ABSTRACT
This study was targeted to characterize the chemical composition and antibacterial properties of Daphne oleoides subsp. oleoides essential oil. The essential oil was analyzed and quantified by gas chromatography and gas chromatography/mass spectrometry. Additionally, the broth dilution method was used to evaluate its antibacterial activity against Bacillus cereus (ATCC 11778), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), Streptococcus faecalis (ATTC 29212), Escherichia coli (ATCC 25922), Proteus mirabilis (ATCC 25933), Proteus vulgaris (ATCC 13315), Pseudomonas aeruginosa (ATCC 27853), and Salmonella typhi Ty2 (ATCC 19430). Seventy-nine compounds were identified, representing 95.2% of the total oil. Nootkatone (18.5%), nootkatin (12.1%), and daphnauranol C (11.7%) were determined as the main constituents in the oil. Oxygenated sesquiterpenes were dominating in the oil (43.0%), followed by fatty acid derivatives (13.7%) and carbonylic compounds (9.6%). The minimal inhibitory/bactericidal concentrations of essential oils of D. oleoides were in the range from 25–100 μg/mL, which can be considered as high activity in comparison with the reference antibiotic which was active in the rangefrom 3.12–100 μg/mL. The greatest minimal inhibitory concentration value was determined as 25 µg/mL against both two Bacillus strains and S. epidermidis, B. cereus, B. subtilis, and S. aureus were the most sensitive strains against essential oils when compared with the minimal inhibitory concentrations of control antibiotic. Consequently, Daphne oleoides subsp. oleoides can be exploited as a source of natural antibacterial agents and nootkatone for the pharmaceutical, food, and agricultural industries.
Introduction
Plants or plant products are a tremendous source for the discovery of new constituents with medicinal importance in the preparation of drug formulations. In plant secondary metabolites, essential oils are highly complex mixtures of volatile components (with mono- and sesqui-terpenoids as the most common constituents).[Citation1] Essential oils are of growing interest both in the industry and scientific research because of their antioxidant, antibacterial, antifungal, antiviral, and antiparasitical activities that make them useful as natural additives in the food, cosmetic, and pharmaceutical industries.[Citation2] In this direction, there is great interest in finding new, safe, and biologically active essential oils from aromatic and medicinal plants.
Daphne is a genus with 70 species, seven of which can be found in Turkey (D. glomerata, D. gnidioides, D. mezereum, D. mucronata, D. oleoides, D. pontica, and D. serica).[Citation3] Members of the Daphne genus have been of interest on account of their notable medicinal value. Several Daphne species such as D. oleoides and D. genkwa are used as traditional drugs for the treatment of several ailments including cancers, rheumatism, gonorrhea, aches, and ulcers.[Citation4–Citation7] Again, phytochemical studies revealed that members of this genus contain different classes of secondary metabolites including coumarins, flavonoids, and terpenoids.[Citation7,Citation8–Citation22] For this reason, much attention has been paid to biological activity and chemical components of Daphne species. Also, essential oil components of some Daphne species including D. oleoides were reported in previous studies.[Citation23] But, the samples were collected in1999 and 2003 in the study conducted by Gurbuz et al.[Citation23] Thus, to the best of our knowledge, there is no actual scientific report on antimicrobial activity and chemical characterization of D. oleoides essential oil. In this point, this study aimed to evaluate antimicrobial properties of essential oil from D. oleoides subsp. oleoides as a new potential source of natural and biologically active agents.
Material and methods
Plant materials
Daphne oleoides Schreber subsp. oleiodes Schreber was collected in July 2013 from Konya (Turkey). The plants were identified by Dr. Murad Aydin Sanda from Department of Field Crops, Agriculture Faculty, Igdir University. A voucher specimen (MS 1001-2013) was deposited in KNYA Herbarium at Department of Biology, Selcuk University.
Essential oils isolation
The air-dried samples of aerial parts were ground in a Waring blender and then subjected to hydrodistillation for 3 h according to the standard procedure described in European Pharmacopoeia (2008). The oils, all with yellowish color and a pleasant smell, were dried over anhydrous sodium sulphate and then stored in sealed vials under N2, at +4°C, ready for the gas chromatography (GC) and gas chromatography–mass spectrometry (GC–MS) analyses. The sample yielded (w/w) 0.39% oil.
GC and GC-MS analysis
Analytical GC was carried out on a Perkin-Elmer Sigma 115 gas chromatograph fitted with a HP-5 MS capillary column (30 m × 0.25 mm), 0.25 μm film thickness. Helium was the carrier gas (1 mL/min). Column temperature was initially kept at 40°C for 5 min, then gradually increased to 250°C at 2°C/min rate, held for 15 min and finally raised to 270°C at 10°C/min. Diluted samples (1/100 v/v, in n-pentane; 1 μL) were injected at 250°C, manually and in the splitless mode. Flame ionization detection (FID) was performed at 280°C. Analysis was also run by using a fused silica HP Innowax polyethylenglycol capillary column (50 m × 0.20 mm), 0.20 μm film thickness and operating as previously described. GC-MS analysis was performed on an Agilent 6850 Ser. II apparatus, fitted with a fused silica DB-5 capillary column (30 m × 0.25 mm), 0.33 μm film thickness, coupled to an Agilent Mass Selective Detector MSD 5973; ionization voltage 70 eV; electron multiplier energy 2000 V. GC conditions were as given; transfer line temperature, 295°C.
Identification of compounds
Most constituents were identified by GC by comparison of their retention indices (Ki) with those of the literature[Citation24,Citation25] or with those of authentic compounds available in our laboratories. The retention indices were determined in relation to a homologous series of n-alkanes (C8-C24) under the same operating conditions. Further identification was made by comparison of their mass spectra on both columns with those stored in NIST 02, Wiley 275 libraries and our homemade library or with mass spectra from literature.[Citation24,Citation26] Component relative percentages were calculated based on GC peak areas without using correction factors.
Antibacterial activity
Bacterial strains
Ten bacterial strains, selected as representative of the class of Gram+ and Gram–, were used: Bacillus cereus (ATCC 11778), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), Streptococcus faecalis (ATTC 29212), Escherichia coli (ATCC 25922), Proteus mirabilis (ATCC 25933), Proteus vulgaris (ATCC 13315), Pseudomonas aeruginosa (ATCC 27853), and Salmonella typhi Ty2 (ATCC 19430). The strains were maintained on Tryptone Soya agar (Oxoid, Milan, Italy); for the antimicrobial tests, Mueller-Hinton Broth (Oxoid, Milan, Italy) was used.
Antimicrobial activity assay
The antibacterial activity was evaluated by determining the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) using the broth dilution method.[Citation27,Citation28] In order to facilitate the dispersion of the oil in the aqueous nutrient medium it was diluted with Tween 20 at a concentration of 10%. Each strain was tested with sample that was serially diluted in broth to obtain concentrations ranging from 100 to 0.8 μg/mL. The sample was previously sterilized with Millipore filter of 0.20 μm. It was stirred, and inoculated with 50 μL of physiologic solution containing 5 × 106 microbial cells. After this process the plates incubated for 24 h at 37°C. The MIC value was determined as the lowest concentration of the sample that does not permit any visible growth of the tested microorganism after incubation. The sterile physiological solution and Tween 20 without essential oil were evaluated for their toxicity and they were not toxic to the microorganisms. The cultures containing only sterile physiologic solution were used as negative control. Chloramphenicol was used as positive antibiotic. MBC was determined by subculture of the tubes with inhibition in 5 mL of sterile nutrient broth. After incubation at 37°C the tubes were observed. When the germs did not grow, the sample denoted a bactericidal action. Oil samples were tested in triplicate.
Results and discussion
Chemical composition of D. oleoides essential oil
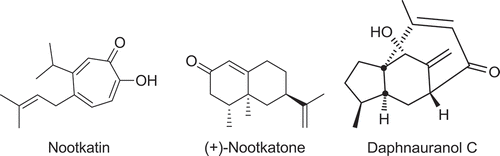
The hydrodistillation of the aerial parts of D. oleoides collected in Konya (Turkey) yielded 0.39% (w/w) of essential oil. GC and GC–MS analysis enabled the identification of 79 compounds representing 95.2% of the total oil. The relative concentrations of the volatile components identified are presented in according to their elution order (RI) on a HP-5 column. The most abundant compounds were the oxygenated sesquiterpenes nootkatone (18.5%), daphnauranol C (11.7%), and nootkatin (12.1%) followed by the phenol p-vinylguiacol (8.2%; and ). Also, the spectral data for nootkatone and nootkatin are shown in . Similarly, nootkotane was reported as a major compound of several essential oils.[Citation29,Citation30] On the whole, the oil was constituted mainly by sesquiterpenes (49.7%), among which oxygenated sesquiterpenes (43.0%) prevailed. Fatty acids and esters (13.7%) were also abundant with a clear prevalence of hexadecanoic acid (4.9%). Carbonylic compounds (9.6%) and phenols (9.2%) were present in quite similar amounts; phenols were represented almost entirely by p-vinylguiacol (8.2%).
Table 1. Chemical composition of D. oleoides essential oil.
In a previous study, Gurbuz et al.[Citation23] studied the volatile compounds of essential oils of D. oleoides and D. pontica isolated by hydro- and microdistillation methods. These authors identified 27 (as being 93.5%) and 25 (as being 99.2%) compounds in the essential oils of D. oleoides from Karaman and Ilgaz, respectively. Nonacosane (42.5 and 27.2%) and hexadecanoic acid (24.4 and 20.0%) were identified as the major components in both Karaman and Ilgaz specimens. Also, hexanhydrofarnesyl acetone (8.6%) and carvacrol (8.5%) were the dominant compounds in essential oil of D. pontica in this study.
The levels of nonacosane (2.5%) and hexadecanoic acid (4.9%) were very low in the present study. Thus, the level of fatty acids (13.7%) and hydrocarbons (8.7%) was determined as low compared to the previous work of Gurbuz et al.[Citation23] In addition, big differences were observed in/between these studies in terms of compound groups. For example, the level of oxygenated sesquiterpene was 2.1% in the previous study while the content was reached to 43.0% in our work ().The observed differences may be explained by different sample collection time, rainfall, soil texture, average temperature, and humidity. In accordance with these approaches, many literature references demonstrated that yields and compositions of several essential oils were significantly affected by environmental factors.[Citation31–Citation35]
Table 2. Comparison of D. pontica and D. oleoides essential oils collected from different region of Turkey in terms of major volatile groups (%).
Nootkatone is a sesquiterpene ketone and known as the main component of the smell and flavor of grapefruit. This compound exhibits important biological activities including anti-platelet, anticholinesterase, and anti-obesity.[Citation36–Citation38] In this sense, the production of nootkatone is performed by chemical synthesis mainly from valencene. However, due to its chiral structure, the preparation of nootkatone via organic synthesis is difficult. Again, the formation of nootkatone cannot be marketed as “natural and safe” product and does not satisfy increasing market demands for natural aromatic compounds.[Citation39] Thus, there is an increasing interest in the investigation of naturally occurring nootkatone from plants. As a result of inhibiting cholinesterase, nonkatone is considered as an effective insecticide or acaricide.[Citation40–Citation42] For instance, nootkatone isolated from A. oxyphylla showed insecticidal activity against larvae of Drosophila melanogaster.[Citation43] Moreover, nootkatone metabolites show antiproliferative activity toward cancer cell lines A549 and HL-60.[Citation44] Similarly, daphnauranols A-C from Daphne aurantiaca were reported by Huang et al.[Citation45] as new, non-toxic, and natural anti-feedant agents. shows the levels of nootkatone of several essential oils. According these results, D. oleoides essential oils have the higher nootkatone levels as compared to the previous studies. Consequently, the studied essential oil could be considered as an excellent source of nootkatone for industrial areas, including agricultural and pharmaceutical.
Table 3. Comparison of different essential oils in terms of nootkatone level (%).
Antimicrobial activity
In this study antimicrobial activity of essential oils of D. oleoides was investigated by broth microdilution method against 10 standard bacteria according to Barry[Citation27] and Zengin et al.[Citation28] The obtained results are presented in . It was inferred from that essential oils of D. oleoides revealed strong antibacterial activity against tested strains. The greatest MICs value was determined as 25 µg/mL against both two Bacillus strains and S. epidermidis. When compared to the control antibiotic, this value was considered significantly effective. For S. aureus, Str. faecalis, and E. coli, essential oils manifested significant activity at a dose of 50 µg/mL. With the exception of E. coli, the other Gram-negative bacteria including K. pneumoniae, P. vulgaris, P. aeruginosa, and S. typhi were more resistant to the essential oils than Gram-positive bacteria and MICs values were determined as 100 µg/mL. When the MBCs were evaluated, S. epidermidis showed the lowest MBC values with a concentration of 25 µg/mL followed by Bacillus species and S. aureus with a dose of 50 µg/mL. It can be stated from the study that, B. cereus, B. subtilis, and S. aureus were the most sensitive strains against essential oils when compared with the MICs of control antibiotic. The MIC values were nearly two-fold as the one of chloramphenicol. But it was determined that Gram-positive bacteria were more affected from the oils when compared to Gram-negative bacteria.
Table 4. MIC and MBC* values (μg/mL) of essential oil from D. oleoides (D) and MIC of reference antibiotic (Ch).
In a study conducted by Jusković et al.,[Citation46] the antimicrobial activity of the methanol extracts from leaves and stems of D. malyana were evaluated by employing both microdilution and disc diffusion methods. The results of the microbiological screening indicated good antimicrobial potential of the extracts of D. malyana and these researchers supposed that flavonoids might be responsible for this activity. Similarly Cottigli et al.[Citation47] reported that the stem methanol extract of D. gnidium revealed antibacterial activity on B. lentus and E. coli at doses close to toxic and on S. aureus, M. morganii, and P. aeruginosa at higher concentrations. The authors proved that the antimicrobial activity of stems of D. gnidium was not only due to coumarins but also to flavonoids. In another study, the methanol extracts of the leaves and twigs of D. cneorum exhibited good antimicrobial activity against P. vulgaris.[Citation48]
In our study, essential oils of D. oleoides manifested strong antimicrobial activities against tested strains. While S. epidermidis exhibited the lowest MIC and MBC values (25 µg/mL), the most sensitive strains were determined as B. cereus and B. subtilis (25 and 50 µg/mL, respectively) when compared to control antibiotics. The minimal inhibitory concentrations (MIC)/minimal bactericidal concentrations (MBC) were in the range from 25–100 μg/mL, which can be considered as high activity in comparison with the reference antibiotic which was active in the range from 3.12–100 μg/mL. Our results have strong consistency with the results obtained by researchers in past studies in terms of antimicrobial activity of Daphne species. It could be supposed that these oxygenated sesquiterpenes mentioned above might be responsible for this antimicrobial activity. Manter et al.[Citation49] proved that nootkatin, carvacrol, valencene, nootkatone strongly inhibited the germination of Phytophthora ramorum zoospores or sporangia, and reduced hyphal growth in culture and they revealed that these compounds isolated from some woods exhibited moderate to strong antimicrobial activities in the following studies.[Citation50,Citation51] In that case, our results and foresights which are related to antimicrobial activity of oxygenated sesquiterpens are supported by the results of Manter et al.[Citation49]
Conclusion
In recent decades, the discovery of new raw materials from plants is one of the most important topics in the food industry. In this direction, especially, the wild plant species could be considered as valuable sources for new and safe raw materials. According to this perspective, this study clearly indicated that the volatiles of D. oeloides could be considered as a potential/natural source of sesquiterpenes (escpecially nootkatone) for new raw materials in agricultural and pharmaceutical areas. These valuable sesquiterpenes demonstrate a broad spectrum of biological effects such as antiplatelet, antiobesity, antiproliferative, and antimicrobial. However, further studies are required to evaluate toxic properties of the tested Daphne essential oil and these sesquiterpenes.
Acknowledgments
The authors want to thank Andrei Mocan for proofreading the present manuscript.
References
- Yayli, N.; Yaşar, A.; Güleç, C.; Usta, A.; Kolaylı, S.; Coşkunçelebi, K.; Ş. Karaoğlu, Composition and Antimicrobial Activity of Essential Oils from Centaurea Sessilis and Centaurea Armena. Phytochemistry 2005, 66(14), 1741–1745.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils–A Review. Food and Chemical Toxicology 2008, 46(2), 446–475.
- Tan, K. Flora of Turkey and the East Aegean Islands, Vol. 7; University Press: Edinburgh, Scotland, 1982; 521–526.
- Taniguchi, M.; Fujiwara, A.; Baba, K.; Wang, N.-H. Two Biflavonoids from Daphne Acutiloba. Phytochemistry 1998, 49(3), 863–867.
- Yeşilada, E.; Sezik, E.; Honda, G.; Takaishi, Y.; Takeda, Y.; Tanaka, T. Traditional Medicine in Turkey IX: Folk Medicine in North-West Anatolia. Journal of Ethnopharmacology 1999, 64(3), 195–210.
- Sezik, E.; Yeşilada, E.; Honda, G.; Takaishi, Y.; Takeda, Y.; Tanaka, T. Traditional Medicine in Turkey X. Folk Medicine in Central Anatolia. Journal of Ethnopharmacology 2001, 75(2), 95–115.
- Xu, W.C.; Shen, J.G.; Jiang, J.Q. Phytochemical and Biological Studies of the Plants from the Genus Daphne. Chemistry and Biodiversity 2011, 8(7), 1215–1233.
- Niwa, M.; Sugino, H.; Takashima, S.; Sakai, T.; Wu, Y.C.; Wu, T.S.; Kuoh, C.S. A New Coumarin Glucoside from Daphne-Arisanensis. Chemical & Pharmaceutical Bulletin 1991, 39(9), 2422–2424.
- Taninaka, H.; Takaishi, Y.; Honda, G.; Imakura, Y.; Sezik, E.; Yesilada, E. Terpenoids and Aromatic Compounds from Daphne Oleoides ssp Oleoides. Phytochemistry 1999, 52(8), 1525–1529.
- Ullah, N.; Ahmad, S.; Anis, E.; Mohammad, P.; Rabnawaz, H.; Malik, A. A Lignan from Daphne Oleoides. Phytochemistry 1999, 50(1), 147–149.
- Ullah, N.; Ahmad, S.; Malik, A. Phenylpropanoid Glycosides from Daphne Oleoides. Chemical & Pharmaceutical Bulletin 1999, 47(1), 114–115.
- Ullah, N.; Ahmed, S.; Ahmed, Z.; Mohammad, P.; Malik, A. Dimeric Guaianolides from Daphne Oleoides. Phytochemistry 1999, 51(4), 559–562.
- Ullah, N.; Ahmed, S.; Malik, A. A Dicoumarin Glycoside from Daphne Oleoides. Phytochemistry 1999, 51(1), 99–101.
- Ullah, N.; Ahmed, S.; Mohammad, P.; Rabnawaz, H.; Malik, A. Chemical Constituents of Daphne Oleoides. Fitoterapia 1999, 70(2), 214–215.
- Ullah, N.; Ahmed, S.; Muhammad, P.; Ahmed, Z.; Nawaz, H.R.; Malik, A. Coumarinolignoid Glycoside from Daphne Oleoides. Phytochemistry 1999, 51(1), 103–105.
- Ullah, N.; Ahmed, Z.; Ahmed, S.; Muhammad, P.; Malik, A. A Pentacyclic Triterpene from Daphne Oleoides. Phytochemistry 1999, 50(5), 839–841.
- Riaz, M.; Ullah, N.; Mehmood, A.; Nawaz, H.R.; Malik, A.; Afza, N. Furanoid and Furofuranoid Lignans from Daphne Oleoides. Z Naturforsch B 2000, 55(12), 1216–1220.
- Riaz, M.; Malik, A.; Sadhozai, S.K.; Hussain, M.; Ullah, N. Daphwazirin, Biscoumarin Glycopyranoside from Daphne Oleoides. Natural Product Letters 2001, 15(6), 433–438.
- Zhang, W.; Zhang, W.; Liu, R.; Shen, Y.; Zhang, C.; Cheng, H.; Fu, P.; Shan, L. Two New Chemical Constituents from Daphne Odora Thunb. Var. Marginata. Natural Product Research 2006, 20(14), 1290–1294.
- Zhang, Q.; Ye, N.; Sun, W.-X.; Zhang, K.-M.; Jiang, J.-Q. Phytochemical Investigation of Daphne Giraldii Nitsche (Thymelaeaceae). Biochemical Systematics and Ecology 2008, 36(1), 63–67.
- Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.D.; Arroo, R.; Baykal, T. Efficacy of Daphne Oleoides Subsp. Kurdica Used for Wound Healing: Identification of Active Compounds Through Bioassay Guided Isolation Technique. Journal of Ethnopharmacology 2012, 141(3), 1058–1070.
- Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.; Baykal, T. Daphne Oleoides Schreber ssp. Oleoides Exhibits Potent Wound Healing Effect: Isolation of the Active Components and Elucidation of the Activity Mechanism. Records of Natural Products 2014, 8(2), 93–109.
- Gurbuz, I.; Demirci, B.; Franz, G.; Baser, K.H.C.; Yesilada, E.; Demirci, F. Comparison of the Volatiles of Daphne Pontica L. and D. Oleoides Schreber Subsp Oleoides Isolated by Hydro- and Microdistillation Methods. Turkish Journal of Biology 2013, 37(1), 114–121.
- Jennings, W.S. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography. Academic Press: New York, NY, 1980.
- N. Davies, Gas Chromatographic Retention Indices of Monoterpenes and Sesquiterpenes on Methyl Silicon and Carbowax 20M Phases. Journal of Chromatography A 1990, 503, 1–24.
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectroscopy. 4th Ed; Allured Publishing Co.: Carol Stream: Illinois, 2007.
- Barry, A.L. The Antimicrobic Susceptibility Test: Principles and Practices. Lippincott Williams & Wilkins: Philadelphia, PA, 1976.
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of Phytochemical Composition and Biological Effects of Three Extracts from a Wild Plant (Cotoneaster Nummularia Fisch et Mey.): A Potential Source for Functional Food Ingredients and Drug Formulations. Plos One 2014, 9(11), 1–13.
- Butkiene, R.; Nivinskiene, O.; Mockute, D.; Miliute, A. Variety of the Essential Oils Composition of Wood, Needles (Leaves), Unripe and Ripe Berries of Juniperus Communis Var. Communis Growing Wild in Druskininkai District. Chemija 2007, 18(3), 35–40.
- Khasawneh, M.A.; Xiong, Y.; Peralta-Cruz, J.; Karchesy, J.J. Biologically Important Eremophilane Sesquiterpenes from Alaska Cedar Heartwood Essential Oil and Their Semi-Synthetic Derivatives. Molecules 2011, 16(6), 4775–4785.
- Perry, N.B.; Anderson, R.E.; Brennan, N.J.; Douglas, M.H.; Heaney, A.J.; McGimpsey, J.A.; Smallfield, B.M. Essential Oils from Dalmatian Sage (Salvia Officinalis L.): Variations Among Individuals, Plant Parts, Seasons, and Sites. Journal of Agricultural Food Chemistry 1999, 47(5), 2048–2054.
- Formisano, C.; Senatore, F.; Bancheva, S.; Bruno, M.; Maggio, A.; Rosselli, S. Volatile Components of Aerial Parts of Centaurea Nigrescens and C. Stenolepis Growing Wild in the Balkans. Natural Product Communications 2010, 5(2), 273–278.
- González-Burgos, E.; Carretero, M.; Gómez-Serranillos, M., Sideritis spp.: Uses, Chemical Composition and Pharmacological Activities—A Review. Journal of Ethnopharmacology 2011, 135(2), 209–225.
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation Among Environmental Factors, Chemical Composition and Antioxidative Properties of Essential Oil and Extracts of Chamomile (Matricaria Chamomilla L.) Collected in Molise (South-Central Italy). Industrial Crops and Products 2015, 63, 256–263.
- Moghaddam, M.; Miran, S.N.K.; Pirbalouti, A.G.; Mehdizadeh, L.; Ghaderi, Y. Variation in Essential Oil Composition and Antioxidant Activity of Cumin (Cuminum Cyminum L.) Fruits During Stages of Maturity. Industrial Crops and Products 2015, 70, 163–169.
- Murase, T.; Misawa, K.; Haramizu, S.; Minegishi, Y.; Hase, T. Nootkatone, a Characteristic Constituent of Grapefruit, Stimulates Energy Metabolism and Prevents Diet-Induced Obesity by Activating AMPK. American Journal of Physiology-Endocrinology and Metabolism 2010, 299(2), E266–E275.
- Seo, E.J.; Lee, D.U.; Kwak, J.H.; Lee, S.M.; Kim, Y.S.; Jung, Y.S. Antiplatelet Effects of Cyperus Rotundus and Its Component (+)-Nootkatone. J Ethnopharmacol 2011, 135(1), 48–54.
- Anderson, J.; Coats, J. Acetylcholinesterase Inhibition by Nootkatone and Carvacrol in Arthropods. Pesticide Biochemistry and Physiology 2012, 102(2), 124–128.
- Palmerín‐Carreño, D.M.; Castillo‐Araiza, C.O.; Rutiaga‐Quiñones, O.M.; Verde Calvo, J.R.; Trejo‐Aguilar, G.M.; Dutta, A.; Huerta‐Ochoa, S. Whole Cell Bioconversion of (+)‐Valencene to (+)‐Nootkatone by Yarrowia Lipolytica Using a Three Phase Partitioning Bioreactor. Journal of Chemical Technology and Biotechnology 2015, 91, 1164–1172.
- Villafañe, E.; Tolosa, D.; Bardon, A.; Neske, A. Toxic Effects of Citrus Aurantium and C. Limon Essential Oils on Spodoptera Frugiperda (Lepidoptera: Noctuidae). Natural Product Communications 2011, 6(9), 1389–1392.
- Bharadwaj, A.; Stafford III, K.C.; Behle, R.W. Efficacy and Environmental Persistence of Nootkatone for the Control of the Blacklegged Tick (Acari: Ixodidae) in Residential Landscapes. Journal of Medical Entomology 2012, 49(5), 1035–1044.
- Jordan, R.A.; Schulze, T.L.; Dolan, M.C. Efficacy of Plant-Derived and Synthetic Compounds on Clothing as Repellents Against Ixodes Scapularis and Amblyomma Americanum (Acari: Ixodidae). Journal of Medical Entomology 2012, 49(1), 101–106.
- Miyazawa, M.; Nakamura, Y.; Ishikawa, Y. Insecticidal Sesquiterpene from Alpinia Oxyphylla Against Drosophila Melanogaster. Journal of Agricultural Food Chemistry 2000, 48(8), 3639–3641.
- Gliszczyńska, A.; Łysek, A.; Janeczko, T.; Świtalska, M.; Wietrzyk, J.; Wawrzeńczyk, C. Microbial Transformation of (+)-Nootkatone and the Antiproliferative Activity of Its Metabolites. Bioorganic & Medicinal Chemistry 2011, 19(7), 2464–2469.
- Huang, S.-Z.; Li, X.-N.; Ma, Q.-Y.; Dai, H.-F.; Li, L.-C.; Cai, X.-H.; Liu, Y.-Q.; Zhou, J.; Zhao, Y.-X. Daphnauranols A–C, New Antifeedant Sesquiterpenoids with a 5/6/7 Ring System from Daphne Aurantiaca. Tetrahedron Letters 2014, 55(27), 3693–3696.
- Jusković, M.; Vasiljević, P.; Manojlović, N.; Mihailov-Krstev, T.; B. Stevanović, Phytochemical and Antimicrobial Screening of Leaves and Stems of Balkan Endemic Species Daphne Malyana Blečić. Biotechnology & Biotechnological Equipment 2012, 26(3), 3010–3015.
- Cottigli, F.; Loy, G.; Garau, D.; Floris, C.; Caus, M.; Pompei, R.; Bonsignore, L. Antimicrobial Evaluation of Coumarins and Flavonoids from the Stems of Daphne Gnidium L. Phytomedicine 2001, 8(4), 302–305.
- Manojlović, N.T.; Mašković, P.Z.; Vasiljević, P.J.; Jelić, R.M.; Jusković, M.Ž.; Sovrlić, M.; Mandić, L.; Radojković, M. HPLC Analysis, Antimicrobial and Antioxidant Activities of Daphne Cneorum L. Hemijska Industrija 2012, 66(5), 709–716.
- Manter, D.; Karchesy, J.; Kelsey, R. The Sporicidal Activity of Yellow‐Cedar Heartwood, Essential Oil and Wood Constituents Towards Phytophthora Ramorum in Culture. Forest Pathology 2006, 36(5), 297–308.
- Manter, D.K.; Kelsey, R.G.; Karchesy, J.J. Antimicrobial Activity of Extractable Conifer Heartwood Compounds Toward Phytophthora Ramorum. Journal of Chemical Ecology 2007, 33(11), 2133–2147.
- Manter, D.K.; Kelsey, R.G.; Karchesy, J.J. Antimicrobial Activity of Extracts and Select Compounds in the Heartwood of Seven Western Conifers Toward Phytophthora Ramorum, The Sudden Oak Death Third Science Symposium, Albany, Gen. Tech. Rep. PSW-GTR-214, Albany, 2008; 375–378.
- Kelsey, R.G.; González-Hernández, M.; Karchesy, J.; S. Veluthoor, Volatile Terpenoids and Tropolones in Heartwood Extracts of Yellow-Cedar, Monterey Cypress, and Their Hybrid Leyland Cypress. Annals of Forest Science 2015, 72(3), 349–355.
- Wang, C.; Yuan, H.; Bao, X.; Lan, M. Chemical Composition, in Vitro Cytotoxic and Antioxidant Activities of the Fruit Essential Oil of Alpinia Oxyphylla. Asian Journal of Chemistry 2014, 26(14), 4201.
- Rana, V.S.; Blazquez, M.A. Compositions of the Volatile Oils of Citrus Macroptera and C. Maxima. Natural Product Communications 2012, 7(10), 1371–1372.
- Xie, J.; Wang, S.; Sun, B.; Ito, Y. Isolation and Purification of Nootkatone from the Essential Oil of Fruits of Alpinia Oxyphylla Miquel by High-Speed Counter-Current Chromatography. Food Chemistry 2009, 117(2), 375–380.
- Zhou, J.-h.; Zhou, C.-s.; Jiang, X.-y.; Xie, L.-w. Extraction of Essential Oil from Shaddock Peel and Analysis of Its Components by Gas Chromatography-Mass Spectrometry. Journal of Central South University of Technology 2006, 13(1), 44–48.
- Leal, L.F.; Miguel, O.G.; Silva, R.Z.; Yunes, R.A.; Santos, A.S.; Cechinel-Filho, V. Chemical Composition of Piper Mikanianum Essential Oil. Journal of Essential Oil Research 2005, 17(3), 316–317.
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile Constituents of Redblush Grapefruit (Citrus Paradisi) and Pummelo (Citrus Grandis) Peel Essential Oils from Kenya. Journal of Agricultural Food Chemistry 2005, 53(25), 9790–9794.