ABSTRACT
The functional properties of pH-shifted protein isolates from bigeye snapper head were evaluated. Alkaline isolate showed a superior salt-solubility and gel forming ability to acid counterpart as indicated by a regular gel structure (i.e., imaged by scanning electron microscope) with higher gel strength and lower expressible drip (p < 0.05). Acid isolate exhibited higher surface hydrophobicity (p < 0.05) and thereby improved interfacial properties. Emulsifying activity index of acid isolate was lower than commercial whey protein and egg-white (p < 0.05) but its emulsion stability was better (p < 0.05). Both protein isolates had lower foamability than commercial proteins but their foam stability was not different (p > 0.05).
Introduction
Bigeye snapper (Priacanthus spp.) is an important fish for surimi production in Thailand due to its good gel forming ability.[Citation1] During surimi processing, a large amount of the head is generated as the low value by-product which is commonly used as animal feeds. It has been reported that the head is a major by-product fraction yielding about 20% of the fish weight.[Citation2] Conversion of those remainders into a value added product can pave the way for full utilization of limited fishery resources. Fish head is a complex raw material containing about 55% muscle, 20% bones, 15% gills, 5% skin, and about 4% eyes, and the average protein content is about 15%.[Citation3] Therefore, fish heads are a good source of protein which can be extracted and used as a functional food ingredient. Like other muscle proteins, fish head proteins comprise of myofibrillar proteins, sarcoplasmic proteins, stroma, and others. However, fish flesh composing in the head is difficult to recover with a typical mechanical processing. Hence, it is desirable to develop a technology that would allow efficient recovery of functional proteins from fish head in order to meet human nutritional needs and reduce environmental stress associated with seafood processing.
Acid- and alkaline-aided solubilization or pH-shift method is a technology that efficiently recovers functional and nutritious protein isolates from sources difficult to process through conventional means.[Citation4] The pH-shift processing has shown significant potential as an effective method for maximal protein recovery from fish residues such as frames, bone, skin, head, saw dust, and cut-off by-products.[Citation5–Citation7] The extraction mechanism of the two processes is to solubilize the muscle proteins at low and high pH to separate soluble proteins, bone, skin, connective tissue, cellular membranes, and neutral storage lipids through the centrifugation. The solubilized proteins are collected and recovered by isoelectric precipitation to give a highly functional and stable protein isolate.[Citation4] From this point-of-view, the pH shift method could likely be applied to efficiently recover functional proteins from fish heads for subsequent development of human food ingredients.
The quality and stability of a final product are affected by functional properties of proteins.[Citation8] It is a commonly held view that denaturing muscle proteins negatively affects their functional properties. Therefore, it goes against common belief that muscle proteins that are subjected to extreme low or high pH, like in the acid- and alkaline-aided processes have improved functionality. Kristinsson and Hultin[Citation9] have shown that the unique structure the proteins possess after pH treatment is responsible for improved functional properties in regard to gelation, emulsification, and solubility. The partially unfolded/folded structure of proteins is more flexible and is, therefore, hypothesized to be able to form better protein networks on heating. Protein gels made from proteins isolated with the acid- and alkaline-aided processes from several species have shown to have equal and sometimes significantly better gelation properties than those produced using conventional surimi processing techniques.[Citation10–Citation13] Several observations indicate that acid treatment has a different effect on the structure of the muscle proteins compared to alkaline treatment, also in a species dependent manner.[Citation14,Citation15] Gels prepared from rockfish and Atlantic croaker from proteins solubilized at alkaline pH exhibited better gel quality than those prepared from the acid-aided process.[Citation16,Citation17] However, the impact of extreme acid and alkaline unfolding processes on bigeye snapper head protein isolate functionality is not well-documented. In the present study, the effect of pH-shifting treatments on protein functionalities including solubility, gel forming ability, emulsifying property, and foaming property from bigeye snapper head was investigated.
Material and methods
Chemicals
Bromophenol blue sodium salt (BPB) was purchased from Sigma (St. Louis, MO, USA). Trichloroacetic acid (TCA), sodium chloride (NaCl), lithium chloride, and potassium chloride were obtained from Merck (Darmstadt, Germany). Sodium hydroxide (NaOH) and hydrochloric acid (HCl) were obtained from Fluka (Buchs, Switzerland). All chemicals were of analytical grade.
Fish head sample
Bigeye snappers (P. tayenus) were caught from the Khanom coast along the gulf of Thailand and off-loaded approximately 24–36 h after capture. Fish were kept in ice, using the fish/ice ratio of 1:2 (w/w), and transported to the Department of Agro-Industry, Walailak University, Thasala, Nakhon Si Thammarat within 1 h. Upon arrival, the fish were washed with cold water (4°C) and beheaded. Fish head (10 kg) was ground by using a Talsa bowl chopper. Ground fish head was vacuum packed and kept at –18°C until used.
Protein recovery from ground bigeye snapper head by acid- and alkaline-aided processes
Recovery of proteins from ground bigeye snapper head by acid- and alkaline-aided processes was performed according to the method of Marmon and Undeland.[Citation18] Ground bigeye snapper head was thawed under running cold water. The ground bigeye snapper head was mixed with 9 volumes of ice-cold distilled water and homogenized for 30 s using an IKA Labortechnik homogenizer (Selangor, Malaysia). The pH of the homogenate was adjusted to either 11.5 (alkaline pH-shift method) or 2.5 (acid pH-shift method) using 2 M NaOH or 2 M HCl during constant manual stirring. The pH was monitored with a calibrated pH meter (Cyberscan 500, Singapore). The pH-adjusted homogenates were centrifuged at 8,000 × g in a RC-5B plus centrifuge (Sorvall, Norwalk, CT, USA) at 4°C for 20 min. The solubilized proteins in the supernatant were collected and separated from the pellet and the floating fat layer by filtering through three layers of cotton sheet. Thereafter, the pH of supernatant was adjusted to 5.5 by using 2 M HCl or 2 M NaOH. A second centrifugation was performed, and the pellet, referred to as protein isolate, was collected and weighed. The moisture content of both protein isolates was equally adjusted to 80% prior to further analyses. The protein yield of both processes was ~30%.
Determination of protein solubility
The solubility of protein obtained from different processes was measured according to the method of Choi and Park[Citation10] with slight modifications. The sample (1 g) was homogenized with 10 mL of distilled water, for 50 s in an ice bath. To prepare the total soluble protein solution, the homogenates (2 mL) were then mixed with 2 M sodium hydroxide (2 mL) and left stirred in a cold room (4°C) overnight. Then, another 2 mL of homogenates were solubilized in 2 mL of lithium chloride buffer (8.4% LiCl and 0.04 M Li2CO3, pH 7.2) and did the same manner as alkaline solubilization. All solutions were centrifuged at 12,000 × g for 20 min at 4°C, and the protein concentration of the supernatant was measured by the Biuret method.[Citation19] Protein solubility (%) was defined as the fraction of protein remaining soluble after centrifugation and calculated as follows:
Determination of protein surface hydrophobicity
Hydrophobicity of non-solubilized myofibrils was determined using BPB for electrophoresis according to the method of Chelh et al.[Citation20] To 1 mL of myofibril suspension prepared, according to the method described by Martinaud et al.,[Citation21] 200 μL of 1 mg/ml BPB (in distilled water) was added and mixed well. A control, without myofibrils, consisted of the addition of 200 μL of 1 mg/mL BPB (in distilled water) to 1 mL of 20 mM phosphate buffer at pH 6. Samples and control were kept under agitation, at room temperature, during 10 min and then centrifuged for 15 min at 2000 × g. The absorbance of the supernatant (diluted 1/10) was measured at 595 nm against a blank of phosphate buffer. The surface hydrophobicity was reported as the amount of BPB bound given by the formula:
where A = absorbance at 595 nm
Gel preparation
To prepare the gels, the frozen samples were thawed at 4°C for 12 h until the core temperature reached 0°C. The samples were then cut into small pieces and the moisture content was adjusted to 80%. The samples were chopped for 5 min in a walk-in cold room at 4°C with and without dry NaCl (2.5%, w/w) to obtain the homogeneous sol. The sol was then stuffed into polyvinylidine casing with a diameter of 2.5 cm and both ends of the casing were sealed tightly. The sol was then incubated at 40°C for 30 min, followed by heating at 90°C for 20 min.[Citation22] The gels were cooled in iced water and stored for 24 h at 4°C prior to analysis.
Measurement of gel properties
Texture analysis
Texture analysis of the gels was performed using a TA-XT2 texture analyzer (Stable Micro Systems, Godalming, Surrey, UK). Gels were equilibrated and evaluated at room temperature (28–30°C). Six cylinder-shaped samples with a length of 2.5 cm were prepared and subjected to determination. Breaking force (gel strength) and deformation (elasticity/deformability) were measured using the texture analyzer equipped with a spherical plunger (diameter 5 mm; depression speed 60 mm/min).
Determination of color and whiteness
Colorimetric values of the samples were obtained, in triplicate, by using a portable Hunterlab Miniscan/EX instrument (10° standard observers, illuminant D65, Hunter Assoc. Laboratory; VA, USA). The instrument was calibrated to a white and black standard. The tristimulus L* (lightness), a* (redness/greenness), and b* (yellowness/blueness) measurement mode was used as it relates to the human eye response to color. The whiteness was calculated as described by Chaijan et al.[Citation22] as follows:
Determination of expressible moisture
Expressible moisture of gels was measured according to the method of Ng.[Citation23] A gel sample with a thickness of 0.5 cm was weighed and placed between two pieces of Whatman filter paper No. 1 at the top and three pieces of the same filter paper at the bottom. The standard mass (5 kg) was placed on the top of the sample and maintained for 2 min. The sample was then removed and weighed again. Expressible moisture was calculated and expressed as percentage of sample weight.
Determination of gel microstructure
Microstructures of gels prepared by the different methods were determined using a scanning electron microscope (SEM; GeminiSEM, Carl Ziess Microscopy, Germany). Samples with a thickness of 2–3 mm were fixed with 2.5% (v/v) glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 2 h. The samples were then rinsed for 1 h in distilled water before being dehydrated in ethanol with serial concentrations of 50, 70, 80, 90, and 100% (v/v). Dried samples were mounted on a bronze stub and sputter-coated with gold. The specimens were observed with an SEM at an acceleration voltage of 10 kV.
Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Protein patterns of protein isolates and their gels prepared with NaCl were visualized by SDS-PAGE according to the method of Laemmli.[Citation24] To prepare the protein sample, 27 mL of 5% (w/v) SDS solution were added to the sample (3 g). The mixture was homogenized for 1 min. The homogenate was incubated at 85°C for 1 h to dissolve total proteins. The sample was centrifuged at 8500 × g for 5 min at room temperature (26–28°C) using a Biofuge primo centrifuge (Sorvall, Hanau, Germany). Protein concentration was determined according to the Biuret method,[Citation19] using bovine serum albumin as a standard. Solubilized samples were mixed at a 1:1 (v/v) ratio with the sample buffer (0.5 M Tris-HCl, pH 6.8, containing 4% SDS and 20% glycerol) in the presence or absence of 10% βME, representing reducing or non-reducing conditions, respectively. Samples (20 μg protein) were loaded onto polyacrylamide gels comprising a 10% running gel and a 4% stacking gel and subjected to electrophoresis at a constant current of 15 mA/gel using a Mini Protein II unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After electrophoresis, the gel was stained with 0.05% (w/v) Coomassie Blue R-250 in 15% (v/v) methanol and 5% (v/v) acetic acid and destained with 30% (v/v) methanol and 10% (v/v) acetic acid. A protein standard (Trial Mix™ Protein Markers, Merck Millipore, Darmstadt, Germany) was used to estimate the molecular weight of the proteins.
Determination of emulsifying properties
Emulsion activity index (EAI) and emulsion stability index (ESI) of protein isolates were determined according to the method of Pearce and Kinsella,[Citation25] with a slight modification. Soybean oil (2 mL) and protein solution (1% protein, 6 mL) were homogenized using an IKA Labortechnik homogenizer (Selangor, Malaysia) at a speed of 20,000 rpm for 1 min. Emulsions were pipetted out at 0 and 15 min and 300-fold diluted with 0.1% SDS. The mixture was mixed thoroughly for 10 s using a vortex mixer. Absorbance at 500 nm of the resulting dispersion was measured using a spectrophotometer (Shimadzu, Model UV-16001, Tokyo, Japan). Commercial whey protein isolate and egg albumen were used as reference materials in order to compare the EAI and ESI with pH-shifted protein isolates. EAI and ESI were calculated by the following formula:
where A: absorbance at 500 nm, DF: dilution factor, l: path length of cuvette (m), ø: oil volume fraction, and C: protein concentration in aqueous phase (g/m3).
where A0: absorbance at 500 nm, ΔA: A0-absorbance at 500 nm for 15 min and Δt: 15 min.
Determination of foaming properties
Foam expansion (FE) and foam stability (FS) of protein isolates were determined, as described by Shahidi et al.,[Citation26] with a slight modification. One percent protein concentration was transferred into 100 ml cylinders. The mixtures were homogenized for 1 min at 13,400 rpm for 1 min at room temperature. The sample was allowed to stand for 0, 30, and 60 min. Whey protein isolate and egg albumen were used as reference materials in order to compare the FE and FS with pH-shifted protein isolates. FE and FS were then calculated using the following equations:
where VT is total volume after whipping; V0 is the original volume before whipping and Vt is total volume after leaving at room temperature for different times (30 and 60 min).
Statistical analysis
Three different lots of protein isolates were produced from bigeye snapper head (n = 3). For each lot, all parameters studied except for texture analysis were determined in triplicate (n = 3). For texture analysis, six replications were conducted (n = 6). Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan’s multiple-range test to identify significant differences (p < 0.05) among treatments.[Citation27] For pairwise comparison, a t-test was used. Statistical analysis was performed using the Statistical Package for Social Science (SPSS 10.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Protein solubility and surface hydrophobicity
The salt-solubility of protein isolates obtained from acid aided- and alkaline aided- process is shown in . Good protein solubility is believed to be a prerequisite for many functional properties, including gelation and emulsification. Protein solubility in fish muscle has been used as a criterion for the alteration of proteins.[Citation28] Among protein isolates, the alkaline-aided process provided higher protein solubility than did the acid-aided process (p < 0.05). Differences in solubility of fish proteins subjected to acid and alkaline treatments have been reported. Zayas[Citation28] reported that protein solubility and yield of extract was greater at alkaline pH than at acid pH. However, Kristinsson and Hultin[Citation9] reported that the acid and alkaline unfolding of cod myosin had no impact on the solubility characteristics of myosin refolded at pH 7.5. This is likely due to the fact that the rod portion of the myosin was in a native configuration after acid and alkaline treatments.[Citation9] Lower protein solubility in acid-aided processes is probably caused by the acid induced denaturation of proteins. Protein may undergo denaturation with a greater degree in acid process when compared to alkaline counterpart. Decrease of protein solubility, as a result of protein denaturation, subsequently caused precipitation of the proteins.[Citation28] Kristinsson and Hultin[Citation29] concluded that the more improperly unfolded the protein, the lower was its solubility. This was in agreement with surface hydrophobicity of protein isolates reported by BPB-bound value which was greater in acid made-protein isolate (p < 0.05; ). The result showed that the higher the hydrophobicity of non-solubilized myofibrils the lower the salt-solubility of protein isolate was observed (). It can be postulated that acid condition may induce the unfolding of muscle proteins to a greater extent than alkaline counterpart.
Table 1. Salt-solubility and surface hydrophobicity reported as the amount of BPB bound of acid- and alkaline-made protein isolates from bigeye snapper head.
Gel forming ability
Breaking force and deformation of gels
Texture is a complex sensory attribute involving such parameters as hardness, springiness, cohesiveness, gumminess, chewiness, and resilience that cumulatively contribute to the mouthfeel/texture experienced by humans.[Citation30,Citation31] Szczesniak[Citation31] defined cohesiveness as “the strength of the internal bonds making up the body of the product” and springiness as “the rate at which a deformed material goes back to its undeformed condition after the deforming force is removed.” Protein isolate sols from bigeye snapper head made with and without NaCl underwent different changes upon heating and revealed major differences in the texture parameters (). No gels were formed but very soft protein aggregates were obtained in the absence of NaCl from both protein isolates. Thus, the textural properties including breaking force and deformation were not detectable in those aggregates. From the result, both acid- and alkaline-made protein isolates required NaCl to gel. Typically, salt (NaCl) addition is usually required to extract myofibrillar proteins to induce protein gelation via formation of disulfide bonds and hydrophobic interactions during heating of muscle protein gel based products. However, several researches demonstrated that pH-shifted protein isolates can be gelled without NaCl addition. This phenomenon was primarily due to the conformational changes in the proteins induced by the pH-shift process favor their gelation. In general, protein solubility and denaturation are the prerequisite for thermal gelation of food protein. In this study, however, proteins underwent the extreme denaturation, indicated by low protein solubility with high surface hydrophobicity (), which had an adverse effect on gelation (). Therefore, protein isolates from bigeye snapper head needed NaCl to improve their solubility and hence gelation. Sodium and chloride ions might interact with the opposite charged groups on surface of protein isolates to form a double layer and thus increase their affinity for water molecules. The greater protein water interaction resulted in a decreased breaking force and increased deformation. Several studies regarding the effect of NaCl on gelling properties of protein isolates obtained by pH shift process has been reported with varying results depending on fish species. For instance, alkaline-aided Atlantic menhaden gel added salt exhibited lower breaking force but higher deformation than that prepared without salt.[Citation32] Choi and Kim[Citation33] reported that the breaking force of alkaline-aided croaker and jack mackerel gels was significantly decreased while the deformation was constant with increasing NaCl content. In contrast, Kim and Park[Citation34] reported that salt addition to acid- and alkaline-extracted proteins from Alaska pollock decreased both breaking force and deformation. Results from this study indicated that salt positively affected gel formation of protein isolate from bigeye snapper head prepared using alkaline and acid solubilization technique.
Table 2. Textural and color properties of acid- and alkaline-made protein isolate gels from bigeye snapper head prepared with and without NaCl.
In the presence of NaCl, both protein isolates can form soft gels (low breaking force and deformation; ). The alkaline-made protein isolate gel showed superior breaking force and deformation to acid counterpart (p < 0.05). Gel strength and elasticity of alkaline-made protein isolate was considered to increase 2.8 and 1.8 folds in comparison with acid-made protein isolate (). The results suggested that fish protein was extremely denatured due to the pH-shift process, especially in acidic condition, leading to the poorer gelling characteristics. Choi and Park[Citation10] reported that acid processing resulted in low breaking force due to the activity of retained cathepsin L enzymes. Shikha et al.[Citation35] concluded that the neutralization of acidified protein did not recover the gel strength to the level before acidification. In addition, apparently acid- or alkaline-induced solubilization leads to substantial changes in the conformation and structure of fish proteins, leading to different properties.[Citation9] Chaijan et al.[Citation36] also reported that the sardine and mackerel surimi gels prepared by the conventional method showed greater breaking force and deformation than those protien isolate gels did from the alkaline solubilizing process. Kim et al.[Citation17] found higher breaking force in fish protein gel treated under alkaline conditions (pH 11) than that under acid conditions (pH 2). Yongsawatdigul and Park[Citation37] also reported that higher breaking force and deformation were obtained in rockfish muscle protein gels prepared by alkaline solubilization. Batista et al.[Citation38] reported the protein recovered after acid- or alkaline-aided processes showed poorer gelling properties than those of surimi and the acidic-recovered protein had the lowest gel strength. However, in the case of better gelation of pH shifted protein isolate compared to conventional surimi, Wright[Citation39] proposed that the pH-shift process disrupts the myofibrillar protein structure more completely than a conventional process then releases individual proteins into dispersion and thus increases the protein surface area for reactivity in gelation.
Color of gels
Proper color and texture are two critical quality attributes of restructured fish food products. From the results, some of the heme proteins and other dark pigment, e.g., melanin were probably extracted and removed from bigeye snapper head during pH shift processing. However, residual pigments were coprecipitated during pI precipitation and retained with the recovered protein isolates; therefore, contributing to high redness (a*) and yellowness (b*) with low lightness (L*) and whiteness of the gels (). Chomnawang and Yongsawatdigul[Citation7] reported that protein isolate gels from tilapia frame by-products showed a dark-brownish appearance regardless of extraction pH applied. It has been showed that addition of NaCl resulted in increase of gel whiteness due to the light scattering of water entrapped in the gel matrix. However, in this case, no significance difference was found in lightness (L*) among protein isolate gels made with and without NaCl (p > 0.05). Gel obtained from acid aided process was lighter and whiter than did alkaline made protein isolate gel (p < 0.05) indicating a greater removal or destruction of pigments such as myoglobin, hemoglobin and melanin after the acid-aided process. In our previous experiment, we found the lower total pigment content in acid-made protein isolate which was supposed to be due to the degradation of heme pigments induced by extreme acid condition rather than removal them from the isolate.[Citation40] It has been reported that at acidic pHs, the proton-catalyzed displacement process and a protoporphyrin IX ring destruction may be responsible for promoting heme loss which limited total pigment detection[Citation40] Additionally, the degradation of myoglobin under extreme acidic condition can be another factor causing the improved whiteness of the resulting protein isolate. Heme-globin dissociation upon acid treatment may result in the colorless heme and globin and thus increase the whiteness resulting in better gel color. Rawdkuen et al.[Citation41] found that acid-made protein isolate gel from tilapia had a whiter color than alkaline counterpart.
Expressible drip
The expressible moisture of gels made from acid- and alkaline aided processes was 40.63 and 33.64%, respectively (), which was quite high when compared to good grade surimi gel. Different expressible moisture suggested difference in the water holding capacity of the gel network. The result indicated that the protein network of both gels was poor in water-holding properties causing by the extreme denaturation of protein during pH-shift processing. Acid process could induce the protein unfolding to a greater extent than alkaline method resulting in a poorer gel network with lower strength and deformation of acid-made protein isolate. Therefore, gel matrices which could not imbibe water show high water releases. In general, the lower expressible moisture was coincidental with the increased breaking force.[Citation36] In addition, adjusting the pH to the pI for recovery the protein isolate can enhance the aggregation of proteins which can further alter the water-binding property of the protein gel. It can be noted that the expressible moisture was not measurable in the samples without NaCl addition due to their inability to retain the proper gel structure ().
Protein patterns
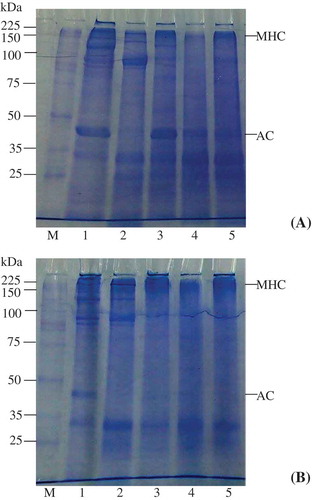
Bigeye snapper head composed of several proteins with different molecular weights as illustrated by SDS-PAGE under non-reducing () and reducing () conditions. It was not that myofibrillar proteins, particularly myosin heavy chain (MHC) and actin, were the major proteins in bigeye snapper head. With pH shift processing, both acid and alkaline treatments can recover most myofibrillar proteins but alkaline version recovered more MHC and actin than the acid process. Additionally, acid version seemed to cause some degradation of MHC and actin as shown by a decrease in MHC band intensity, a fading in actin band and the increase in protein bands with molecular weight lower than that of MHC or actin. Choi and Kim[Citation33] suggested that degradation of MHC in croaker and jack mackerel, under acidic conditions, was higher than that under alkaline conditions. This result was likely to contribute to a decrease in breaking force of the heat-induced gel from acidic processing (). After thermal gelation, a decrease in MHC band intensity with the appearance of crosslinked protein band with molecular weight higher than MHC was observed in gels from all treatments compared with fish head and the protein isolates suggesting the formation of polymerized proteins ( and ). Different protein patterns of both acid- and alkaline-made protein isolate gels under reducing () and non-reducing () conditions suggested that the networks of protein isolate gels were presumably stabilized by disulfide bonds. When comparing the MHC intensity of gels made by the acid and alkaline pH-shifting processes, it was found that more MHC was retained in sample prepared by the acid method, indicating the lower crosslinking ability of myosin in acid-made protein isolate. This result confirmed that alkaline-made protein isolate had a superior gel forming ability to the acid counterpart.
Figure 1. SDS-PAGE of bigeye snapper head pH-shifted protein isolates and their gels under (a) non-reducing and (b) reducing conditions. M: Standard molecular weight marker; 1: Original bigeye snapper head; 2: Acid-made protein isolate; 3: Alkaline-made protein isolate; 4: Gel of alkaline-made protein isolate; 5: Gel of acid-made protein isolate. MHC: myosin heavy chains; AC: actin.
Microstructure of gels
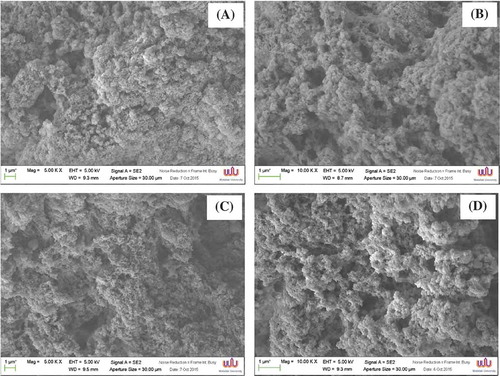
The microstructures of protein isolate gels, prepared using acid- and alkaline-aided processes, were observed by an SEM. SEM has been widely imaged in food microstructure as it gives a 3D view of the sample and makes the differences in structure easily understood. – demonstrated the differences in microstructure of acid-made and alkaline-made protein isolate gels with and without NaCl addition. Without NaCl addition, both acid-made and alkaline-made protein isolate formed aggregates with the dense surface and a disrupt structure indicating agglutination of protein instead of gelation ( and ). Addition of NaCl during gel preparation in either acid aided- or alkaline aided-protein presented a gel network with many sizable holes, cluster structure and strong frames formed by protein molecules through undergoing aggregation as gel properties ( and ). It was noted that slightly larger strands of network were observed in gel from both protein isolates. Aggregated protein induced by acid-alkaline solubilization more likely provided those strands in the gel network. Among the protein isolate, alkaline made protein isolate gel () showed a structure with aggregates of sparse packed spherical proteins, arranged in clusters and smother surface which also confirmed the higher values of breaking force and deformation (). This may be a causal factor in the change of textural properties, for example lower gel strength of both protein isolate gels with a relative high in expressible moisture (). The pH-shift processing with both versions recovered low concentration of myofibrillar proteins as visualized by SDS-PAGE () leading to poor gelling properties with disorganized network. In general, the microstructure of myofibrillar proteins gel had finer and longer strands with fibrous and ordered 3D networks.[Citation42]
Figure 2. Electron microscopic images of protein isolate gels/aggregates prepared by using different conditions (magnification: 10,000 × EHT: 10 kV). (a) Acid-made protein isolate without NaCl; (b) Acid-made protein isolate with NaCl; (c) Alkaline-made protein isolate without NaCl; (d) Alkaline-made protein isolate with NaCl.
Emulsifying properties
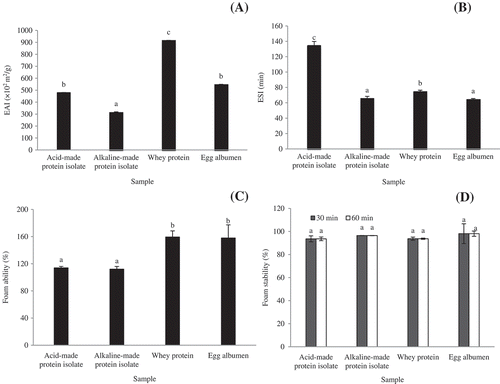
Proteins are surface active agents that can form and stabilize the emulsion by creating electrostatic repulsion on oil droplet surface.[Citation43] The ability of proteins to form emulsion is important owing to the interactions between proteins and lipids in the food systems. The principal role of emulsifying agents is to reduce the interfacial tension at the oil-water interface and to form an interfacial film to protect droplets against coalescence.[Citation44] Also, the ability of muscle proteins to emulsify fat in minced meat products is of much importance.[Citation45] In order to know how well of protein isolates can emulsify and stabilize oil in water emulsion, the EAI of both protein isolates compared to other well-established emulsifying proteins including whey protein concentrate and egg albumen was demonstrated (). The highest EAI was found in whey protein concentrate which was greater than acid- and alkaline- made protein isolates of 47.7 and 65.3%, respectively. Generally, small globular proteins like whey protein are excellent in emulsifying capacity because of its ability to expose the interior structure and subsequently move and adsorb at the interface. Interestingly, acid treated protein isolate had an emulsifying ability in comparable to egg albumen (p > 0.05). Acid induced partially unfolding of muscle protein was likely responsible for protein integration into oil droplets membrane. The result was in accordance with higher surface hydrophobicity of acid treated isolate (). This may also explain why the acid-made protein isolate showed superior emulsifying activity to an alkaline-made one. From the results, it was noted that greater surface hydrophobicity of muscle proteins had a positive effect on emulsification. The ability of a protein to rapidly lower free energy of a newly created interface is controlled by (1) how rapidly it can adsorb to the interface and (2) how rapidly and easily it can undergo conformational rearrangement and reorientation at the interface.[Citation15] The latter is the rate-controlling step, as it determines the rate at which a protein can decrease the interfacial tension between oil and water.[Citation46] Li-Chan et al.[Citation47] demonstrated that emulsifying properties of a preparation of crude salt soluble muscle proteins correlated well with their surface hydrophobicity. The relative increase in surface hydrophobicity and emulsification activity shows an almost perfect relationship in cod myosin subjected to pH-shift process suggesting that surface hydrophobicity can be a sensitive measure of the emulsification ability of myosin.[Citation15] Misfolding of protein as a result of acid treatment, which led to increased surface hydrophobicity as more hydrophobic clusters became exposed,[Citation9] is likely the cause for the increased ability of refolded protein to form and stabilize the oil-in-water emulsion. This hypothesis is supported by the fact that acid unfolded protein was more misfolded and hydrophobic and in turn had better emulsifying properties than the alkaline-unfolded protein. The mechanism by which this occurs is possibly first due to the more rapid adsorption of the relatively hydrophobic globular head of the pH-treated protein to the nonpolar lipid globules. The higher the hydrophobicity, the more energetically favorable would be the interaction of the protein with the nonpolar oil surface. The process of conformational changes at the interface is hypothesized to be through loss in tertiary structure rather than secondary structure,[Citation48] as the interfacial energy at the oil-water interface is probably insufficient to overcome the activation energy barrier for complete unfolding.[Citation49] It is also possible that the dissociation of the light chains of myosin, the major muscle protein, previously reported on acid and alkali pH treatment[Citation9] may have aided in emulsification, as they could have left exposed hydrophobic patches on the myosin head. Also, the increased reactivity of the thiol groups may have facilitated the formation of a stable protein network at the interface, thus aiding in the stabilization of the emulsion.[Citation9] It was not surprising that the acid made protein isolate had the highest emulsifying stability index (p < 0.05; ). The major proteins composing in both protein isolates are myofibrillar proteins mainly myosin (), in which they could form continuous thin films along the interface.
Figure 3. (a) Emulsion activity index (EAI), (b) emulsion stability index (ESI), (c) foam ability, and (d) foam stability of acid-and alkaline-made protein isolates and commercial whey protein isolate and egg albumen. Bars represent the standard deviation from triplicate determinations. Different letters indicate significant differences (p < 0.05).
Foaming properties
Foamability is an important functional property of proteins by which proteins form flexible cohesive film to entrap air bubbles. Proteins that rapidly unfold and adsorb at the freshly formed air/liquid interface during bubbling exhibit improved foamability.[Citation46] Foams are important in food formulations. FE is mainly related to the solubility of proteins, balance between flexibility and rigidity of proteins at the air water interface, hydrophobicity, pH, ionic strength, and temperature, etc.[Citation50] Egg albumen is the most frequently used standard for foaming comparisons among proteins because of its good foaming properties.[Citation51] No significant difference in foam ability between acid- and alkaline-made protein isolates was noticed (p > 0.05; ). Acid- and alkaline-made protein isolates had the capacity to form foam as 72 and 70% in comparison with egg albumen and whey protein, respectively, indicating an effective foaming agent of both isolates. It should be noted that pH adjustment during solubilization and precipitation may affect the foaming ability of protein isolates from bigeye snapper head. Grahams and Phillips[Citation52] linked good foamability with flexible protein molecules that can reduce surface tension, while highly ordered globular proteins, which are relatively difficult to surface-denature give low foamability. Hence, one may suggest that pH either acid or alkaline may induce the conformational changes of myofibillar proteins (major proteins in the protein isolates) which can easily expose their interior hydrophobic amino acid residues and then subsequently adsorb at air-water interface like flexible protein do. FS of both protein isolates was greater than 95% which was similar to the foaming standards, egg albumen, and whey protein (p > 0.05), indicating the excellent capacity to stablilize foam against collapse (). Generally, foam collapse takes place by any of these three mechanisms including (1) indisproportionation of bubbles; (2) coalescence of bubbles due to instability of thin film between them; and (3) drainage of water from the surface of the bubbles down to the liquid layer, thereby leading to the removal of protein from film around the bubble.[Citation53,Citation54] The excellent foam stabilizer should be enhanced greater protein-protein interaction, which increases viscosity and facilitates formation of multilayer cohesive protein film at the interface. The formation of a cohesive multilayer film offers resistance to disproportionation and coalescence of bubbles. In addition, it could lead to formation of thicker films, which limits the effect of drainage of protein from films. According to these results, the protein isolates from bigeye snapper head prepared by either acid or alkaline pH-shift method exhibited a high potential to form and stabilize foam which may be applied into foam-based food products.
Conclusion
This study demonstrated a feasibility to develop functional food products made of muscle protein isolate recovered with isoelectric solubilization/precipitation from bigeye snapper head. Protein isolates are the most refined form of protein products containing the greatest concentration of protein. Alkaline aided protein isolate exhibited better salt-solubility and gel forming ability in the presence of NaCl than acid aided protein isolate. On the other hand, acid made protein isolates displayed a good emulsifying and foaming properties with principally due to the proper surface hydrophobicity of protein. Therefore, the pH-shift processing can be used as a powerful tool to recover functional proteins from bigeye snapper head. Protein isolates may be used in the development of new class of formulated foods with enhanced nutritional properties. However, functionalities of the protein isolates were strongly influenced by solubilization method with related to the degree of molecular alteration.
Funding
The authors are grateful for the financial support from the office of the Higher Education Commission under HERP 2015 program.
Additional information
Funding
References
- Benjakul, S.; Visessanguan, W.; Riebroy, S.; Ishizaki, S.; Tanaka, M. Gel‐Forming Properties of Surimi Produced from Bigeye Snapper, Priacanthus Tayenus and P Macracanthus, Stored in Ice. Journal of the Science of Food and Agriculture 2002, 82, 1442–1451.
- Gildberg, A. Digestive Enzyme Activities in Starved Pre-Slaughter Farmed and Wild-Captured Atlantic Cod (Gadus Morhua). Aquaculture 2004, 238, 343–353.
- Valdimarson, G.; James, D. World Fisheries-Utilization of Catches. Ocean & Coastal Management 2001, 44, 619–633.
- Matak, K.E.; Tahergorabi, R.; Jaczynski, J. A Review: Protein Isolates Recovered by Isoelectric Solubilization/Precipitation Processing from Muscle Food By-Products as a Component of Nutraceutical Foods. Food Research International 2015, 77, 697–703.
- Chen, Y.C.; Jaczynski, J. Protein Recovery from Rainbow Trout (Oncorhynchus Mykiss) Processing By-Products Via Isoelectric Solubilization/Precipitation and Its Gelation Properties as Affected by Functional Additives. Journal of Agricultural and Food Chemistry 2007, 55, 9079–9088.
- Gehring, C.K.; Gigliotti, J.C.; Moritz, J.S.; Tou, J.C.; Jaczynski, J. Functional and Nutritional Characteristics of Proteins and Lipids Recovered by Isoelectric Processing of Fish By-Products and Low-value Fish—A Review. Food Chemistry 2011, 124, 422–431.
- Chomnawang, C.; Yongsawatdigul, J. Protein Recovery of Tilapia Frame by-Products by pH-Shift Method. Journal of Aquatic Food Product Technology 2013, 22, 112–120.
- Xiong, Y.L. Meat Processing. In Food Proteins, Processing Applications; Nakai, S., Modler, H.W., Eds.; Wiley-VCH: New York, NY, 2000; 89–145.
- Kristinsson, H.G.; Hultin, H.O. Changes in Conformation and Subunit Assembly of Cod Myosin at Low and High pH and After Subsequent Refolding. Journal of Agricultural and Food Chemistry 2003, 51, 7187–7196.
- Choi, Y.L.; Park, J.W. Acid-Aided Protein Recovery from Enzyme-Rich Pacific Whiting. Journal of Food Science 2002, 67, 2962–2967.
- Hultin, H.O.; Kelleher, S.D. Surimi Processing from Dark Muscle Fish. In Surimi and Surimi Seafood; Park, J.W., Ed.; Marcel Dekker: New York, NY, 2000; 59–77.
- Kristinsson, H.G.; Demir, N. Functional Fish Protein Ingredients from Fish Species of Warm and Temperate Waters: Comparison of Acid and Alkali-Aided Processing vs. Conventional Surimi Processing. In Advances in Seafood Byproducts; Alaska Sea Grant College Program: Fairbanks, AK, 2003.
- Undeland, I.; Kelleher, S.D.; Hultin, H.O.; McClements, J.; Thongraung, C. Consistency and Solubility Changes in Herring (Clupea Harengus) Light Muscle Homogenates as a Function of pH. Journal of Agricultural and Food Chemistry 2003, 51, 3992–3998.
- Davenport, M.P.; Kristinsson, H.G. In Low and High pH Treatments Induce a Molten Globular Structure in Myosin Which Improves Its Gelation Properties, abstract of the IFT Annual Meeting, Chicago, July 12–16, 2003.
- Kristinsson, H.G.; Hultin, H.O. Effect of Low and High pH Treatment on the Functional Properties of Cod Muscle Proteins. Journal of Agricultural and Food Chemistry 2003, 51, 5103–5110.
- Perez-Mateos, M.; Amato, P.M.; Lanier, T.C. Gelling Properties of Atlantic Croaker Surimi Processed by Acid or Alkaline Solubilization. Journal of Food Science 2004, 69, FCT328–FCT333
- Kim, Y.S.; Park, J.W.; Choi, Y.J. New Approaches for the Effective Recovery of Fish Proteins and Their Physiochemical Characteristics. Fisheries Science 2003, 69, 1231–1239.
- Marmom, S.K.; Undeland, I. Protein Isolation from Gutted Herring (Clupea Harengus) Using pH-Shift Processes. Journal of Agricultural and Food Chemistry 2010, 58, 10480–10486.
- Robinson, H.W.; Hodgen, C.G. The Biuret Reaction in the Determination of Serum Protein I. A Study of the Condition Necessary for the Production of the Stable Color Which Bears a Quantitative Relationship to the Protein Concentration. The Journal of Biological Chemistry 1940, 135, 707–725.
- Chelh, I.; Gatellier, P.; V. Santé-Lhoutellier, Technical Note: A Simplified Procedure for Myofibril Hydrophobicity Determination. Meat Science 2006, 74, 681–683.
- Martinaud, A.; Mercier, Y.; Marinova, P.; Tassy, C.; Gatellier, P.; Renerre, M. Comparison of Oxidative Processes on Myofibrillar Proteins from Beef During Maturation and by Different Model Oxidation Systems. Journal of Agricultural and Food Chemistry 1997, 45, 2481–2487.
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Characteristics and Gel Properties of Muscles from Sardine (Sardinella Gibbosa) and Mackerel (Rastrelliger Kanagurta) Caught in Thailand. Food Research International 2004, 37, 1021–1030.
- Ng, C.S. Measurement of Free and Expressible Drips. In Manual on Analytical Methods and Procedure for Fish and Fish Products Laboratory; Hasegawa, H., Ed.; Southeast Asian Fisheries Development Center: Singapore, 1987.
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage. Nature 1970, 227, 680–685.
- Pearce, K.N.; Kinsella, J.E. Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique. Journal of Agricultural and Food Chemistry 1978, 26, 716–723.
- Shahidi, F.; Xiao-Qing, H.; J. Synowiecki, Production and Characteristics of Protein Hydrolysates from Capelin (Mallotus Villosus). Food Chemistry 1995, 53, 285–293.
- Steel, R.G.D.; Torrie, J.H. Principle and Procedure of Statistics; MacGraw-Hill: New York, NY, 1980; 633.
- Zayas, J.F. Functionality of Proteins in Food; Spring-Verlag: Berlin, Germany, 1997; 373.
- Kristinsson, H.G.; Hultin, H.O. Changes in Trout Hemoglobin Conformations and Solubility after Exposure to Acid and Alkali pH. Journal of Agricultural and Food Chemistry 2004, 52, 3633–3643.
- Haard, N.F. Control of Chemical Composition and Food Quality Attributes of Cultured Fish. Food Research International 1992, 25, 289–307.
- Szczesniak, A.S. Texture is a Sensory Property. Food Quality and Preference 2002, 13, 215–225.
- Pérez-Mateos, M.; Lanier, T.C. Comparison of Atlantic Menhaden Gels from Surimi Processed by Acid or Alkaline Solubilization. Food Chemistry 2007, 101, 1223–1229.
- Choi, J.Y.; Kim, J-S. Fish Protein Recovered Using pH Shifting Method and Its Physicochemical Properties. Journal of Ocean University of China 2005, 4, 224–228.
- Kim, Y.S.; Park, J.W. Negative Role of Salt in Gelation Properties of Fish Protein Isolate. Journal of Food Science 2008, 73, C585–C588.
- Shikha, F.H.; Hossain, M.I.; Morioka, K.; Kubota, S.; Itoh, Y. Effect of pH Shifting on the Gel Forming Characteristics of Salt-Ground Meat from Walleye Pollack. Fisheries Science 2006, 72, 870–876.
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Physicochemical Properties, Gel-forming Ability and Myoglobin Content of Sardine (Sardinella Gibbosa) and Mackerel (Rastrelliger Kanagurta) Surimi Produced by Conventional Method and Alkaline Solubilization Process. European Food Research and Technology 2006, 222, 58–63.
- Yongsawatdigul, J.; Park, J.W. Effects of Alkali and Acid Solubilization on Gelation Characteristics of Rockfish Muscle Proteins. Journal of Food Science 2004, 69, 499–505.
- Batista, I.; Pires, C.; Nelhas, R. Extraction of Sardine Proteins by Acidic and Alkaline Solubilization. Food Science and Technology International 2007, 13, 189–194.
- Wright, BJ. Effect of ultrastructural disruption and protein dispersion on gel forming in myofibrillar gels. DPhil dissertation, North Carolina State University, Raleigh, NC, November 6, 2007; 256.
- Chaijan, M.; Undeland, I. Development of a New Method for Determination of Total Heme Protein in Fish Muscle. Food Chemistry 2015, 173, 1133–1141.
- Rawdkuen, S.; Sai-Ut, S.; Khamsorn, S.; Chaijan, M.; S. Benjakul, Biochemical and Gelling Properties of Tilapia Surimi and Protein Recovered Using an Acid-Alkaline Process. Food Chemistry 2009, 112, 112–119.
- Tachasirinukun, P.; Chaijan, M.; Riebroy, S. Effect of Setting Conditions on Proteolysis and Gelling Properties of Spotted Featherback (Chitala Ornata) Muscle. LWT Food Science and Technology 2016, 66, 318–323.
- Makri, E.; Papalamprou, E.; Doxastakis, G. Study of Functional Properties of Seed Storage Proteins from Indigenous European Legume Crops (Lupin, Peas, Broad Bean) in Admixture Within Polysaccharides. Food Hydrocolloids 2005, 19, 583–594.
- Phillips, L.G.; Whitehead, D.M.; Kinsella, J. Structure-Function Properties of Food Proteins; Academic Press: San Diego, CA, 1994; 131–178.
- Xiong, Y.L. Structure-Function Relationships of Muscle Proteins. In Food Proteins and Their Applications; Damodaran, S., Paraf, A., Eds.; Marcel Dekker: New York, NY, 1997; 341–392.
- Damodaran, S. Protein-Stabilized Foams and Emulsion. In Food Proteins and Their Applications; Damodaran, S., Paraf, A., Eds.; Marcel Dekker: New York, NY, 1997; 25–56.
- Li-Chan, E.; Nakai, S.; Wood, D.F. Hydrophobicity and Solubility of Meat Proteins and Their Relationship to Emulsifying Properties. Journal of Food Science 1984, 49, 345–350.
- Zhu, H.; Damodaran, S. Heat-Induced Conformational Changes in Whey Protein Isolate and Its Relation to Foaming Properties. Journal of Agricultural and Food Chemistry 1994, 42, 846–848.
- Kristinsson, H.G.; Rasco, B.A. Biochemical and Functional Properties of Atlantic Salmon (Salmo Salar) Muscle Proteins Hydrolyzed with Various Alkaline Proteases. Journal of Agricultural and Food Chemistry 2000, 48, 657–666.
- Jiang, L.; Wang, Z.; Li, Y.; Meng, X.; Sui, X.; Qi, B.; Zhou, L. Relationship Between Surface Hydrophobicity and Structure of Soy Protein Isolate Subjected to Different Ionic Strength. International Journal of Food Properties 2015, 18, 1059–1074.
- Symers, K.C. The Relationship Between the Covalent Structure of the Xanthomonas Polysaccharide (Xanthan) and Its Function as a Thickening, Suspending and Gelling Agent. Food Chemistry 1980, 6, 63–76.
- Graham D.E.; Phillips M.C. The Conformation of Proteins at the Air-Water Interface and Their Role in Stabilizing of Foam. In Foams; Akers, R.J., Ed.; Academic Press: London, 1976; 237–253.
- Adebowale, K.O.; Lawal, O.S. Foaming, Gelation and Electrophoretic Characteristics of Mucuna Bean (Mucuna Pruriens) Protein Concentrates. Food Chemistry 2003, 83, 237–246.
- He, Z.; Li, W.; Guo, F.; Li, W.; Zeng, M.; Chen, J. Foaming Characteristics of Commercial Soy Protein Isolate as Influenced by Heat-Induced Aggregation. International Journal of Food Properties 2015, 18, 1817–1828.
