ABSTRACT
Chinese jasmine tea, a type of flower-scented tea, is produced by repeatedly mixing the base tea with the aromatic flowers of Jasminum sambac. The aim of this study was to analyze the changes in the volatiles, chemical components, and antioxidant activities of Chinese jasmine tea during six rounds of the scenting processes. The results indicated that benzyl alcohol, linalool, benzyl acetate, (Z)-3-hexenyl benzoate, methyl anthranilate, indole, and α-farnesene were seven major volatile compounds of jasmine tea. Moreover, the total amount of the volatile compounds increased gradually with increasing scenting rounds. The absorption of linalool became saturated quickly, while those of the other six major volatile compounds exhibited nearly linear increases throughout all six repeated scenting rounds. Importantly, the value of the jasmine tea flavor index, an evaluating indicator of the aroma quality, gradually increased with the progression of the repeated scenting rounds. The change of each detected taste component was less than 15% during six rounds of the scenting process. The antioxidant activities of the tea samples decreased in the first two rounds and later increased in the succeeding four rounds of the scenting process. However, the antioxidant activity of the finished tea was lower than that of the base tea, being significantly correlated with the change of catechin concentration. The findings provided insight into the changes in the volatiles, chemical components, and antioxidant activity of Chinese jasmine green tea during the repetitious scenting process, which could provide beneficial insight on improving the quality grade of the tea.
Introduction
Chinese jasmine tea has been enjoyed for several centuries in China because of its unique aroma and sedative effects on autonomic nerves and mood states.[Citation1,Citation2] The flower-scented tea is produced by repeatedly mixing the base tea with the aromatic flowers of Jasminum sambac, transferring the fragrant compounds from the fresh flowers into the base tea. Each round of the jasmine tea scenting processes includes eight steps, namely, preprocessing the base tea, maintaining the fresh flowers, layering the flowers with the base tea, spreading out the mixture for heat dissipation, re-heaping up and scenting, separating the flowers from the tea, heating, and cooling. In order to increase the intensity of the jasmine fragrance, the resultant tea from the last scenting round is mixed with the proper amount of the fresh jasmine flowers to obtain the finished tea. This specific step is named Ti-hua.
There are many quality grades of jasmine tea, which are determined by the quality of the base tea, environmental temperature, moisture content, ratio of the jasmine flowers to the base tea, and the repeated rounds of the scenting processes.[Citation3] Generally, the more rounds of the scenting process the tea undergo, the higher the quality grade. However, the cost increases markedly with an increase in repeated rounds of the scenting process because more fresh jasmine flowers are required and a longer scenting time is needed because the absorption efficiency of the base tea decreases gradually during the scenting processes. For cost reduction, large batches of the jasmine tea are produced with two to four rounds of the scenting process, usually yielding a low quality grade tea. To obtain scented tea with a high quality grade, six to seven rounds of the scenting processes have to be repeated. This indicates that the number of scenting rounds has a significant impact on the quality of the jasmine tea. Thus, it is of significance to carefully study the effects of repeated scenting rounds on the quality grade of jasmine tea. However, the changes in flavor substances were researched in jasmine tea produced with no more than four scenting rounds, and no research on the corresponding changes in jasmine tea with higher numbers of scenting rounds has been reported so far.
The aroma of jasmine tea is one of the most important factors determining its quality. Therefore, many researches of jasmine tea focused on its aromatic volatile compounds to understand the aroma characteristics. It is believed that the aroma of jasmine tea is absorbed from the fragrant components of the fresh J. sambac flowers by the base tea, but rarely from the base tea itself.[Citation4] Some researchers have evaluated the potential correlation between the volatile compounds and the quality grade of the flower-scented tea. Yamanishi described the correlation between the quality grade of jasmine tea and the ratio of the total concentration of benzyl alcohol, benzyl acetate, (Z)-3-hexenyl benzoate, methyl benzoate, and methyl anthranilate to that of linalool.[Citation5] Lin et al. proposed the “jasmine tea flavor” (JTF) index, defined as the ratio of the peak area percentage of α-farnesene, (Z)-3-hexenyl benzoate, methyl anthranilate and indole to that of linalool, to be used for quality evaluation of jasmine tea.[Citation6] In all cases, the aroma quality of jasmine tea increases as the value of the index or ratio increases.
The chemical components containing polyphenols, amino acids, methylxanthines, and soluble sugars also influence the quality of the tea.[Citation7] Flavanols and flavonols are the important active polyphenols in green tea and tea catechins may account for over 10% of the dry tea weight. Caffeine, theophylline, and theobromine constitute the main tea alkaloids. The chemical structures of tea catechins and methylxanthines are shown in . Previous studies show that these chemical components are changed during the scenting processes.[Citation8] However, little is known about the effects of repeated scenting rounds on the chemical components in jasmine tea. While jasmine tea, a flower-scented tea, is one of the most popular beverages consumed in China, to date there are only about ten references in the literature concerning the beneficial effects of drinking jasmine tea.[Citation1,Citation2,Citation9–Citation16] Additionally, the difference in bioactivity between the base tea extract and the finished jasmine tea extract is still not clear.
Thus, the objective of this study is to investigate the changes of the volatile compounds, chemical components and antioxidant activities of jasmine tea during six rounds of the repeated scenting processes, and to study the differences in jasmine tea made by the traditional and modern scenting techniques. In order to reach this goal, a research plan was developed to: (1) investigate the changes of the volatile compounds in Chinese jasmine tea; (2) determine the level of the total polyphenols, catechins, gallic acid (GA), and methylxanthines; and (3) examine the antioxidant activity of jasmine tea in vitro using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay.
Materials and methods
Chemicals and materials
GA, caffeine, theobromine, theophylline, (+)-catechin (C), (–)-epicatechin (EC), (–)-gallocatechin (GC), (–)-epigallocatechin (EGC), (–)-epicatechin gallate (ECG), (–)-epigallocatechin gallate (EGCG) and (–)-gallocatechin gallate (GCG) were purchased from Sigma (St. Louis, MO). DPPH was obtained from Aldrich (Milwaukee, WI, USA). Acetonitrile, methanol, and formic acid were high-performance liquid chromatography (HPLC)-grade and obtained from Merck (Darmstadt, Germany). Deionized water was prepared using a Millipore Milli Q-Plus system (Millipore, Billerica, MA, USA).
Fresh flowers of the double-petalled jasmine planted in Fuzhou, Fujian, China, were harvested and used for scenting. The jasmine tea, Yin Hao Jasmine, was produced using both the traditional scenting technique (named as tea A) and the modern scenting technique (named as tea B) in Fujian Chun Lun Tea Ltd. (Fuzhou, China) in September 2012. For the modern scenting technique, the steps of separating the jasmine flowers from the resultant tea and the heating were performed in a machine. For the traditional scenting technique, the heating step was performed in a charcoal baking cage. The scenting processes were both repeated with six rounds for the two scenting techniques. At the end of each round of the scenting process, the jasmine flowers were removed from the resulting tea. The tea then underwent the drying step and 200 g tea was collected for each sample. The tea samples made using the traditional scenting technique were designated as A1R to A6R for each sample from the first through sixth round, respectively; the corresponding tea samples made by the modern scenting technique were designated as B1R to B6R, respectively. The final scented teas made using the traditional and modern scenting techniques were designated as finished tea A and B, respectively. All of the tea samples were stored in aluminum foil bags at 4°C.
Determination of the volatile compounds using gas chromatography-mass spectrometry (GC-MS) combined with solid-phase microextraction (SPME)
SPME fiber coated with 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS; Supelco, USA) was preconditioned at 270°C in the GC injector and used in this work. One gram of jasmine tea (the base tea or the resulting tea from each scenting round) or three fresh jasmine flowers were placed into a 20 mL glass bottle with the SPME fiber and incubated in a water bath at 60°C (for tea) or 30°C (for flowers) for 5 min. The SPEM fiber was then inserted into the headspace of the bottle for extraction for 20 min.
After extraction, the SPEM fiber was took out and inserted into the pre-heated injector port of the GC for desorption at 250°C. The desorbed volatile constituents were separated and analyzed using a 7890A GC equipped with an HP-5MS capillary column and coupled with a 5975C mass-selective detector (Agilent Technologies, USA). The oven temperature was: (1) held at 50°C for 2 min; (2) increased from 50 to 120°C at a rate of 5°C/min and held at 120°C for 15 min; (3) increased by 5°C/min to 180°C and held at 180°C for 2 min; and (4) increased to 280°C at a rate of 30°C and maintained at 280°C for 2 min. Electron impact (EI) spectra were recorded, the temperature of the ion source was 230°C and mass spectra were full-scanned in an m/z range from 50 to 550 mass units. All experiments were performed in triplicate.
Determination of n-alkane mixture (C8~C40, DRH-008S-R2; American Accu Standard Inc) was performed under the same GC conditions, and the temperature-programmed retention indices were calculated according to the method described by van Den Dool and Kratz.[Citation17] The components were identified by comparing their mass spectra with NIST05 mass spectral database and their calculated linear retention indices with the published data. The percentage of the aroma compounds was calculated by peak-area normalization.
In our preliminary studies, we used ethyl decylate as an internal standard for quantitative determination of the volatiles, but poor repeatability and stability of its peak area were observed. The same results were also reported by Wang et al.[Citation18] and Lv et al.[Citation19] who used ethyl heptanoate and ethyl decylate, respectively, as internal standards in the SPME–GC analysis. Therefore, the internal standard method was not used in this study.
Determination of total polyphenols, GA, catechins, and methylxanthines in the tea samples
The total polyphenolic content of each sample was determined according to the Folin-Ciocalteu method described by Anesini et al.[Citation20] which was calculated according to the standard curve of GA solution and defined as: milligram of GA equivalents/gram of tea sample (mg GAE/g). All experiments were performed in triplicate.
The contents of GA, theobromine, theophylline, caffeine, C, EC, EGC, ECG, GC, EGCG, and GCG were determined by HPLC. HPLC analysis was performed on an Agilent series 1100 (Agilent Technologies) liquid chromatograph, equipped with a vacuum, a quaternary pump, a G1314A variable wavelength detector, a hand-sampler and a column oven. A Welch Ultimate XB-C18 column (250 mm × 4.6 mm i.d., 5 μm) protected by a precolumn was used in this study. The separation was achieved by a gradient elution of 0.1% formic acid solution (solvent A) and acetonitrile (solvent B) at a flow rate of 1 mL/min: 0–20 min, 5–17% B; 20–40 min, 17–17% B. The equilibration time was 20 min. The injection volume was 20 μL, and the detection wavelength was set at 278 nm. The column was re-equilibrated with the initial conditions for 15 min before the next run.
DPPH free radical-scavenging activity of the tea extracts
The antioxidant capacity was determined by the DPPH radical scavenging assay according to the method described by Gaulejac et al., with minor changes.[Citation21] Briefly, 0.1 mL of tea extract solution at various concentrations was mixed with 3.0 mL of 6×10−Citation5 mol/L methanolic solution of DPPH and incubated at room temperature in the dark for 30 min. The absorbance of each sample at 517 nm was measured using a ultraviolet-visible (UV-Vis) spectrophotometer. Pure methanol was used both as a control and for the baseline correction. The radical scavenging activity was defined using the following equation: DPPH radical scavenging activity (%) = 100 × (Acontrol − Asample) /Acontrol, where Asample and Acontrol are the absorbance at 517 nm of the sample and the methanol control, respectively. The values of antioxidant capacity were expressed as Trolox Equivalent. All experiments were repeated three times.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) with the statistical software SPSS 19.0. Significant differences were determined by Duncan’s multiple range tests (p < 0.05). Correlations (r) was evaluated using Pearson’s correlation analysis.
Results and discussion
Volatile compounds
Jasmine tea is a type of flower-scented tea which is made by repeatedly mixing basic tea with fresh J. sambac flowers. The aroma characteristics of jasmine tea are formed mainly during the scenting processes, which have been proven by some previous researches.[Citation4,Citation6] In this study, the volatile compounds in the base tea, the fresh jasmine flowers, and the finished jasmine tea were characterized using the HS-SPME-GC-MS. As summarized in and Table S1, 64 volatile compounds were identified in the base tea, the finished jasmine tea, and the fresh jasmine flowers, representing 92.44–96.13% of total volatile substances. It is noteworthy that seven compounds (.tau.-cadinol, methyl nonanoate, hexyl benzoate, cadina-1,3,5-triene, cadalene, tridecane, and butylated hydroxytoluene) were identified for the first time as the flavor compounds of jasmine tea (marked by “*” in ). The results showed that benzyl alcohol, linalool, benzyl acetate, (Z)-3-hexenyl benzoate, methyl anthranilate, indole, and α-farnesene were the major aroma components in both the finished jasmine tea A and B. Furthermore, the aroma compounds identified in the finished jasmine tea were in agreement with those found in the fresh jasmine flowers (), indicating that these aroma compounds were absorbed by the base tea from the jasmine flowers.
Table 1. Volatile compounds identified in the aroma concentrate of basic tea, finished jasmine tea and fresh flower of J. sambac.
As shown in , 37 compounds were found to exist in both the finished jasmine tea and the fresh flowers of J. sambac. Except benzyl alcohol, copaene, caryophyllene, α-caryophyllene, δ-cadinene and cadina-1,3,5-triene, the other 31 compounds are not found to exist naturally in the basic green tea, suggesting that they were absorbed from the jasmine flowers. It is inferred that the absorption of aromatic compounds into the tea-leaves was achieved through both physical absorption and chemical absorption.[Citation22,Citation23] The moisture is thought to play an important role during the scenting process of jasmine tea. At the end of each scenting round, the moisture must be removed from the resulting tea through the heating step to prevent the tea from spoiling. It is worth mentioning that the cis-3-hexenyl acetate concentration was approximately 1% in the resulting jasmine tea, while it could reach up to 11.79% in the fresh jasmine flowers. This could possibly be attributed to a loss of the aromatic components during the heating step.
The major compounds, such as linalool (17.89–19.59%), benzyl acetate (24.17–24.99%), benzyl alcohol (9.17–13.07%), methyl anthranilate (5.55–5.78%), and indole (5.18–6.03%), detected in the present study have previously been reported as the odor-active compounds of jasmine tea.[Citation4,Citation24] Among them, linalool endows many aromatic plants with a flowery and fruity odor and methyl anthranilate endows the jasmine flower J. sambac with a sweet grape-like odor.[Citation25] In addition to these two, some minor and trace compounds can also contribute to the flavor of jasmine tea because of their unique odors.[Citation4] For example, methyl salicylate can confer the black tea with a unique minty, sweet, wintergreen-like odor;[Citation18,Citation26] methyl benzoate has a pleasant fruity odor and is the most abundant scenting compound in the majority of snapdragon varieties.[Citation27]
Changes of the volatile compounds during the scenting process
In the production of jasmine tea, the scenting processes are generally repeated for as many as six or seven rounds to ensure a strong fragrance in the high-quality scented tea. Thus, not only a large number of fresh jasmine flowers but also longer scenting times are used, inevitably increasing its production cost. Unfortunately, there is not yet enough scientific data to support the necessity of the repeated scenting rounds to ensure a high quality of finished tea. In order to examine the effects of repeated rounds of the scenting process on the aroma quality of jasmine tea, changes in the aroma volatiles during the six scenting rounds were determined systematically.
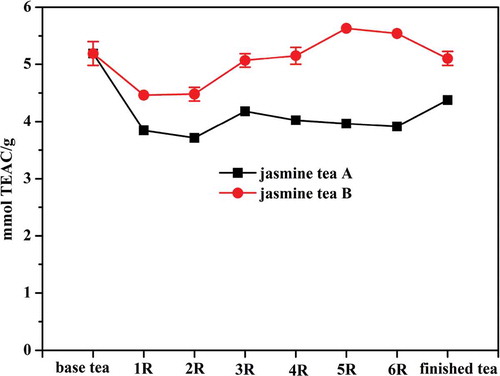
As shown in , the total peak area of the detected tea volatiles exhibited a positive correlation with the number of repeated scenting rounds, which increased separately by 194.7% for the tea A sample and 161.8% for the tea B sample from the first round (1R) to the sixth round (6R). Moreover, it was observed that the volatile concentration of the resulting tea increased faster in the first three scenting rounds than in the last three scenting rounds. Changes in the seven major volatile compounds in both tea A and tea B were monitored throughout the six scenting rounds ( and ). The peak areas of benzyl alcohol, linalool, benzyl acetate, indole, methyl anthranilate, α-farnesene and cis-3-hexenyl benzoate in the tea A and B were increased separately by 304.2 and 189.3%, 71.8 and 73.7%, 175.4 and 265.2%, 884.4 and 210.7%, 1121.7 and 144.3%, 564.1 and 235.7%, and 1007 and 193.8%, respectively, from 1R to 6R. The content of linalool was significantly increased from 1R to 3R, and then remained almost unchanged during the last three scenting rounds, indicating that the absorbance of linalool became quickly saturated. The concentrations of the other six major volatiles, benzyl alcohol, benzyl acetate, indole, methyl anthranilate, α-farnesene, and cis-3-hexenyl benzoate, exhibited nearly linear increases throughout all six scenting rounds.
Figure 2. (a) Changes of total peak area of jasmine tea samples during scenting process; (b) Changes of peak area of the seven main volatile compounds in tea A during scenting process; (c) Changes of peak area of the seven main volatile compounds in tea B during scenting process; (d) JTF index of jasmine tea samples at each round of scenting process. Values were expressed as mean value (n = 3).
In this study, the JTF index proposed by Lin et al. was chosen to describe the correlation between the quality grade of jasmine tea and the flavor components.[Citation6] The JTF index values of the finished tea A and B were 1.63 and 1.32, respectively, indicating that the two jasmine tea samples were well-scented due to a JTF index >1. The JTF index values of the finished tea A and B were increased by 391 and 74% from 1R to 6R, respectively. Furthermore, the JTF index values increased gradually with the added rounds of the scenting processes (). Lin et al. previously reported that the JTF index value increased with the quality grade of jasmine tea.[Citation6] Therefore, our results indicated that the more rounds of the scenting processes that were repeated, the better the quality of the jasmine tea, which is consistent with the practical experience of the traditional jasmine tea scenting process.
Changes of the chemical components during the scenting processes
Methylxanthines and polyphenolic compounds have been considered as the two main important biochemical components in green tea that are beneficial to human health.[Citation28–Citation31] During the scenting processes, the increased moisture and temperature inevitably cause changes in the biochemical components in the base tea,[Citation8] further influencing the bioactivity of tea. Therefore, it is necessary to analyze the changes in the biochemical components during the scenting processes.
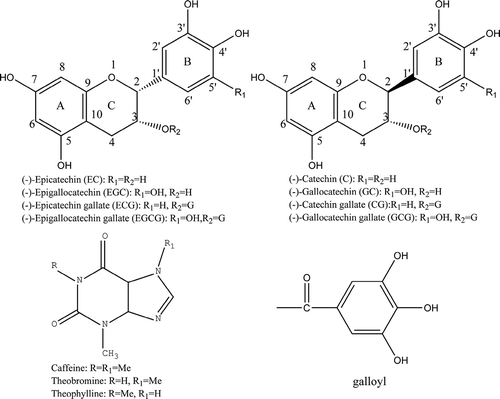
The HPLC results showed that the content of theophylline was lower than its limit of quantification. As shown in and , the total content of the detected catechins was higher in the finished tea B than the finished tea A with a concentration of 96.67 mg/g versus 88.65 mg/g, respectively, while the contents of both GA and theobromine are almost the same in both finished tea A and B. No significant regularity was observed in the changes of the total soluble phenolic components during the scenting process. The finished tea A seems to have a lighter bitter and astringency flavor than the finished tea B due to the lower levels of polyphenols and caffeine.[Citation32]
Table 2. Concentrations of gallic acid (mg/g DW), theobromine (mg/g DW), caffeine (mg/g DW), total soluble phenolics (TPS, mg gallic acid/g DW), and total catechins (TC, mg/g DW) during scenting process of jasmine tea A samples.
Table 3. Concentrations of gallic acid (mg/g DW), theobromine (mg/g DW), caffeine (mg/g DW), total soluble phenolics (TPS, mg gallic acid/g DW), and total catechins (TC, mg/g DW) during scenting process of jasmine tea B samples.
As shown in and , the contents of both theobromine and caffeine showed no significant difference (p > 0.05) in both tea A and B samples during six rounds of the scenting process, whereas the GA concentrations of the tea A and B increased by 1.16- and 1.13-fold, respectively, from the base tea to the finished tea, owing to its liberation from catechin gallates.[Citation28]
As shown in , the most predominant catechin was EGCG, following by EGC and ECG. Moreover, the three major catechins accounted for approximately 80% of the total catechins. The results showed that the contents of the total catechins EGCG, EC, GCG, and EGC decreased separately by 2.3, 2.4, 4.4, 2.0, and 13.0%, while those of ECG, C, and GC increased by 7.8, 3.1, and 7.4%, respectively, in the finished tea A compared with the base tea. During the scenting process of the tea A, the contents of EGCG, ECG, and GCG gradually decreased from the base tea to the fourth round (4R) of the scenting process and then increased during the last two scenting rounds. On the contrary, those of EGC, EC, C, and GC were increased to a certain degree from the base tea to the A4R and then decreased during the last two scenting rounds (). El-Hady and Albishri reported that some catechins might undergo isomerization at the C-2 position of flavan-3-ol during the tea making process (), for examples, EGCG, ECG, EGC, and EC could be converted into GCG, CG, GC, and C, respectively.[Citation33] However, no increase in the GCG content was observed with the reduction of EGCG, likely due to the transfer of EGCG into the EGCG dimers.[Citation34] showed the changes of the catechins during the scenting processes of the jasmine tea B samples. The results showed that the concentrations of all the detected catechins except EGC increased from the base tea to the finished tea B. Our data indicated that the content change of each detected component in the tea extracts was less than 15% from the base tea to the finished tea.
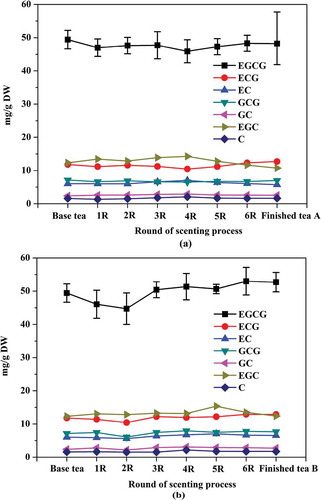
Changes of the antioxidant activity during the scenting processes
Antioxidant activity of jasmine tea is closely related to the base tea used. However, the water-soluble chemical components might be changed during the scenting process of jasmine tea, potentially affecting its health benefits. Therefore, the DPPH assay was used to examine the effects of the repeated scenting rounds on the antioxidant capacity of jasmine tea. The results showed that the antioxidant capacities of tea A and tea B decreased by 15.7 and 1.7%, respectively, from the base tea to the finished tea. As shown in , the antioxidant activities of the tea A1R and B1R extracts decreased respectively by 25.9 and 14.0% compared with that of the base tea. It is noteworthy that the tea A2R and B2R extracts exhibited the weakest inhibition activity of oxidation. On the whole, the antioxidant capacity of the tea samples initially decreased during the first two scenting rounds and then increased from the third scenting round. During the six rounds of the scenting processes, the antioxidant capacity of the tea A was significantly correlated with the changes of the total catechins EGCG and GCG (r = 0.64, 0.68, and 0.69, respectively), while that of the tea B was markedly correlated with the changes of the total catechins, EGCG, ECG and EC (r = 0.87, 0.83, 0.76, and 0.86, respectively). The results demonstrated that the antioxidant capacities of the finished tea A and B were different, likely owing to the differences in the catechin concentrations.
Conclusions
To the best of our knowledge, this is the first report to reveal the changes in volatile compounds, chemical components, and antioxidant activities of jasmine tea during a higher number of repeated rounds of the scenting process. After six scenting rounds, the concentrations of the major volatile compounds absorbed from fresh J. sambac flowers were increased dozens of times in the finished tea compared with the base tea, and the chemical components also underwent changes (less than 15%). Meanwhile, the antioxidant capacities of the tea samples appeared to decline during the first two scenting rounds and then recovered from the third scenting round, which notably correlated with the change in catechin concentration. In general, increased repeated scenting rounds obtained a better aroma quality in the finished jasmine tea and recovery of the antioxidant capacity. Although the aroma quality grade of the finished tea produced by the modern-scented technique is slightly lower than the one made by the traditional-scented technique, the modern-scented technique exhibits higher production efficiency and can endow the finished tea with a much stronger antioxidant activity compared with the traditional-scented technique. Therefore, the modern-scented technique to produce jasmine tea is worth a wide popularization and application in practical industrial production.
Our results indicated that the aroma quality of the jasmine teas was greatly enhanced with an increase in scenting rounds, and their antioxidant capacity was not significantly lost after increasing repeated scenting rounds. Therefore, we strongly recommend the necessity of using more scenting rounds to ensure a high quality of the finished tea in the production practice of jasmine tea. Our work could provide fundamental and practical information for improvement of the quality grade of Chinese jasmine tea.
Supplementary files
Download MS Word (125 KB)Funding
The authors are grateful for financial support from the Special Fund of the Fujian key science and technology special projects—key agricultural science and technology special project (No.2015NZ0003) and Fuzhou Agricultural Bureau. We thank Tian-long Fu at Fujian Chun Lun Tea Ltd. for supplying the jasmine-scented tea samples.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Inoue, N.; Kuroda, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Autonomic Nervous Responses According to Preference for the Odor of Jasmine Tea. Bioscience Biotechnology and Biochemistry 2003, 67, 1206–1214.
- Kuroda, K.; Inoue, N.; Ito, Y.; Kubota, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Sedative Effects of the Jasmine Tea Odor and (R)-(-)- Linalool, One of Its Major Odor Components, on Autonomic Nerve Activity and Mood States. European Journal of Applied Physiology 2005, 95, 107–114.
- Zhang, J.; Gong, S.Y.; Tang, D.S.; Zhang, Y.B.; Chen, M.L. Quality Evaluation and Price Discrimination of Jasmine-Scented Tea. Journal of Zhejiang University (Agriculture and Life Sciences) 2015, 41, 577–585.
- Ito, Y.; Sugimoto, A.; Kakuda, T.; Kubota, K. Identification of Potent Odorants in Chinese Jasmine Green Tea Scented with Flowers of Jasminum Sambac. Journal of Agriculture and Food Chemistry 2002, 50, 4878−4884.
- Yamanishi, T. Aroma of Chinese Scented Green Tea. In Frontiers of FlaVor. Proceedings of 5th International Flavor Conference, Porto Karras, Chalkidiki, Greece, July 1–3, 1987; Charalambous, G., Ed.; Elsevier Science Publishers: Amsterdam, pp 181–190.
- Lin, J.; Chen, Y.; Zhang, P.; Ren, M.G.; Xu, H.R.; Wang, X.C. A Novel Quality Evaluation Index and Strategies to Identify Scenting Quality of Jasmine Tea Based on Headspace Volatiles Analysis. Food Science and Biotechnology 2013, 22, 331–340.
- Liang, Y.R.; Wu, Y.; Lu, J.L.; Zhang, L.Y. Application of Chemical Composition and Infusion Color Difference Analysis to Quality Estimation of Jasmine-Scented Tea. International Journal of Food Science & Technology 2007, 42, 459–468.
- Ye, Q.P.; Jin, X.Y.; Li, X.L.; Xu, X.D.; Huang, D.X; Chen, S.H. Study on Variation of Physicochemical Indexes and Tea Quality During Scented Process of Jasmine Tea. Science and Technology of Food Industry 2015, 36, 99–103.
- Liu, J.; Yang, J.F. Primary Studies on the Anti-Depression Function of Jasmine Tea. Journal of Shanxi Agricultural University (Natural Science Edition) 2013, 33, 493–497.
- Liu, J.; Gao, S.L.; Yang, J.F. Antidepressant Effect of Jasmine Tea. Journal of Fujian Agriculture and Forestry University (Natural Science Edition) 2014, 43, 139–145.
- Chan, P.T.; Fong, W.P.; Cheung, Y.L.; Huang, Y.; Ho, W.K; Chen, Z.Y. Jasmine Green Tea Epicatechins Are Hypolipidemic in Hamsters (Mesocricetus Auratus) Fed a High Fat Diet. The American Society for Nutritional Sciences 1999, 129, 1094–1101.
- Surono, I.S.; Hosono, A. Bacterial Mutagenicity of Terasi and Antimutagenicity of Indonesian Jasmine Tea Against Terasi. International Journal of Food Microbiology 1996, 32, 49–58.
- Chen, Z.Y.; Chan, P.T. Antioxidative Activity of Green Tea Catechins in Canola Oil. Chemistry and Physics of Lipids 1996, 82, 163–172.
- Chen, Z.Y.; Wang, L.Y.; Chan, P.T.; Zhange, Z.S.; Chung, H.Y.; Liang, C. Antioxidative Activity of Green Tea Catechin Extract Compared with that of Rosemary Extract. Journal of the American Oil Chemists’ Society 1998, 75, 1141–1145.
- Zhang, A.; Chan, P.T.; Luk, Y.S.; Ho, W.K.K.; Chen, Z.Y. Inhibitory Effect of Jasmine Green Tea Epicatechin Isomers on LDL-Oxidation. The Journal of Nutritional Biochemistry 1997, 8, 334–340.
- Zhang, A.; Zhu, Q.Y.; Luk, Y.S.; Ho, K.Y.; Fung, K.P.; Chen, Z.Y. Inhibitory Effects of Jasmine Green Tea Epicatechin Isomers on Free Radical-Induced Lysis of Red Blood Cells. Life Sciences 1997, 61, 383–394.
- van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. Journal of Chromatography A 1963, 11, 463–471.
- Wang, L.F.; Lee, J.Y.; Chung, J.O.; Baik, J.H.; So, S.; Park, S.K. Discrimination of Teas with Different Degrees of Fermentation by SPME–GC Analysis of the Characteristic Volatile Flavour Compounds. Food Chemistry 2008, 109, 196–206.
- Lv, H.P.; Zhong, Q.S.; Lin, Z.; Wang, L.; Tan, J.F.; Guo, L. Aroma Characterisation of Pu-Erh Tea Using Headspace-Solid Phase Microextraction Combined with GC/MS and GC–Olfactometry. Food Chemistry 2012, 130, 1074–1081.
- Anesine, G.; Ferraro, G.E.; Filip, R. Total Polyphenol Content and Antioxidant Capacity of Commercially Available Tea (Camellia Sinensis) in Argentina. Journal of Agriculture and Food Chemistry 2008, 56, 9225–9229.
- Gaulejac, N.S.C.; Provost, C.; Vivas, N. Comparative Study of Polyphenol Scavenging Activities Assessed by Different Methods. Journal Agricultural Food Chemistry 1998, 47, 425–431.
- Tang, Y. Aroma Absorbing and Keeping Mechanism of Tea. Journal of Tea 2000, 26, 132–135.
- Watanabe, N.; Watanabe, S.; Nakajima, R.; Moon, J.H.; Shimokihara, K.; Inagaki, J.J; Etoh, H.; Asai, T.; Sakata, K.; Ina, K. Formation of Flower Fragrance Compounds by Enzymatic Action During Flower Opening. Bioscience Biotechnology and Biochemistry 1993, 57, 1101–1106.
- Ito, Y.; Kubota, K. Sensory Evaluation of the Synergism Among Odorants Present in Concentrations Below Their Odor Threshold in a Chinese Jasmine Green Tea Infusion. Molecular Nutrition & Food Research 2005, 49, 61–68.
- Katsuno, T.; Kasuga, H.; Kusano, Y.; Yaguchi, Y.; Tomomura, M.; Cui, J.L.; Yang, Z.Y.; Baldermann, S.; Nakamura, Y.; Ohnishi, T.; Mase, N.; Watanabe, N. Characterisation of Odorant Compounds and Their Biochemical Formation in Green Tea with a Low Temperature Storage Process. Food Chemistry 2014, 148, 388–395.
- Abraham, K.O.; Shankaranarayana, M.L.; Raghavan, B.; Natarajan, C.P. Determination of Methyl Salicylate in Black Tea. Microchimica Acta 1976, 65, 11–15.
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental Regulation of Methyl Benzoate Biosynthesis and Emission in Snapdragon Flowers. The Plant Cell 2000, 12, 949–961.
- Cabreta, C.; Artacho, R.; Giménez R. Beneficial Effects of Green Tea—A Review. Journal of the American College of Nutrition 2006, 25, 79–99.
- Ahmad, R.S.; Butt, M.S.; Huma, N.; Sultan, M.T.; Arshad, M.U.; Mushtaq, Z.; Saeed, F. Quantitative and Qualitative Portrait of Green Tea Catechins (Gtc) Through Hplc. International Journal of Food Properties 2014, 17, 1626–1636.
- Oi, Y.; Fujita, H. Body Weight and Body Mass Index (BMI) in Preobese and Overweight Japanese Adults Treated with Black Chinese Tea Water Extract (BTE) Using a More Appropriate Statistical Analysis Method. International Journal of Food Properties 2015, 18, 1345–1349.
- Huang, Y.Y.; Liu, C.; Xiao, X.D. Quality Characteristics of a Pickled Tea Processed by Submerged Fermentation. International Journal of Food Properties 2016, 19, 1194–1206.
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Total Polyphenols, Catechin Profiles and Antioxidant Activity of Tea Products from Purple Leaf Coloured Tea Cultivars. Food Chemistry 2013, 136, 1405–1413.
- El-Hady, D.A.; Albishri, H.M. Alkyl Imidazolium Ionic Liquid Based Sweeping-Micellar Electrokinetic Chromatography for Simultaneous Determination of Seven Tea Catechins in Human Plasma. Journal of Chromatography B 2014, 969, 224–229.
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of Tea Polyphenol (−)-Epigallocatechin-3-Gallate and Formation of Dimers and Epimers Under Common Experimental Conditions. Journal of Agricultural and Food Chemistry 2005, 53, 9478–9484.