10% acetic acid for 1 h and then destained in 10% methanol and 10% acetic acid for 12 h.
Free amino acids (FAAs) analysis
Analysis of FAAs was performed according to Han et al.[Citation12] The sample homogenates were dissolved in sulphosalicylic acid, and supernatants were applied to the analyser using sodium citrate buffer system (pH 2.2, 3.3, 4.3, and 5.4) with post-column ninhydrin detection for FAA. A known mixture of different FAA was applied as an external standard for the calculation.
Trichloroacetic acid-soluble nitrogen (TCA-N) analysis
Ten grams of surimi was blended in 25 mL of distilled water and centrifuged at 5000 × g for 5 min. The supernatant was used for TCA-N contents determination. The general protein precipitation method of the Association of Official Analytical Chemists (AOAC) was used with the modification that trichloroacetic acid (2.5 g/L) was used instead of phosphotungstic acid,[Citation9] and the results were calculated on a dry-weight basis.
Scanning electron microscopy (SEM) analysis
Surimi samples were prepared and subjected to freeze-drying in a lyophilizer (Telstar-Cryodos, Spain) for 24 h. After coating the samples with gold-palladium, the microstructure of surimi was observed by SEM (S-4700, Hitachi, Tokyo, Japan). The samples were inspected at the acceleration voltages of 15 kV, and the scanned images were amplified to 800 times.
Instrumental texture profile analysis (TPA)
TPA was performed using a software-controlled penetrometer, TA-XT Plus texture analyzer (Stable Micro System, UK) equipped with a 5 kg load cell. At least 15 rectangular surimi samples per treatment were used. Surimi was subjected to two-cycle compression at 50% compression using texture analyzer with a 70-mm TPA compression plate attachment moving at a speed of 127 mm/min. From the resulting force-time curves, hardness, adhesiveness, springiness, and cohesiveness were determined.
Color evaluation
The rectangular surimi samples were cut in half and the color of the internal parts were measured using an automatic color difference meter colorimeter (HunterLab ColorQuest.XE, Reston, VA, USA). The values of L*, a*, and b* were used to evaluate the surimi color.
Sensory evaluation
Changes in sensory qualities of taste, flavor, texture, and overall acceptability of the ripened surimi were evaluated by 15 trained specialists acquainted with seafood tastes and the subtleties of surimi degradation. The 9-point hedonic scale was used for evaluation, with 9 being extreme liking, 5 being neither like or dislike, and 1 being extreme dislike.
Microbiological analysis
Microbiological analysis was conducted according to Han et al.[Citation13] Briefly, 50 g of each sample were aseptically weighed into a sterile blender jar (Oster) containing 450 mL sterile 1% NaCl diluent (pH 7.2). Samples were blended at high speed for 2 min and subsequent decimal dilutions were prepared in 1% NaCl diluents[Citation14] for microbiological analysis. For aerobic plate count (APC) enumeration, samples were enumerated in pour-plates of Plate Count Agar (PCA, Oxoid, England) after incubation at 22°C for 72 h. For bacterial endospores (spores) enumeration, samples were pasteurized (80ºC, 10 min) and spores were enumerated in pour-plates of PCA after incubated at 30ºC for 72 h. LAB were enumerated in pour-plates of MRS Agar Base (Merck Oxoid, England) after 3–4 days incubation at 30ºC. Selective enumeration of viable Enterobacteriaceae was carried out in pour-plates of Violet Red Bile Glucose agar (VRBG, Oxoid, England) after 36 h incubation at 30ºC.
Statistical analysis
All experiments were performed in triplicate and the results were presented as mean ± standard deviation (SD) of replicated measurements. One-way analysis of variance (ANOVA) was performed using SPSS 21 computer program (SPSS Inc., Chicago, IL, USA), and differences in mean values were determined with the least significant difference (LSD, p < 0.05) procedure of the statistical analysis system.
Results and discussion
Basic components
Changes in basic components of surimi curd, surimi pehtze, salted surimi and ripened surimi are shown in . Compared with the original surimi curd, crude fat, and protein components of the final products were all decreased. Enzymes secreted by the fermentation starter, including lipases and proteases, could promote the decomposition of fats and proteins, and the added salt had a great impact on the composition of the dry matter.[Citation15] Ash content sharply increased after salting because of water loss and salt addition, but showed an apparently decrease after ripening which was mainly attributed to the water infiltration. During ripening, water in the brine kept infiltrating into surimi pehtze until the salinity between the surimi and brine balanced. At the end of the ripening, NaCl content in surimi remained at 8.58%, which could keep the surimi from spoilage during shelf life.
Table 1. Changes in basic components, texture profile, and color properties of surimi at different stages of fermentation.
The water loss during surimi pehtze preparation stage was mainly because of the growth consumption of the fungal mycelia and the water evaporation. Water content consistently decreased and reached a minimum value of 62.1% after salting, due to water diffusion caused by the salt concentration difference.[Citation16] The lowered water content might help maintain the shape and texture of the surimi curd during the later ripening stage. However, water loss and salt uptake would affect the conformation of muscle proteins, causing aggregation, and denaturation, and resulted in greater hardness.[Citation17] A much denser structure might affect the protein degradation by protease during the ripening stage, and further affect the nutritional compositions of the final surimi product.
Physicochemical characteristics
Changes in pH and TA of the surimi during fermentation are shown in . The initial pH of the surimi curd was 6.82. As the fermentation proceeded, pH value constantly decreased and reached a minimum value of 4.62 at the end of ripening. Lower pH was important for the control of surimi spoilage and pathogen proliferation.[Citation18] TA of the fermented surimi increased from 0.09 to 0.40% during pehtze preparation stage, decreased to 0.21% after salting, and then increased to a maximum value of 0.64% after 60 d of ripening. The decrease of pH during pehtze preparation stage was partly resulted from the production of organic acids, including oxalic acid, lactic acid, acetic acid, and citric acid, by Mucorrecemosus molds.[Citation8] Other microbes such as LAB and Bacillus cereus, which could produce organic acids, amino acids, and fatty acids, might also exist as raw material does or from contaminants in environment. The production of organic acids was responsible for the formation of special flavor in surimi, especially during ripening.[Citation19]
Table 2. Physiochemical properties and protease activity of surimi at different stages of fermentation.
Protein hydrolysis
Fungal fermentation starters could produce lots of enzymes which can promote protein hydrolysis and thus degrade large protein molecule into FAAs, peptides, and other non-protein nitrogenous substances.[Citation6] It could be seen from that A. elegans XH-22 inoculated in surimi was capable of producing extracellular protease and its activity reached 69.34 U/g after pehtze preparation stage, which was only 0.97 U/g in the surimi curd where A. elegans was not inoculated yet, while in Han’s study of fermented soybean curd, protease activity reached a bit higher value of 85.4 U/g after the 36 h of pehtze preparation stage.[Citation20] Afterwards, protease activity decreased intensely during the salting stage, resulted from a negative inhibition of protease activity by salt. At the stage of ripening, the intracellular proteases of A. elegans XH-22 mold in the surimi were gradually released, and as a result, protease activity increased gradually even under the dual inhibitory of salt and alcohol in the dressing mixture.
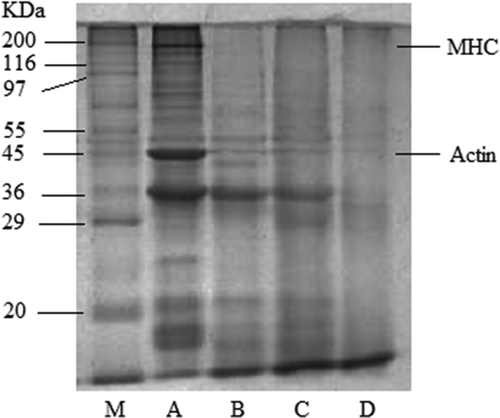
SDS-PAGE was performed to analysis the changes in protein patterns of surimi during the fermentation. As shown in , bands intensities showed obvious differences in the protein composition among the four samples, especially between samples of the pehtze preparation stage and ripening stage. It could be obviously seen that the myosin heavy chain (MHC) band disappeared after the first 36 h of fermentation, and the intensity of actin band and the band at 36 KDa which probably corresponding to troponin-T[Citation21] decreased at the meantime, indicating obvious degradation of large protein molecule took place as the fermentation proceeded. This could be explained by the increased protease activity produced by A. elegans as previously described. Although salt is beneficial for the release of intracellular protease during ripening stage, it also partly inhibited the protease activity, and as a result, little variation in band intensities were observed during the salting stage.
Figure 1. SDS-PAGE patterns of surimi protein at different stages of fermentation. MHC: myosin heavy chain; M: molecular weight marker; (a) surimi curd; (b) surimi pehtze; (c) salted surimi; (d) ripened surimi.
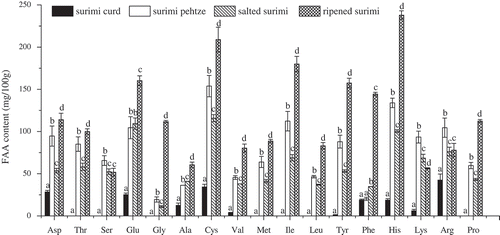
FAAs content and TCA-N levels are indicators representing the degree of proteolysis in fermented product, and their variations were thought to reflect the activity of proteases.[Citation22] Changes in FAAs content of surimi during fermentation are shown in . Total FAAs content increased from 192.15 mg/100 g to 1327.55 mg/100 g after 36 h of fermentation, indicating the hydrolysis of surimi protein by A. elegans XH-22 protease. A slightly decrease was found after the salting stage, which could be explained that the FAAs were consumed or transformed at a greater rate than they were formed by proteolytic activity.[Citation12] The content of total FAAs reached a maximum value of 2026.15 mg/100 g at the end of the ripening, this could be mainly due to the effects of microbial degradation of surimi protein.[Citation23] After ripened for 60 d, aspartic acid, glutamic acid, cysteine, isoleucine, tyrosine, phenylalanine, and histidine were prominent in the surimi product, with contents of 114.00, 160.30, 208.96, 179.94, 157.48, 144.45, and 237.92 mg/100 g, respectively. TCA-N levels of surimi during the fermentation were found to change in the same way as that of the FAAs (). It increased intensely and reached 0.68 g/100 g after pehtze preparation stage, dropped a little after salting, and then increased to the maximum of 0.97 g/100 g, futher reflecting hydrolysis of surimi protein during the fermentation.
Textual properties
Texture profiles of surimi during fermentation were analyzed and the results are shown in . Except for adhesiveness which increased at the surimi pehtze preparation stage, all the other parameters tested decreased during the 36 h of fermentation, increased after salting and then decreased again after ripening. According to Riebroy, Benjakul,[Citation19] the development of hardness was related to pH reduction and moisture removal. Regardless of the obviously decrease of pH value and water content during the 36 h of fermentation, the decrease of hardness value could be explained by protein degradation and the disrupt of the protein network. Afterwards, low water content led to the increase of hardness during salting, while the protein degradation and high water content led to the decrease of hardness which ultimately resulted in a much loosen structure of the product after ripening.
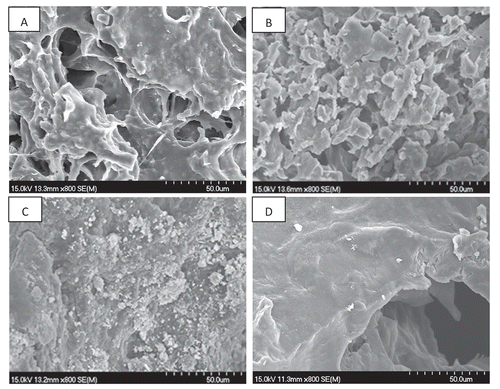
The microstructure evolutions of surimi during fermentation were investigated by SEM. SEM has been widely applied in food analysis as it gives a 3D-view of the sample and makes the differences in structure easily understood.[Citation24] As shown in , SEM images demonstrated differences in surimi structure as affected by the fermentation starters, salt, alcohol, and other fermentation conditions such as temperature and humidity. At the beginning, surimi curd presented a gel network with many sizable holes, cluster structure, and strong frames formed by surimi protein undergoing aggregation,[Citation25] which was also consistent with the high value of springiness as shown in . Coincided with the decreased hardness, springiness, and cohesiveness, surimi pehtze showed a loose surface with a disrupt structure after 36 h of fermentation and this was mainly related to protein degradation and loss of water as previously described. Salted surimi showed a dense structure due to the loss of water, while ripened surimi showed a dense but much softer structure.
Color properties
Changes in L*, a*, and b* values of the surimi color during fermentation are shown in . Obvious color changes were found, and in general, L* values decreased while a* and b* values increased gradually. Proterin hydrolysis, water loss, and the reaction of some substances like the oxidation of soy isoflavone in isolated soy protein might be responsible for the increase of a* and b* values. Park[Citation26] reported that moisture contents would affected L* and b* values, the higher the moisture, the lighter and the less yellow the color. Furthermore, the addition of brine brought the significantly decrease of L* value and the increase of a* and b* values in ripening (p < 0.05).
Sensory evaluation
The sensory score of surimi during ripening is shown in . Taste, flavor and texture scores of surimi generally increased with the increase of ripening time, and the overall acceptability reached the best score of 8.25 ± 0.28 at the end of the ripening. High content of FAAs, appropriate amounts of alcohols and salts, and large amount of volatile compounds might lead to the high overall acceptability of the fermented surimi.
Table 3. Sensory scores of surimi with different ripening time.
Microbiological analysis
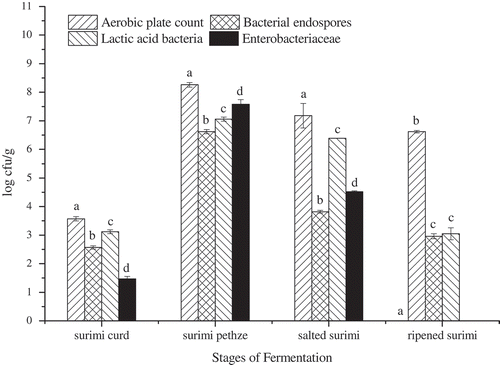
Microbial changes of surimi during fermentation are shown in . Initial counts of aerobic bacteria, bacterial endospores, LAB, and enterobacteriaceae were in low levels, while they all increased and reached maximum after 36 h of fermentation during surimi pehtze preparation stage, and then decreased during salting and ripening stages, respectively. The opening fermentation environment might cause rapid growth of microorganisms, however, appropriate levels of salt and alcohol could control the survival or growth of pathogens.[Citation7] APC showed a sharp increase from 3.57 log CFU/g in surimi curd to 8.26 log CFU/g after pehtze preparation stage, and then decreased to 7.18 log CFU/g after salting. At the end of the ripening, LAB reached a minimum value of 3.05 log CFU/g, while the APC did not change much. Enterobacteriaceae was not detectable in surimi after 60 d of ripening. It could be inferred that bacterial endospores, LAB, and enterobacteriaceae were more susceptible to salt and alcohol. Count of bacterial endospores changed in the similar way as other microbes in surimi, but the intense reduction during salting was presumably attributed to germination, and the resulting vegetative cells subsequently died.[Citation13]
Conclusions
A new value-added surimi product was developed by solid-state fermentation technique. The high levels of protease activity, FAAs content and TCA-N content, and the decrease of protein band intensity in SDS-PAGE directly revealed the protein hydrolysis occurred in surimi fermentation. TPA and SEM images demonstrated changes in the surimi texture and further reflected effectively protein degradation of surimi. High sensory scores indicated high overall acceptability of the final surimi product. On the whole, A. elegans was confirmed to be an ideal fermentation starter for the solid-state fermentation of surimi. However, the characteristics of surimi during fermentation were relevant with the concentration of starters, levels of salt and alcohols and other fermentation conditions, such as fermentation temperature and time. Further investigations are in progress to further improve the nutritional characteristics of the final product.
Funding
This work was supported by the National Science Foundation of China (31471613) and Natural Science Foundation of Zhejiang Province (LY13C200010).
Additional information
Funding
References
- Yoon, I.H.; Matches, J.R.; Rasco, B. Microbiological and Chemical Changes of Surimi-Based Imitation Crab During Storage. Journal of Food Science 1988, 53, 1343–1346.
- Martin-Sanchez, A.M.; Navarro, C.; Perez-Alvarez, J.A.; Kuri, V. Alternatives for Efficient and Sustainable Production of Surimi: A Review. Comprehensive Reviews in Food Science and Food Safety 2009, 8, 359–374.
- Coton, M.; Denis, C.; Cadot, P.; Coton, E. Biodiversity and Characterization of Aerobic Spore-Forming Bacteria in Surimi Seafood Products. Food Microbiology 2011, 28, 252–260.
- Xu, Y.S.; Xia, W.S.; Yang, F.; Nie, X.H. Physical and Chemical Changes of Silver Carp Sausages During Fermentation with Pediococcus Pentosaceus. Food Chemistry 2010, 122, 633–637.
- Medina, J.R.; Garrote, R.L. The Effect of Two Cryoprotectant Mixtures on Frozen Surubi Surimi. Brazilian Journal of Chemical Engineering 2002, 19, 419–424.
- Nout, M.R.; Aidoo, K.E. Asian Fungal Fermented Food. In Industrial Applications; Springer: Springer, Berlin, Heidelberg, 2011; 29–58.
- Han, B.Z.; Rombouts, F.M.; Nout, M.J.R. A Chinese Fermented Soybean Food. International Journal of Food Microbiology 2001, 65, 1–10.
- Zhou, X.X.; Zhao, D.D.; Liu, J.H.; Lu, F.; Ding, Y.T. Physical, Chemical and Microbiological Characteristics of Fermented Surimi with Actinomucor Elegans. LWT–Food Science Technology 2014, 59, 335–341.
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, 1984.
- Keay, L.; Wildi, B.S. Proteases of the Genus Bacillus. I. Neutral Proteases. Biotechnology and Bioengineering 1970, 12, 179–212.
- Gornal, A.G. Determination of Serum Proteins by Means of the Biuret Reaction. The Journal of Biological Chemistry 1949, 177, 751–766.
- Han, B.Z.; Rombouts, F.M.; Nout, M.J.R. Amino Acid Profiles of Sufu, a Chinese Fermented Soybean Food. Journal of Food Composition and Analysis 2004, 17, 689–698.
- Han, B.Z.; Cao, C.F.; Rombouts, F.M.; Nout, M.J.R. Microbial Changes During the Production of Sufu—A Chinese Fermented Soybean Food. Food Control 2004a, 15, 265–270.
- Hollingworth, T.A.; Kaysner, C.A.; Colburn, K.G.; Sullivan, J.J.; Abeyta, C.; Walker, K.D.; Torkelson, J.D.; Throm, H.R.; Wekell, M.M. Chemical and Microbiological Analysis of Vacuum-Packed, Pasteurized Flaked Imitation Crabmeat. Journal of Food Science 1991, 56, 164–167.
- Han, B.Z.; Wang, J.H.; Rombouts, F.M.; Nout, M.J.R. Effect of NaCl on Textural Changes and Protein and Lipid Degradation During the Ripening Stage of Sufu, a Chinese Fermented Soybean Food. Journal of the Science of Food and Agriculture 2003, 83, 899–904.
- Thorarinsdottir, K.A.; Arason, S.; Thorkelsson, G.; Sigurgisladottir, S.; Tornberg, E. The Effects of Presalting Methods from Injection to Pickling, on the Yields of Heavily Salted Cod (Gadus Morhua). Journal of Food Science 2010, 75, E544–E551.
- Martinez-Alvarez, O.; Gomez-Guillen, C. Influence of Mono- and Divalent Salts on Water Loss and Properties of Dry Salted Cod Fillets. LWT–Food Science Technology 2013, 53, 387–394.
- Swetwiwathana, A.; Leutz, U.; Lotong, N.; Fischer, A. Controlling the Growth of Salmonella Anatum in Nham-Effect of Meat Starter Cultures, Nitrate, Nitrite and Garlic. Fleischwirtschaft 1999, 79, 124–128.
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of Iced Storage of Bigeye Snapper (Priacanthus Tayenus) on the Chemical Composition, Properties and Acceptability of Som-Fug, a Fermented Thai Fish Mince. Food Chemistry 2007, 102, 270–280.
- Han, B.Z.; Ma, Y.; Rombouts, F.M.; Nout, M.J.R. Effects of Temperature and Relative Humidity on Growth and Enzyme Production by Actinomucor Elegans and Rhizopus Oligosporus During Sufu Pehtze Preparation. Food Chemistry 2003a, 81, 27–34.
- Zhang, Q.Q.; Ye, K.P.; Wang, H.H.; Xiao, H.M.; Xu, X.L.; Zhou, G.H. Inhibition of Biofilm Formation of Pseudomonas Aeruginosa by an Acylated Homoserine Lactones-Containing Culture Extract. LWT–Food Science Technology 2014, 57, 230–235.
- Riebroya, S.; Benjakula, S.; Visessanguan, W. Properties and Acceptability of Som-Fug, a Thai Fermented Fish Mince, Inoculated with Lactic Acid Bacteria Starters. LWT–Food Science Technology 2008, 41, 569–580.
- Jiang, J.J.; Zeng, Q.X.; Zhu, Z.W.; Zhang, L.Y. Chemical and Sensory Changes Associated Yu-Lu Fermentation Process—A Traditional Chinese Fish Sauce. Food Chemistry 2007, 104, 1629–1634.
- Groves, K.; Parker, M.L. Appendix: Electron microscopy: Principles and Applications to Food Microstructures. In Food Microstructures; Groves, V.J.M.; Ed.; Witney, Oxford: Woodhead Publishing, 2013; 386–428.
- Ma, X.S.; Yi, S.M.; Yu, Y.M.; Li, J.R.; Chen, J.R. Changes in Gel Properties and Water Properties of Nemipterus Virgatus Surimi Gel Induced by High-Pressure Processing. LWT–Food Science Technology 2015, 61, 377–384.
- Park, J.W. Surimi Gel Colors as Affected by Moisture Content and Physical Conditions. Journal of Food Science 1995, 60, 15–18.