ABSTRACT
Extraction and biochemical properties of a new lipase from the hepatopancreas of Pacific white shrimp were studied. Recovery of the hepatopancreas powder with 50 mM Tris-HCl, pH 7.0 containing 0.2% (v/v) Brij35 gave a higher recovery of lipase activity than other extractants tested (p < 0.05). The optimal pH and temperature for lipase activity were 8.5 and 60°C, respectively, when p-nitrophenyl palmitate was used as a substrate. The enzyme was stable to heat treatment up to 40°C and over a pH range of 7.0–10.0 for 30–120 min. Lipase activities continuously decreased as the sodium deoxycholate (NaDC) concentration increased, but activities increased as NaCl concentration increased up to 3.0 M. Hydrolytic activity was enhanced by NaN3, but strongly inhibited by Hg2+, Cu2+, Al3+, and phenylmethanesulfonyl fluoride. The lipase was evaluated as highly stable against surfactants (Tween 20, Tween 80, Triton X-100, and gum arabic). However, the enzyme was unstable against sodium dodecyl sulphate. Stability of the lipase with commercial liquid and solid detergents (Attack®, Bres®, Omo®, and Pao®) was also investigated. The lipase exhibited substantial stability and compatibility with tested commercial liquid and solid laundry detergents for 30–60 min. The overall properties of the lipase from Pacific white shrimp hepatopancreas, thus leading us to propose that it is an excellent candidate for use as biocatalysts for better detergent formulation.
Introduction
Because of their delicacy, shrimp is in high demand in many countries including Japan, the United States, and Europe.[Citation1] Thailand is the world’s leading shrimp-farming country and has become the top supplier of farmed shrimp. Pacific white shrimp (Litopenaeus vannamei) is an important commercial species primarily cultured in Thailand and accounts for 90% of the global aquaculture shrimp production.[Citation2] By the year 2012, the total amount of frozen Pacific white shrimp and products was 550,147 metric tons and the products were mainly exported to the United States and Japan.[Citation2] Among all shrimp products, there is currently an increasing demand for whole shrimp without hepatopancreas. The hepatopancreas is removed using a vacuum sucking machine.[Citation3] However, this material is a potential source of enzymes such as trypsin,[Citation3] chymotrypsin,[Citation4] and lipase.[Citation5]
Lipases (triacylglycerol acylhydrolases EC 3.1.1.3) are enzymes that catalyze the hydrolysis of triacylglycerols at the oil-water interface to release glycerol and free fatty acids.[Citation6] Lipases have become increasingly important in the last two decades for industrial applications. Some novel biotechnological applications have been successfully established using lipases for the synthesis of biopolymers and biodiesel, the production of enantiopure, pharmaceuticals, agrochemical, flavor compound, and the conversion of natural fat and oil into high value products such as cocoa butter equivalent and oil enriched with omega-3-fatty acids.[Citation7] As for industrial enzymology, lipases are commonly included in detergents.[Citation8] As a detergent additive, the increasing usage of lipase is mainly due to its affiliation with the non-phosphate detergents. Ideally, lipase in detergent should have high activity and stability over a broad range of temperature and pH, and should also be compatible with different components in a detergent including metal ions, surfactants, oxidants, and proteases.[Citation9] Thus, there is growing interest in discovering new sources of these enzymes with appropriate characteristics to suit particular applications.
Compared with other hydrolytic enzymes (e.g., proteases and carbohydrases), lipases from fish are relatively less studied. Lipases from aquatic animals are less known compared to their counterparts from mammalian, plant, and microbial sources.[Citation10] Because of their habitats, marine animals might have enzymes that display distinguishable properties that make them better suited for specific applications. Lipases have been isolated and characterized from the viscera of grey mullet,[Citation5] viscera of cod,[Citation11] and hepatopancreas of crab.[Citation12] Until now, no studies have been reported on lipase from Pacific white shrimp hepatopancreas. The present article describes the extraction and characterization of lipases from Pacific white shrimp hepatopancreas, as well as their compatibility with commercial laundry detergents, and surfactants agents.
Materials and methods
Chemicals
Acetone, tris (hydroxymethyl) aminomethane, Brij35, Triton X-100, and gum arabic were obtained from Merck (Darmstadt, Germany). Bovine serum albumin (BSA) and p-nitrophenyl palmitate (p-NPP) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Sodium dodecyl sulphate (SDS) was obtained from Fluka (Buchs, Switzerland). The surfactants and other chemicals with the analytical grade were procured from Merck (Darmstadt, Germany).
Preparation of hepatopancreas and crude extract from Pacific white shrimp
Fresh hepatopancreas of Pacific white shrimp was collected from the Sea Wealth Frozen Food Co., Ltd., Songkhla Province, Thailand. Samples were placed in polyethylene bags and imbedded in polystyrene boxes containing ice with an ice/sample ratio of 2:1 (w/w) during transportation for approximately 2 h. Upon arrival, the samples were homogenized into powder in three volumes of acetone at –20°C for 30 min according to the method of Klomklao et al.[Citation13] The homogenate was filtered in vacuo on Whatman No.4 filter paper. The residue obtained was then homogenized in two volumes of acetone at –20°C for 30 min, and then the residue was left at room temperature until dried and free of acetone odor.
Effect of extraction media on the recovery of Pacific white shrimp lipase
Effect of extractants on the recovery of Pacific white shrimp lipase
Difference extraction media, including distilled water, 50 mM Na-Phosphate, pH 7.0, and 50 mM Tris-HCl, pH 7.0 were used to extract lipase from hepatopancreas of Pacific white shrimp. The medium was added into the defatted hepatopancreas powder with at a ratio of 1:9 (w/v) and stirred continuously at 4°C for 30 min.[Citation14] The supernatant was recovered by centrifuging the slurry at 5000 × g at 4°C for 30 min. The lipase activity and protein content in the extracts were determined, and the yield and specific activity of the extracts obtained using different media were compared. The extraction medium rending the highest yield was chosen for further steps.
Effect of surfactant on the recovery of Pacific white shrimp lipase
Defatted hepatopancreas powder was suspended in 50 mM Tris-HCl, pH 7.0 containing 0.2% (v/v) different surfactants (Brij35, Tween20, Triton X-100, and SDS) at a ratio of 1:9 (w/v) and stirred continuously at 4°C for 30 min. The supernatant was recovered by centrifuging the slurry at 5000 × g at 4°C for 30 min. The lipase activity and protein content in the extracts were measured. The extraction yield and specific activity of the extracts were calculated. The extractant giving the highest yield was chosen for preparation of crude lipase extract.
Enzyme assay and protein determination
Lipase activity was determined spectrophotometrically using p-NPP as substrate according to the method of the Kademi et al.[Citation15] with a slight modification. One volume of 8.0 mM substrate solution in isopropanol was mixed just before use with nine volumes of 50 mM Tris-HCl buffer pH 7.5 containing 0.4% (w/v) Triton X-100 and 0.1% gum arabic. This solution (0.9 mL) was equilibrated at 37°C and the reaction was started by addition of 0.1 mL of the enzymatic solution. The variation of optical density at 410 nm was monitored against a blank without enzyme using a Shimadzu ultraviolet (UV)-1700 spectrophotometer (Kyoto, Japan). The amount of liberated p-NPP was determined at 410 nm during the first 5 min of reaction. One unit of enzyme activity was defined as the amount of enzyme that liberated 1 µmol of p-nitrophenol per minute under the assay conditions. Protein concentration was determined by the Biuret method[Citation16] using BSA as a standard.
pH and temperature profiles
The activity of Pacific white shrimp hepatopancreas lipase was measured within the pH range of 6.0-10.0. Different buffers were used for different pH conditions: 0.2 M McIlvain’s buffer (0.2 M sodium phosphate–0.1 M sodium citrate) for pH 6.0–7.5, 50 mM Tris-HCl for pH 7.5–8.5 and 0.1 M glycine-NaOH for pH 8.5–10.0. The activity was assayed at 37°C for 5 min. For temperature profile study, the activity was assayed at different temperatures (20, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, and 80°C) for 5 min at pH 8.5.
Thermal and pH stability
Crude extracts were incubated at various temperatures (0, 10, 20, 30, 40, 50, 60, 70, 80, and 90°C) for 30, 60, or 120 min, followed by cooling in iced water. The residual activity was assayed using p-NPP as a substrate and reported as the relative activity (%) compared with the original activity. The effect of pH on enzyme stability was evaluated by measuring the residual enzyme activity after incubation at various pHs (2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0) for 30, 60, and 120 min at room temperature. The residual activity was assay with p-NPP as previously described.
Effect of sodium deoxycholate
The effect of varying concentrations of sodium deoxycholate (NaDC) on activity of the lipase was studied by incubating the crude extract with the NaDC at room temperature (28–30°C) for 30 min. The remaining activity was assay with p-NPP.
Effect of sodium chloride
The effect of NaCl on lipase activity was studied. NaCl was added to the standard reaction assay to obtain the final concentration of 0, 1, 2, 3, 4, and 5 M. The remaining activity was determined at room temperature using p-NPP as previously described.
Effect of some chemicals
Different chemicals were mixed with the enzyme solution to obtain the concentration designated (0, 1, and 10 mM for MgCl2, CaCl2, MnCl2, HgCl2, CuCl2, AlCl3, NaN3, ethyldiaminetetraacetic acid (EDTA), and phenylmethanesulfonyl fluoride). The mixtures were kept at room temperature for 30 min and the remaining activity was determined using p-NPP as a substrate.
Effect of some surfactants
Different surfactants (Tween20, Tween80, Triton X-100, SDS, and gum arabic) were added to the crude lipase extract to obtain a final concentration of 1 and 10 mM. The lipase activity was assayed after incubation for 30 min at room temperature. The residual activity was determined and reported as the relative activity compared with the original activity.
Compatibility and stability with commercial laundry detergents
The compatibility and stability of crude lipase with commercial solid and liquid laundry detergents were investigated. The detergents tested were Attack® (Kao, Thailand), Bres® (Uniliver, Thailand), Omo® (Uniliver, Thailand), and Pao® (Lion, Thailand). The solid detergents were diluted with tap water to give final concentration of 7 mg/mL and the liquid detergents were diluted 100-fold to simulate washing conditions.[Citation17] The lipase contained in these detergents were inactivated by heating the diluted detergents for 1 h at 65°C prior to addition of the enzyme preparation. The crude lipase extract was incubated with different detergents for 30 and 60 min at room temperature, and then the remaining activities were determined under the standard assay conditions. The enzyme activity of control without detergent, incubated under the similar conditions, was taken as 100%.
Statistical analysis
A completely randomized design was used throughout this study. Data were subjected to analysis of variance and mean comparison was carried out using Duncan’s multiple range test.[Citation18] Statistical analysis was performed using a SPSS package (SPSS 11.5 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Effect of extraction media in the recovery of Pacific white shrimp hepatopancreas lipase
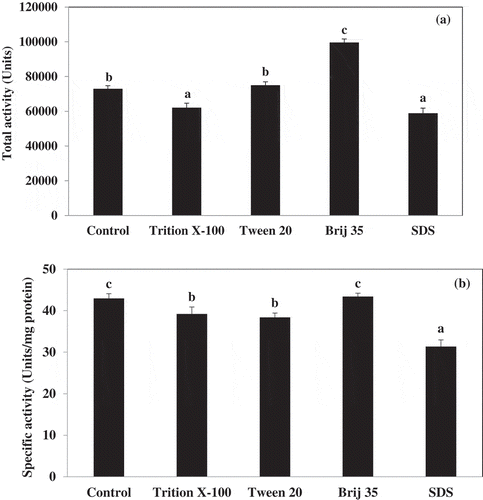
showed the effect of extractants on the recovery of Pacific white shrimp hepatopancreas lipase. Hepatopancreas extract using 50 mM Tris-HCl, pH 7.0 showed the higher lipase activity than those extracted with distilled water and 50 mM Na-Phosphate buffer, pH 7.0 when p-NPP was used as substrate (p < 0.05). The results suggested that Tris-HCl buffer had a greater ability to extract lipase than Na-Phosphate and distilled water. Tris in the buffer might favor the solubilization of lipase associated with the cell membrane by increasing charge of enzymes and protein.[Citation14] The repulsion between the enzymes and tissue might lead to the ease of lipase isolation from the hepatopancreas. Mukherjee et al.[Citation19] reported that 200 mM Tris-HCl buffer, pH 7.5 was the optimum medium to dissolve the Thermomyces lanuginose lipase. From this work, 50 mM Tris-HCl, pH 7.0 was selected as the extraction medium for Pacific white shrimp hepatopancreas lipase because the extract had the maximum lipase activity.
Tris-HCl buffer containing different surfactants were used to extract lipase from hepatopancreas of Pacific white shrimp ( and ). Addition of Triton X-100, Tween20, and SDS in 50 mM Tris-HCl, pH 7.0 mainly decreased the yield and specific activity of lipase. In contrast, the lipase recovery was strongly enhanced in the presence of Brij35. The Brij35 was added to facilitate improved extraction of soluble cell material and to emulsify the small amount of lipid present in Pacific white shrimp hepatopancreas extract to prevent lipid interference with the lipase activity. Kurtovic et al.[Citation20] reported that addition 0.2% (v/v) of Brij35 to extract from Chinook salmon intestine resulted in a small increase in trypsin activity. From the results, 50 mM Tris-HCl, pH 7.0 containing 0.2% (v/v) Brij35 was chosen as the extraction medium for lipase in Pacific white shrimp hepatopancreas.
Table 1. Effect of extraction media on the recovery of lipase from Pacific white shrimp hepatopancreas*.
pH and temperature profiles
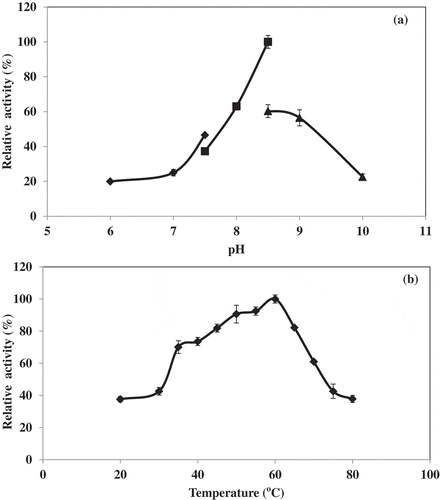
Lipase activity from hepatopancreas of Pacific white shrimp was investigated at different pH values (6.0–10.0). The maximal lipase activity was obtained at pH 8.5 (). This activity was reduced drastically above pH 8.5. The optimum pH value for the Pacific white shrimp lipase activity was close to those described for other fish species such as grey mullet viscera (Mugil auratus; pH 8.0),[Citation21] crab hepatopancreas (Carcinus mediterraneus; pH 8.0)[Citation12] and juvenile redclaw crayfish digestive gland (Cherax quadricarinatus; pH 8.5).[Citation22]
The temperature profile of Pacific white shrimp hepatopancreas extract is shown in . The lipase activity was tested at temperatures ranging from 20 to 80°C using p-NPP as substrate. The optimum temperature of lipase from hepatopancreas of Pacific white shrimp was 60°C, which is higher than the values reported for other fish lipases. For instance, the reported optimum temperature of lipase from sardine and cod were 37 and 25°C, which tributyrin and p-nitrophenyl myristate were used as substrate, respectively.[Citation23,Citation24] The difference in temperature optima might be due to several factors including the varying mechanical properties of the homologous lipases, the different substrate used for measurements, since different substrates do exhibit temperature activity differences with enzymes.[Citation6] A sharp decrease in activity was observed at temperature above 60°C The decrease in activity at high temperature was possibly due to thermal denaturation of the enzyme.[Citation13]
Thermal and pH stability
The thermal stability of Pacific white shrimp hepatopancreas lipase is depicted in . The enzyme was stable when incubated at temperatures up to 40°C for 30–120 min. Nevertheless, the sharp decrease in activity was noticeable at temperatures above 50°C. Generally, the stability of lipase decreased with increasing the heating time. A heating time of 120 min caused the highest loss of activity at every temperature used above 40°C.[Citation21] Enzymes are inactivated at high temperature due to the partial unfolding of the enzyme molecule. Aryee et al.[Citation6] reported that lipase form grey mullet viscera was active within the temperature range of 20–60°C. Zarai et al.[Citation25] found that thermal stability of the marine snail digestive lipase retained about 85% of its maximum activity after incubation for 30 min at 50°C and when the enzyme was incubated at 65°C, it was completely inactive.
Figure 3. Thermal (a) and pH (b) stability of lipase from hepatopancreas of Pacific white shrimp. Bars represent the standard deviation from triplicate determinations.
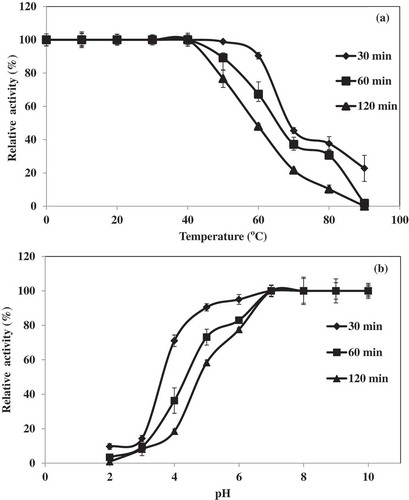
For pH stability, the hepatopancreas extract was stable in the pH range of 7–10 with the exposure time of 30–120 min (). With an extended incubation time, the lipase activity was lost to a greater extent. At pH value below 7.0, the stability of the enzyme sharply decreased. The grey mullet lipase was stable between pH 7.0 and 10.0 during incubation at 25°C for 30 min.[Citation6] Both lipase and phospholipase from grey mullet viscera were found to be mostly stable in alkaline pH value ranging from 7.5 to 9.0.[Citation21] Zarai et al.[Citation25] reported that marine snail digestive lipase was found to be most stable at higher pH values and maintained 100% of its activity after 30 min of incubation at pH 10.0. At acidic pHs, it is suggested that conformational changes in the enzyme affected the proper binding of the enzyme to the substrate.[Citation13]
Lipases that are alkaline tolerant are highly desirable in detergent formulations because enzyme-based detergents possess higher cleaning ability than do chemical-based detergents. Since the results showed that lipase from Pacific white shrimp hepatopancreas was alkaline tolerant, it can be considered as a potential candidate for application in processes that are conducted in the alkaline range such as detergent applications.[Citation26]
Effect of NaDC
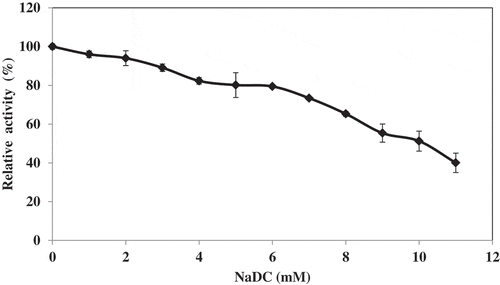
The activity of lipase decreased gradually with increasing NaDC concentration (). Smichi et al.[Citation21] reported that grey mullet digestive lipase maintained 30% of its initial activity in the presence of NaDC concentration as high as 8 mM when olive oil was used as a substrate. Lipase from grey mullet viscera was inactive within the NaDC at concentration of 1–20 mM.[Citation6] NaDC have been shown to inhibit hydrolysis of triacylglycerols in concentration below their critical micellar concentration. This inhibition is attributed to electrostatic repulsions between the negatively charged surfactant and the lipase.[Citation25]
Effect of NaCl
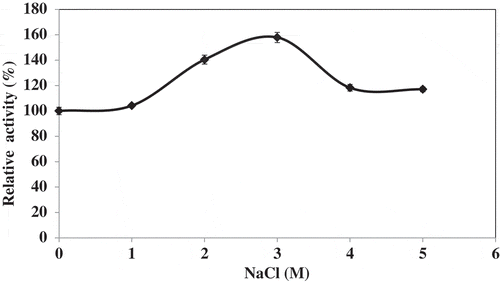
Lipase activities from hepatopancreas of Pacific white shrimp increased with addition of NaCl (). When the concentration of NaCl was increased from 1 to 3 M, activity apparently increased. However, there was no further increase in the activity with NaCl above 3 M. Little is known about the effect of salinity on fish lipolytic activity. Most of fish lipase activities were recorded at NaCl concentrations lower than 100 mM.[Citation27–Citation29] Grey mullet digestive lipase maintained 90% of its initial activity in the presence of NaCl concentration as high as 1 M, unlike sardine digestive lipase, which lost 50% of its initial activity in the presence of 1 M NaCl.[Citation21] López-López et al.[Citation22] reported that lipase from juvenile redclaw crayfish digestive gland (Cherax quadricarinatus) was strongly resistant to the presence of high NaCl concentrations of 5 and 100 mM. Salt-tolerant lipolytic enzymes are gaining attraction for their potential in the food industry and microalgae-based biofuel field. Therefore, lipase from hepatopancreas might be a good candidate for catalysis of lipolytic reaction occurring at high salt concentrations.
Effect of some chemicals
In this study, the enzyme was incubated with various compounds and residual activities were measured after 30 min of incubation at room temperature (). NaN3 at levels of incorporation of 1 and 10 mM activated lipase from hepatopancreas of Pacific white shrimp by 2 and 17%, respectively. Aryee et al.[Citation6] reported that 1 and 10 mM NaN3 increased grey mullet digestive lipase by 23 and 30%, respectively.
Table 2. Effect of selected chemicals on the stability of lipase from hepatopancreas of Pacific white shrimp.
In general, lipases are strongly inhibited by HgCitation2+ (a thiol group inhibitor). This is likely due to the proximity of SH group to the catalytic and interfacial binding site, but spatially remote from the catalytic site, this may have induced the marked loss of activity.[Citation30] The catalytic triad of lipases has been recognized to consist of Ser, His, and Glu or Asp.[Citation6] Thus, the bulky HgCitation2+group might cause steric interference to the approach of the substrate to the active site. From the results, after 30 min incubation with 1 mM and 10 mM HgCitation2+, the lipase retained approximately 83.37 and 22.99% residual activity, respectively.
In addition, treatment with CuCitation2+ and AlCitation3+ at both 1 and 10 mM significantly inhibited the activity of lipase from hepatopancreas. MgCitation2+ and MnCitation2+ had negligible effect on lipase activity even though the concentration increased. CaCitation2+ also had little effect on the activity of the enzyme with only 8 and 11% inhibition at 1 and 10 mM, respectively. Thus, lipase from hepatopancreas of Pacific white shrimp did not seem to require CaCitation2+ for catalysis, similar to other lipases.[Citation6,Citation24] Other metal ions slightly inhibited lipase activity, which may be due to the fact that transition metal ions change the conformation of the protein to less stable form.[Citation31]
EDTA had no appreciable effect on lipase activity at the 1 mM level, although 10 mM EDTA caused slight (26%) decrease in activity. Lipase activity was inhibited by 48-73% in the presence of 1-10 mM PMSF. According to Tripathi et al.,[Citation32] lipase activity from Microbacterium sp. was inhibited by 79% in the presence of 1 mM PMSF. Understanding the role of lipase inhibitors may provide a better perceptive of their mechanism of action[Citation6] and successful identification of potent and specific inhibitors have resulted in their applications in certain treatments.[Citation24,Citation32]
Effect of some surfactants on lipase activity
As shown in , the crude lipase from hepatopancreas of Pacific white shrimp showed a stability toward 1% non-ionic surfactants such as Tween20, Tween80, and Triton X-100 with the residual activity higher than 86.80%. However, a decrease in activity was observed at the non-ionic surfactants concentration of 10%. As similar to the results, Tripathi et al.[Citation32] reported reduction of lipolytic activity in the presence of 1% Tween20, Tween80, and Triton X-100. Lima et al.[Citation33] reported that increasing concentrations of Triton X-100 decreased lipase activity and suggested that this detergent could be incompatible with some lipase assay.[Citation33] The crude lipase from hepatopancreas of Pacific white shrimp showed an extreme stability toward 1 and 10% gum arabic. Ungcharoenwiwat et al.[Citation7] found that 1.0% gum arabic increased the lipase activity of Burkholderia sp. EQ3 with a 120% relative activity. Gum arabic is the most commonly used for hydrolysis of triacrylglycerol, giving emulsion which can be stored for several weeks.[Citation35] It has been generally been assumed that gum arabic was simply an emulsifying agent stabilizing emulsions without interfering with the lipase assay itself.[Citation35] However, after 30 min of incubation of the crude lipase with 1 and 10% SDS, the lipase exhibited 76 and 39% of the initial activity, respectively. SDS at 10 mM had strong inhibitory effect on activity of grey mullet viscera (Mugil auratus) lipase with only 47% residual activity after incubation at 25°C for 30 min.[Citation6] SDS inhibition is probably due to the high negative charge it imparts to the interface increase electrostatic repulsion between the enzyme and the interface formed by emulsified substrate.
Table 3. Effect of different surfactants on the stability of lipase from Pacific white shrimp hepatopancreas.
The objective of the present study was to produce lipase, which could be suitable for application in detergents for the use in laundry and/or cleaning of hard surfaces. Thus, it is relevant to test lipase stability in the presence of this additive. The results of the assay revealed high lipase resistance toward both 1% surfactants for 30 min, since retained activity was above 76.65%. Overall, hepatopancreas lipase was evaluated as highly stable against surfactants, delineating itself as desirable additive for better detergent formulation.
Stability of the lipase with commercial liquid and solid detergents
The high activity and stability of the hepatopancreas lipase in alkaline pH range and its relative toward different surfactants are very useful for its eventual application as detergent additive. Therefore, we checked the compatibility of hepatopancreas lipase with some commercial liquid and solid detergents (Attack®, Bres®, Omo®, and Pao®). The crude lipase was pre-incubated in the presence of various commercial laundry detergents for 30 and 60 min at room temperature. The data presented in showed that the hepatopancreas lipase was stable in the presence of all liquid detergents tested. The results of the assay revealed high lipase resistance for 30 and 60 min, since retained activity was above 75%. The enzyme retained 100% of its activity in the present of Attack® after 30 min incubation at room temperature. In the cause of liquid detergents, the crude lipase was found more stable in Attack® than those (Bres®, Omo®, and Pao®) of all liquid detergents at all times.
Figure 6. Stability of crude lipase from hepatopancreas of Pacific white shrimp in presence of various commercial liquid (a) and solid (b) laundry detergents. The different letters indicate significant differences (p < 0.05).
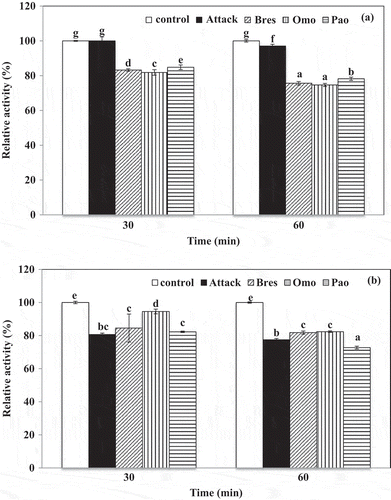
The crude lipase displayed an extreme stability in the presence of all commercial solid laundry detergents tested (). It particularly showed excellent compatibility and stability with Attack®, Bres®, and Omo® at room temperature for 60 min, remaining more than 77.49% of its initial activity (p < 0.05). However, the crude lipase was less stable in the presence of Pao® with only 72.73% residual activity after 60 min incubation. Since the hydrolytic activity varied with each laundry detergent, the obtained results clearly indicate that performance of enzyme in detergents depends on a number of factors, including the detergents’ compounds. Thus, the choice of lipase for use in detergent formulations is dependent on stability and activity as well as compatibility under the harsh conditions associated with the washing process, the composition of detergents and the temperature and pH.[Citation8] The results thus indicate that the crude lipase from hepatopancreas of Pacific white shrimp is superior for the laundry detergent industry, especially for most formulations available in Thailand.
Conclusions
The hepatopancreas of Pacific white shrimp would be a potential source of lipase for certain food processing operations that require high alkaline and high salt condition. In addition, considering the high activity and stability in high alkaline pH, relative stability in the presence of surfactants and several commercial laundry detergents, Pacific white shrimp hepatopancres lipase may find application in laundry detergents.
Funding
This research was supported by the Thailand Research Fund (TRF) and Thaksin University. The TRF distinguished research professor grant was also acknowledged.
Additional information
Funding
References
- Lebel, L.; Mungkung, R.; Gheewala, S.H.; Label, P. Innovation Cycles, Niches and Sustainability in the Shrimp Aquaculture Industry in Thailand. Environmental Science & Policy 2010, 13, 291–302.
- Senphan, T.; Benjakul, S. Compositions and Yield of Lipids Extracted from Hepatopancreas of Pacific White Shrimp (Litopenaeus Vannamei) as Affected by Prior Autolysis. Food Chemistry 2012, 134, 829–835.
- Sriket, C.; Benjakul, S.; Visessanguan, H.; Hara, K.; Yoshida, A.; Liang, X. Low Molecular Weight Trypsin from Hepatopancreas of Freshwater Prawn (Macrobrachium Rosenbergii): Characteristic and Biochemical Properties. Food Chemistry 2012, 134, 351–358.
- Hernandez-Cortes, P.; Whitaker, J.R.; Garcia-Carreno, F.T. Purification and Characterization of Chymotrypsin from Penaeus Vannamei (Crustacea: Decapoda). Journal of Food Biochemistry 1997, 21(1), 497–514.
- Perez, C.R.; Toro, A.N.; Carreno, F.G. Purification and Characterization of an Intracellular Lipase from Pleopods of White Leg Shrimp (Litopenaeus Vannamei). Comparative Biochemistry and Physiology—Part B: Biochemistry & Molecular Biology 2011, 158, 99–105.
- Aryee, A.N.A.; Benjamin, B.K.; Villalonga, R. Lipase Fraction from the Viscera of Grey Mullet (Mugil Cephalus): Isolation, Partial Purification and Some Biochemical Characteristic. Enzyme and Microbial Technology 2007, 40, 394–402.
- Ungcharoenwiwat, P.; H-Kittikun, A. Purification and Characterization of Lipase from Burkholderia Sp. EQ3 Isolated from Wastewater from a Canned Fish Factory and Its Application for the Synthesis of Wax Esters. Journal of Molecular Catalysis B: Enzymatic 2015, 115, 96–104.
- Salihu, A.; Alam, Z. Solvent Tolerant Lipases: A Review. Process Biochemistry 2015, 50, 86–96.
- Wang, Y.X.; Srivastava, K.C.; Shen, G.J.; Wang H.Y. Thermostable Alkaline Lipase from a Newly Isolated Thermophilic Bacillus, Strain A30-1 (ATCC 53841). Journal of Fermentation and Bioengineering 1995, 79, 433–438.
- López-Amaya, C.; Marangoni, A.G. Lipases: Seafood Enzymes. Utilization and Influence on Postmortem Fish Quality. New York, NY: Press, 2001; p. 121–146.
- Sovik, S.L.; Rustad, T. Effect of Season and Fishing Ground on the Activity of Lipases in Byproducts from Cod (Gadus Morhua). LWT–Food Science and Technology 2005, 38, 867–876.
- Cherif, S.; Fendri, A.; Miled, N.; Trabelsi, H.; Mejdoub, H.; Gargouri, Y. Crab Digestive Lipase Acing at High Temperature: Purification and Biochemical Characterization. Biochimie 2007, 89, 1012–1018.
- Klomklao, S.; Kishimura, H.; Yabe, M.; Benjakul, S. Purification and Characterization of Two Pepsin from Stomach of Pectoral Rattail (Coryphaenoides Pectoralis). Comparative Biochemistry and Physiology—Part B: Biochemistry & Molecular Biology 2007, 147, 682–689.
- Klomklao, S.; Benjakul, S.; Kishimura, H. Proteinases in Hybrid Catfish Viscera: Characterization and Effect of Extraction Media. Journal of Food Biochemistry 2010, 34, 711–729.
- Kademi, A.; Aït-Abdelkader, N.; Fakhreddine, L.; Baratti, J.C. A Thermostable Esterase Activity from Newly Isolated Moderate Thermophilic Bacterial Strains. Enzyme and Microbial Technology 1991, 24, 332–338.
- Robinson, H.W.; Hodgen, C.G. The Biuret Reaction in the Determination of Serum Protein. I. A Study of the Condition Necessary for the Production of the Stable Color Which Bears a Quantitative Relationship to Protein Concentration. Journal of Biological Chemistry 1940, 135, 707–725.
- Ali, N.E.H.; Hmidet, N.; Bougatef, A.; Nasri, R.; Nasri, M. A Laundry Detergent-Stable Alkaline Trypsin from Striped Seabream (Lithognathus Mormyrus) Viscera: Purification and Characterization. Journal of Agricultural and Food Chemistry 2009, 57, 10943–10950.
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics. New York, NY: McGraw-Hill, 1980.
- Mukherjee, J.; Gupta, M.N. Molecular Bioimprinting of Lipase with Surfactants and Its Functional Consequence in Low Water Media. International Journal of Biological Macromolecules 2015, 81, 544–551.
- Kurtovic, I.; Marshall, S.N. Simpson, B.K. Isolation and Characterization of a Trypsin Fraction from the Pyloric Ceca of Chinook Salmon (Oncorhynchus Tshawytscha). Comparative Biochemistry and Physiology—Part B: Biochemistry & Molecular Biology 2006, 143, 432–440.
- Smichi, N.; Gargouri, Y.; Miled, N.; Fendri, A. A Grey Mullet Enzyme Displaying Both Lipase and Phospholipase Activities: Purification and Characterization. International Journal of Biological Macromolecules 2013, 58, 87–94.
- López-López, S.; Nolasco, H.; Vega-Villasante, F. Characterization of Digestive Gland Esterase-Lipase Activity of Juvenile Redclaw Crayfish Cherax Quadricarintus. Comparative Biochemistry and Physiology—Part B: Biochemistry & Molecular Biology 2003, 135, 337–347.
- Gjellesvik, D.R.; Lombrado, D.; Wather, B.T. Pancreatic Bile Salt Dependent Lipase from Cod (Gadus Morhua): Purification and Properties. Biochimica et Biophysica Acta (BBA) 1992, 1124, 123–134.
- Mukundan, M.K.; Gopkumar, K.; Nair, M.R. Purification of a Lipase from the Hepatopancreas of Oil Sardine (Sardinella Longiceps Linnaceus) and Its Characteristic and Properties. Journal of the Science of Food and Agriculture 1985, 36, 191–203.
- Zarai, Z.; Ali, M.B.; Fendri, A. Louati, H.; Mejdoub, H.; Gargouri, Y. Purification and Biochemical Properties of Haxaplex Trunulus Digestive Lipase. Process Biochemistry 2012, 47, 2434–2439.
- Balaji, L.; Jayaraman, G. Metal Ion Activated Lipase from Halotolerant Bacillus sp. VITL8 Displays Broader Operational Range. International Journal of Biological Macromolecules 2014, 67, 380–386.
- Iijima, M.; Chosa, S.; Uematsu, K.; Goto, T.; Hoshita, T.; Kayama, M. Purification and Characterization of Phospholipase A2 from the Pyloric Caeca of Red Sea Bream, Pagrus Major. Fish Physiology and Biochemistry 1997, 16, 487–498.
- Uchijama, S.; Fujikawa, Y.; Uematsu, K.; Matsuda, H.; Aida, S.; Iijima, N. Localization of Group IB Phospholipase A2 Isoform in the Gills of the Red Sea Bream, Pagrus (Chrysophrys) Major. Comparative Biochemistry and Physiology—Part B: Biochemistry & Molecular Biology 2002, 132, 671–683.
- Chatzifotis, S.; Polemitou, I.; Divanach, P.; Antonopoulou, E. Effect of Dietary Taurine Supplementation on Growth Performance and Bile Salt Activated Lipase Activity of Common Dentex, Dentex Dentex, Fed a Fish Meal/Soy Protein Concentrate-Based Diet. Aquaculture 2008, 275, 201–208.
- Gargouri, Y.; Julien, R.; Bois, A.G.; Verger, R.; Sarda, L. Studies on the Detergent Inhibition of Pancreatic Lipase Activity. Journal of Lipid Research 1983, 24, 1336–1342.
- Joseph, B.; Shrivastava, N.; Ramteke, P.W. Extracellular Cold-Active Lipase of Microbacterium Luteolum Isolated from Gangotri Glacier, Western Himalaya: Isolation, Partial Purification and Characterization. Journal of Genetic Engineering and Biotechnology 2012, 10, 137–144.
- Tripathi, R.; Sigh, J.; Bharti, R.K.; Thakur, I.S. Isolation, Purification and Characterization of Lipase from Microbacterium sp. and Its Application in Biodiesel Product. Energy Procedia 2014, 54, 518–529.
- Lima, V.M.G.; Krieger, N.; Mitchell, D.A.; Fontana, J.D. Activity and Stability of a Crude Lipase from Penicillium Aurantiogriseum in Aqueous Media and Organic Solvents. Biochemical Engineering Journal 2004, 18, 65–71.
- Tiss, A.; Carriére, F.; Douchet, I.; Patker, S.; Svendsen, A.; Verger, R. Interfacial Binding and Activity of Lipase at the Lipid-Water Interface: Effect of Gum Arabic and Surface Pressure. Colloids and Surfaces B: Biointerfaces 2002, 26, 135–145.
- Glicksman, M.; Sand, R. Industrial Gums; Whistler, R.L.; Ed.; Academic Press: New York, NY, 1973, pp. 197–263.