ABSTRACT
In this study, the effects of different cooking methods on health-promoting phytochemicals and antioxidant activity of fresh kale were investigated. Results showed that water soluble phytochemicals were significantly decreased by boiling. While stir-frying gave the highest degradation ratio for all phytochemicals, and steaming gave the lowest degradation ratio. The thermal degradation ratios of ascorbic acid, total carotenoids, total chlorophylls, total flavonols, and total phenolics content after stir-frying were 54.9, 28.2, 71.0, 81.3, and 39.3%, respectively. The results showed that steaming can be considered as the best method of cooking as it preserve the bioactive compounds and the antioxidant activity.
Introduction
Brassica vegetables can be cultivated in different seasons and environments, and are widely consumed in many parts of the world. Therefore, they are both economically and nutritionally important vegetables.[Citation1,Citation2] Brassica oleracea L. includes important vegetables, such as kale, broccoli, cauliflower, and others. Kale (Brassica oleracea var. acephala) is one of the oldest forms of Brassica species, originating from the eastern Mediterranean.[Citation3] This non-heading leafy green vegetable has been introduced to many parts of the world during centuries by travelers and immigrants. At the present time, kale has many varieties grown in several geographic areas.[Citation4–Citation7] Kale has become increasingly popular because of its beneficial effects on health. Consumption of kale has been associated with reduced risk of degenerative and chronic diseases such as heart disease, some types of cancers, diabetes, etc.[Citation4] Kale has high content of health-promoting phytochemicals such as ascorbic acid, carotenoids, flavonoids, and other phenolics.[Citation5,Citation8] These components are related to high antioxidant activity of kale. Kale exhibits the highest antioxidant capacity in comparison to Brasicca oleraceae members and several other vegetables.[Citation9]
While tender leaves of kales are used for human consumption, older ones are used in feed production.[Citation3] They are ingredients of several traditional and artisanal foods, such as soups, fillings of pastries, wraps, side dishes, and green leafy vegetable juices.[Citation5,Citation6] However, kale is commonly consumed in cooked form. Cooking methods such as blanching, boiling, steaming, microwaving, and frying are generally used to prepare most of the dishes. It is well-known that cooking induces profound changes in chemical composition, affecting bioavailability and the amount of bioactive compounds in vegetables.[Citation10] Zhang and Hamauzu[Citation11] reported that after cooking, the phenolic compounds, ascorbic acid and total antioxidant activity of broccoli florets and broccoli stems were retained by up to 28.1–28.4% and 55.6–57.8%, 34.1–34.4%, and 29.1–29.5%, and 34.7–35.0% and 34.6–34.7%, respectively. Effects of cooking methods on antioxidant activity and bioactive compounds have been extensively studied in Brassica vegetables.[Citation10,Citation12,Citation13] So far, there are few reports about the influence of thermal processing on the antioxidant activity and bioactive compounds of kale.[Citation7,Citation14,Citation15] However, there is no information reported about the effects of cooking on free and bound phenolic content of kale, and the contribution of free and bound phenolics on antioxidant capacity of kale. This study was carried out in order to determine the effects of common cooking methods on free and bound phenolic content and other bioactive compounds of kale. In addition, the contributions of free and bound phenolic contents on antioxidant capacity of kale were investigated.
Materials and methods
Plant materials
Kale (Brassica oleracea var. acephala) were purchased from a local truck farm in Trabzon, in the east Black Sea coast of Turkey. Kale was harvested in December 2014. After harvesting, the kale was immediately transported to the laboratory and processed. After removing outer leaves of kale, tender dark green leaves were washed, dried with paper towels, and chopped into homogeneous pieces (3 × 3 cm) with a knife and randomly used for each treatment. Each treatment was carried out in three replicates.
Chemicals and reagents
Acetonitrile and water for chromatography (high-performance liquid chromatography [HPLC] grade), and methanol, ethanol, acetone, sodium hydroxide, hydrochloric acid, and acetic acid were obtained from Merck (Darmstadt, Germany). Standards of gallic acid, quercetine, kaempferol, sinapic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) stable radical, 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); (ABTS); diammounium salt, potassium persulfate, tert-butylhydroquinone, 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Cooking treatments
Four different cooking methods, namely boiling, steaming, microwaving, and conventional stir-frying were tested. For boiling, 200 g of kale pieces were immersed in 800 mL of boiling water for 5 min. For stir-frying, sunflower oil (15 mL) was preheated for 3 min in a frying-pan and kale pieces (200 g) were stir-fried for 5 min in preheated oil. For microwave cooking, 200 g of samples were placed in a glass plate, and then kept in microwave oven (Arçelik, MD554, China) at 600 W for 5 min. Steaming was conducted by suspending 200 g of kale above 300 mL of boiling water for 5 min in a steamer with a lid. At the end of each cooking treatment, the leaves were cooled to room temperature, freeze-dried, and kept in polyethylene bags at –30°C for further analyses.
Determination of water, °Brix, ph, titratable acidity, ash, and protein content
Water, °Brix, pH, titratable acidity, ash, and protein content were determined according to the methods given by AOAC.[Citation16]
Color measurement
The Hunter color parameters (L, a, and b) were determined using a Lovibond Tintometer (Model RT 300, The Tintometer Ltd., Salisbury, UK) with a 10° observer window and a D-65 light source. Color parameters were expressed as L (100 whiteness/0 darkness), a (+redness/–greenness), and b (+blueness/–yellowness).
Total chlorophylls
Total chlorophylls was extracted from 0.1 g freeze-dried kale by homogenizing (12,000 rpm, 30 s) the samples in 9.9 mL of 80% cold acetone using a homogenizer (Heidolph, SilentCrusher M, Schwabach, Germany). The homogenate was centrifuged at 2000 × g for 15 min at 4°C. Supernatant (2 mL) was diluted to 10 mL with acetone:water (80:20, v/v). Absorbance was read at 663 and 645 nm. Results were calculated using this formula: total chlorophylls = (20.2 × Abs645) + (8.02 × Abs663).[Citation17]
Total carotenoids
Total carotenoids content was determined using a spectrophotometric method.[Citation18] Nearly 0.3 g of freeze-dried sample was homogenized (20,000 rpm, 30 s) with methanol:water (70:30, V/V), the homogenate was centrifuged for 5 min at 4000 × g and 4°C. Residue was mixed with 7.5 mL acetone:petrolium ether (1:1, V/V), and content was vortexed (1 min) and centrifuged (5 min, 4000 × g, 4°C). This procedure was repeated twice. Supernatants were pooled in a separator funnel and saponified with 10% methanolic KOH for 60 min, and then washed with water until alkali free. Upper layer in separator funnel was separated and its volume was adjusted to 25 mL with petroleum ether. Absorbance was measured at 444 nm in an Agilent 8453 spectrophotometer (Santa Clara, CA, USA). Results were expressed as milligrams of β-carotenoid equivalent (β-car eq) per kilogram of kale on dry weight basis (dw).
Ascorbic acid
Ascorbic acid was determined according to Lee and Coates[Citation19] with some modifications. Freeze-dried kale (0.1 g) was homogenized (30,000 rpm, 30 s) in ice bath with 2 mL of 4% metaphosphoric acid by a tissue homogenizer (Cat, x120, Staufen, Germany). Homogenate was centrifuged for 4 min at 10,000 g and 4°C. Supernatant was filtered using a 0.45 µm poly(vinylidene fluoride) syringe filter, and then, immediately injected into a HPLC system. HPLC system (Shimadzu, Kyoto, Japan) consisted of a LC-20 AD gradient pump, a Rheodyne 7725i valve furnished with 20 μL loop, a SPD-M20A diode array detector and CTO-10AS VP column oven. Hypersil Gold Aqua Q (150 × 44 mm, 5 µm) was utilized with a mobile phase (water:H2SO4, pH 2.54) at a flow rate of 0.7 mL/min. Detection was made at 244 nm and 25°C. The calibration curve was constructed by plotting the known solutions of ascorbic acid dissolved in extraction solvent. The compounds appearing in chromatograms were identified on retention times and spectral data by comparison with ascorbic acid standard. A typical HPLC chromatogram of ascorbic acid compound of cooked kale sample was shown in .
Flavonols
Flavonols was determined according to Park et al.[Citation20] with some modifications. Freeze-dried kale (0.25 g) was mixed with 10 mL of 62.5% aqueous methanol containing 2 g/L tert-butylhydroquinone. After sonication for 20 min, 2.5 mL of 8 M HCl was added to the reaction mixture. For hydrolysis of flavonol glycosides, the mixture was incubated in a water bath at 90°C for 3.5 h. After cooling, hydrolyzed samples were sonicated for 5 min. The aliquots were filtered using 0.45µm PTFE syringe filter (Millipour). This filtrate injected into the HPLC system (Shimadzu, Kyoto, Japan) for quantification and determination of flavonols. Separation of flavonols was carried out using a Symetry C18 (250 × 4.6 mm id, particle size 5 μm) column (Waters, USA) at 25°C. The method utilizes a binary mobile phase consisting of 2% acetic acid in water (A) and 0.5% acetic acid in water:acetonitrile (1:1, v/v; B). Gradient program was as follows: 0 min 50% A; 20 min 10% A; 28 min 0% A. Detection was made at 360 nm. Quercetin and kaempferol standard solutions were prepared by dissolving quercetin and kaempferol in methanol at a concentration ranging from 0.5 to 10 mg/L and 5 to 50 mg/L, respectively. The compounds appearing in chromatograms were identified on retention times and spectral data by comparison with standards.
Preparation of free and bound phenolic extracts
For free phenolic extracts, nearly 0.5 g of freeze-dried kale was put into a centrifuge tube and extracted by shaking with 7 mL of 80:20 (v/v) acetone:water for 2 h at 175 rpm in dark at room temperature. After this, the mixture was centrifuged at 8000 × g for 10 min at 4°C and then, supernatant was transferred into an amber bottle. The above procedure was repeated twice using the residue. Supernatants were combined, and then final volume was adjusted to 25 mL with extraction solvent.[Citation10] For bound phenolic extract, the residue in the centrifuge tubes mixed with 30 mL of 2.6 M NaOH in 53% aqueous methanol and incubated for 20 h at room temperature. After incubation, pH of the sample solution was adjusted to 1-2 with HCl. Sample were filtered through a Whatman No:1 filter paper.[Citation20] For phenolic acids, phenolic content, DPPH, and ABTS assays, both free and bound phenolic extracts were stored at –30°C for analysis.
Phenolic content
Phenolic content was determined by the Folin–Ciocalteau method.[Citation21] While bound phenolic extracts were directly used for phenolic content analysis, free phenolic extracts were diluted with extraction solvent (2:8, v/v). Results were expressed as mg gallic acid equivalent (ga eq) per kg kale (dw).
Determination of phenolic acids
Chromatographic analyses were carried out using the HPLC system (Shimadzu, Kyoto, Japan). The method reported by Coloric et al.[Citation22] was used with some modifications. For phenolic acids, free and bound phenolic extracts of kales were diluted with acetone:water (80:20; 1:1, v/v). Diluted extract was filtered through a 0.45 μm PTFE membrane filter (Millipore, Bedford, USA) and analyzed by HPLC. Separation of phenolic compounds was carried out using a Symetry C18 (250 × 4.6 mm id, particle size 5 μm) column (Waters, USA) at 25°C. The method utilizes a binary mobile phase consisting of 2% acetic acid in water (A) and 0.5% acetic acid in water:acetonitrile (1:1, v/v; B). Gradient program was as follows: 0 min 90% A; 30 min 80% A; 60 min 65% A. Detection was made at 320 nm for hydroxycinnamic acids. The compounds appearing in chromatograms were identified on retention times and spectral data by comparison with standards.
DPPH assay
DPPH assay was performed, with a slight modification, according to Pyo et al.[Citation23] Phenolic extract (0.3 mL) was added to 3.7 mL of DPPH solution (0.025 g/L in methanol). The mixture was left for 60 min in dark at room temperature until the reaction reached a plateau. The absorbance at 515 nm was measured by spectrophotometer after 120 min. The results were expressed as Trolox equivalent antioxidant capacity (mmol Trolox eq/g dw).
ABTS assay
The ABTS assay was performed according to the method described by Re et al.[Citation24] Briefly, ABTS.+ radical cation was generated by reacting 7 mM ABTS and 2.45 mM potassium persulfate via incubation at room temperature in the dark for 12-16 h. The ABTS.+ solution was diluted with ethanol to an absorbance of 0.700 ± 0.05 at 734 nm and ABTS.+ solution (1980 μL) was mixed with the 20 μL of the extract. The mixture was allowed to stand for 6 min at ambient temperature and the absorbance of the mixture was measured at 734 nm. The results were expressed as Trolox equivalent antioxidant capacity (mmol Trolox eq./g dw).
Statistical analysis
All cooking experiments were performed in triplicate. The obtained data were analyzed using the SPSS package program version 15.0 (SPSS Inc. Chicago, IL, USA) for one-way analysis of variance (ANOVA). Duncan’s multiple range test procedure was used to identify significant differences (p < 0.05).
Results and discussion
Some compositional properties of kale leaves used in this study are given in . Kale has low °Brix, titratable acidity and protein content. Armesto et al.[Citation25] found that pH, titratable acidity, dry matter, and °Brix values of smooth-leaved varieties of Galega kale were 6.2, 0.1% citric acid, 15.0% and 13.2°Brix, respectively. Our results, except titratable acidity, were lower than the findings of Armesto et al.[Citation25] These variations may be due to use of different parts of the plant, its maturity, variety, and harvest time. Effects of different cooking methods on surface color are evaluated with Hunter color values and the results are shown in . Base on the results, it can be argued that L-values of cooked kale were decreased compared with that of fresh kale (p < 0.05). However, b-values were not significantly changed among all samples. These results were similar with findings of Pellegrini et al.[Citation12] for L value and that of Xu et al.[Citation10] for b-value. To the contrary of the expectations, both microwaving and stir-frying increased greenness value (p < 0.05). The increase of greenness in the surface of spinach and peas after microwave processing was reported by Turkmen et al.[Citation26] This may be related to color difference in plant parts or alteration of surface reflection properties and depth of light penetration of cooked leaves. A decrease in opacity by replacement of intercellular air with cell juice released due to cell membrane deterioration could be a possible explanation for increase in greenness during cooking.[Citation26]
Table 1. Some chemical properties of kale leaves.
Table 2. Changes in the surface color of kale cooked by different methods.
Effects of cooking methods on total chlorophylls content of kale sample were shown in . The color of green vegetables was mainly related to chlorophyll content in plant material. Total chlorophylls content of kale was found as 8890.1 mg/kg sample dw, and our result was in the range of findings of Ferioli et al.[Citation5] Especially, average chlorophyll content (8130 mg/kg dw) of kale samples from Turkey was similar with our result. The degradation rates in total chlorophylls content after steaming, boiling, microwaving, and stir-frying were 18.7, 21.5, 55.3, and 71.0%, respectively. Microwaving and stir-frying caused a significant decrease in total chlorophylls content in comparison with fresh kale (p < 0.05). However, there were no significant difference in total chlorophylls content among fresh, steamed and boiled samples. Pellegrini et al.[Citation12] found that total chlorophylls content of broccoli were reduced by 40.0 and 74.6% in boiling and microwaving treatments, respectively.
Table 3. Effects of cooking methods on chlorophyll, carotenoid, and vitamin-C content of kale.
Carotenoids are accessory pigments in the light-harvesting steps of photosynthesis. They play an important role in human diet by virtue of their metabolism to vitamin-A. In addition, high antioxidant properties of carotenoids have also been implicated in the protection against heart disease and cancer.[Citation27] Carotenoid is one of the major classes of phytochemicals found in kale. In the present study, amount of total carotenoids obtained from fresh and cooked kale samples were given in . Total carotenoids level of fresh kale was found to be 560.1 mg/kg dw. Total carotenoids content was reported by Murador et al.[Citation7] to be 155.4 µg/g in raw kale. Ferioli et al.[Citation5] noted that total levels of carotenoids in kale samples from Portugal, Italy and Turkey were 631, 959 and 1321 mg/kg dw, respectively. While the results obtained for the sample of Italy and Turkey were higher than our results, the results for the samples of Portugal were similar with our results. All the cooking treatments, except stir-frying, caused negligible alterations in total carotenoids contents. While these changes among fresh, steamed, boiled and microwave cooked samples were not statistically significant, total carotenoids content significantly decreased in the samples cooked by stir-frying (p < 0.05). Stir-frying reduced the total carotenoids content in kale by 28.2%. Murador et al.[Citation7] found that while high decrease in total carotenoids content of kale sample was observed in boiling (77%), followed by steaming (72%) and stir-frying (55%), no significant effect of cooking methods on carotenoid content of red cabbage was observed. Pellegrini et al.[Citation12] reported that total carotenoids content of broccoli was significantly reduced by boiling and microwaving, while total carotenoids content was not influenced by basket steaming. In the relevant literatures, there are controversial findings about the effect of cooking methods on carotenoid content.[Citation7,Citation12,Citation28] This may be due to different food matrices, varying carotenoid levels, and deposition of carotenoids in different types of plastid.[Citation7]
Ascorbic acid is commonly recognized as a major nutrient and antioxidant in vegetables. However, ascorbic acid is very sensitive compound, and may be lost due to oxidation or leaching into the water during cooking.[Citation8,Citation10] Cruciferous vegetables, especially kale, are a good source of ascorbic acid for the diet. shows the effect of cooking methods on ascorbic acid content of kale. The ascorbic acid content of fresh kale was found to be 4917.8 mg/kg dw. Vitamin-C (ascorbic and dehydroascorbic acid) contents of Winterbor and Maribor kale cultivars were reported to be 237.8 and 572.6 mg/kg fresh weight, respectively, by Bacerra-Moreno et al.[Citation29] Sikora et al.[Citation15] found that kale contains the highest amount of vitamin-C (1007 mg/kg on fresh weight basis [fw]) among Cruciferous vegetables. In this study, the lowest ascorbic acid content after cooking was observed for stir-frying, followed by boiling, microwaving, and steaming. Decreases for ascorbic acid content of kales after steaming, boiling, microwaving, and stir-frying were 2.9, 53.1, 10.6, and 54.9%, respectively. The reducing effects of different cooking treatments on vitamin-C in red cabbage noted by Xu et al.[Citation10] followed the same order. Stir-fried and boiled samples showed significantly lower ascorbic acid content than fresh kale (p < 0.05). However, the changes in ascorbic acid contents of microwaved and steamed samples were not significant. While the lowest amount of loss (20%) for vitamin-C content was given by blanching at 80°C for 3 min, boiling for 12–15 min caused the greatest decrease in vitamin-C levels (80%).[Citation15] Our results showed that the decrease in ascorbic acid by boiling was lower compared to that reported by Sikora et al.[Citation15] most probably due to shorter boiling time.
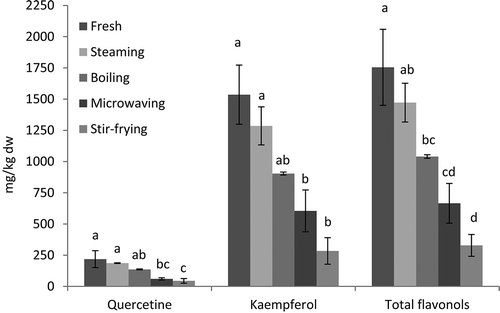
Phenolics are the most important bioactive compounds in kale. Major phenolics in kale are flavonols and hiydroxycinnamic acids. However, flavonols are more abundant than hydroxycinnamic acids and have high degrees of glycosylation and acylation. Ferioli et al.[Citation5] declared that flavonols were over 80% of phenolics in all kale samples. In the present investigation, two flavonols, namely quercetin and kaempferol, were identified and quantified. The effects of cooking treatments on these flavonols were shown in . Fresh kale contained 218.8 mg/kg dw quercetin, 1535.6 mg/kg dw kaempferol, and 1754.4 mg/kg dw total flavonols. Our results regarding the flavonols content were lower than the results reported by Korus and Lisiewska[Citation8] (143 mg/kg fw for quercetin and 596.4mg/kg fw for kaempferol) and Hagen et al.[Citation30] (3190 mg/kg dw for quarcetin, 3430 mg/kg dw for kaempferol in curly kale). This can be related to the high variability of phenolics in Brassica species.[Citation12] While, in our study, steaming resulted in an insignificant decrease in individual and total flavonols contents compared to fresh kale, boiling, microwaving, and stir-frying resulted in significant decreases (p < 0.05). High amount of loss in flavonols was detected in stir-frying (79.7% for quercetin, 81.5% for kaempferol, and 81.3% for total flavonoils). Effect of processing on Flavonols in onion, green beans, and peas was studied by Ewald et al.[Citation31] In onion, high amount of loss in flavonols was determined during the pre-processing step (peeling, trimming, and chopping). In addition, minor changes in flavonols content were reported for onion samples during thermal processing. The samples cooked in microwave oven had lower loss than the samples cooked in water. For peas, boiling resulted in limited reduction in quercetin content. For blanched green beans, different heat treatments caused no consistent difference in flavonids.[Citation31] Pellegrini et al.[Citation12] investigated the effects of different cooking methods (boiling, microwaving, basket steaming, and oven steaming) on flavonols of Brassica vegetables (broccoli, Brussel sprouts, and cauliflower). High ratio of degradations in flavonols (quercetin and kaempferol) of broccoli and kaempferol of cauliflower were observed in micowaved and boiled samples, respectively. On the other hand, cooking procedures led to an increase in flavonoids of Brussel sprouts. These contradictory results were explained by the variant plant matrix and flavonoid derivatives. Flavonoid glucosides and aclayted derivatives were inefficiently extracted from the tissue by the cooking process compared to glucuronide derivatives.[Citation32]
Phenolic compounds have been extensively studied due to their diverse health benefits. Phenolics in plant tissue present in free and bound forms. Fruits and vegetables have the most of their phytochemicals in free and soluble conjugated forms. Bound phenolics comprise an average of 24% of total phenolics present in these food matrices.[Citation33] Kale is a good source of phenolic compounds. However, there is little information about free and bound phenolic content of kale. The free, bound and total phenolics and antioxidant activities of the fresh and cooked kale samples were given in . As mentioned above, another phenolic group in kale is hydroxycinnamic acids. However, kale contains a small amount of hydroxycinnamic acids. In this study, we identified only sinapic acid among hydroxycinnamic acids. This may be due to the absence or very low levels of other hydroxycinnamic acids, which might be lower than the detection limits of photodiode array detector. The concentration of free, bound and total sinapic acids in fresh kale sample were 2.8, 47.6, and 50.5 mg/kg dw, respectively. There was no significant difference in free sinapic acid among fresh, steamed, and microwaved kale. However, free sinapic acid was not detected in boiled and stir-fried kale samples. Compared to fresh kale samples, bound and total sinapic acids significantly decreased after microwaving and stir-frying. Ayaz et al.[Citation34] identified four hydroxycinnamic acid derivatives, which present in kale at the levels of 343, 355, 4746, and 6069 ng/g fw in free, ester, glycoside, and ester-bound forms, respectively. The ratio of the sum of identified phenolic acids by HPLC-MS was accounted as 0.9%. Free and bound sinapic acid values of kale in the present study were higher than the findings of Ayaz et al.[Citation34] (18.7 ng/g fw for free and 8.7 ng/g fw for ester-bound sinapic acid). Sinapic acid value of raw kale sample after enzymatic hydrolysis was calculated as 176.2 mg/kg fw by Korus and Lisiewska.[Citation8] Our result for total sinapic acid content of kale was lower than the finding of Korus and Lisiewska.[Citation8] Chemical hydrolysis may be the cause of the high amount of loss in phenolics, because of rigid conditions such as high temperature and low pH when compared to enzymatic hydrolysis. Fresh kale sample had high total phenolics content (20868.2 mg GAE/kg dw). Ratio of bound phenolics in total phenolics content was 38.1%. Boiling, microwaving, and stir-frying caused significant reductions in free, bound, and total phenolics content of kale when compared to fresh kale sample (p < 0.05). However, the differences between fresh kale and steaming kale samples for free, bound, and total phenolics content were not statistically significant. The highest decrease in bound and total phenolics content of kale was observed in stir-fried samples. 39.3% of total phenolics content and 43.1% of bound phenolic content disappeared in 5 min of stir-frying treatment. However, for free phenolic content, the highest decrease was observed in boiled kale samples (41.6%). Soluble phenolic content of kale sample was probably leached into water. While high decrease in bound phenolic content of kale was observed in microwave processing, low decrease in free phenolic content was detected. This may be due to the release of free phenolics from bound phenolics by the breakdown of cellular constituents and cell walls during microwave process. While free phenolic content of kale decreased in the following order: microwaving > steaming > stir-frying > boiling, bound phenolic content decreased in the following order: steaming > boiling > microwaving > stir-frying. The decline rates of total phenolics content after steaming, boiling, microwaving, and stir-frying were 8.2, 29.2, 15.5, and 39.3%, respectively. The free phenolic content of kale were similar to the data reported by Ilyasoğlu and Burnaz[Citation14] (12690 mg GAE/kg dw). They declared that total phenolics content decreased in the following order: blanching > microwaving > steaming > boiling. The total phenolics contents of kale varieties grown in Italy, Portugal and Turkey were in the range of 785–3430, 2313–5509,1 and 1386–3069 mg/kg fw, respectively.[Citation5] Murador et al.[Citation7] reported that the total phenolics content in fresh kale was 492 mg GAE/kg, and it significantly decreased (31%) by boiling when compared to fresh kale. The change in the DPPH and ABTS values of fresh and cooked kale samples were also given in . Total DPPH radical scavenging activity of fresh kale was 30.4 mmol Trolox eq/g dw. The contribution of free and bound phenolics to the total DPPH radical scavenging activity was 61.2 and 38.8%, respectively. DPPH radical scavenging activity was decreased by all cooking methods. While boiling and stir-frying significantly reduced DPPH values of free phenolic extracts when compared to fresh kale (p < 0.05), steaming and microwaving did not cause any notable loss in DPPH values. Microwaving and stir-frying significantly decreased DPPH values of bound phenolic extracts compared to fresh sample (p < 0.05) but the differences among cooking methods were found to be not significant. The decreases in total DPPH values were 13.2, 29.9, 19.1, and 38.8% for steaming, boiling, microwaving, and stir-frying, respectively. The DPPH value and phenolic content of kale were similarly affected by cooking methods. ABTS values of free and bound phenolic extracts were not significantly influenced by different cooking methods. The reported literatures generally show that phenolic content and antioxidant activity of vegetables decreased during different cooking treatments.[Citation8,Citation10,Citation13] However, several studies indicated that some of the cooking methods enhanced the phenolic content and antioxidant activity of different vegetables.[Citation7,Citation12,Citation35] In this study, phenolic content and antioxidant activity of kale decreased in all samples cooked by different methods. This may be due to the selected processing parameters and their levels in our study.
Table 4. Effects of cooking methods on phenolics and antioxidant activity of kale.
Conclusion
The findings in this study indicate that kale is a good dietary source of health-promoting phytochemicals (ascorbic acid, carotenoid, chlorophyll, and phenolics) and antioxidant activity. Therefore, kale can be considered as a functional food due to its useful ingredients for nutrition support therapy and prevention of different chronic and degenerative disease. While there are some reports indicating increase in the amount of some phytochemicals of plants during cooking, all cooking treatments in this study caused loss in phytochemicals, in comparison with fresh kale. Stir-frying gave the highest degradation ratio for all phytochemicals. Water soluble phytochemicals (ascorbic acid and free phenolics) were significantly decreased by boiling. However, steaming resulted in minor effects on phytochemicals and antioxidant activity of kale. The results show that to preserve the nutritional quality of kale during cooking, steaming is the best procedure.
Funding
Financial support provided by Yüzüncü Yıl University Research Found (2015-FBE-YL204) is gratefully acknowledged.
Additional information
Funding
References
- Singh, B.K.; Sharma, S.R.; Singh, B. Heterosis for Mineral Elements in Single Cross-Hybrids of Cabbage (Brassica Oleracea Var. Capitata L.). Scientia Horticulturae-Amsterdam 2009, 122(1), 32–36.
- Erdem, H.Ü.; Kalın, R; Özdemir, N.; Özdemir, H. Purification and Biochemical Characterization of Peroxidase Isolated from White Cabbage (Brassica Oleracea Var. Capitata F. Alba). International Journal of Food Properties 2015, 18(10), 2099–20109.
- Balkaya, A.; Yanmaz, R. Promising Kale (Brassica Oleracea Var. Acephala) Populations from Black Sea Region, Turkey. New Zealand Journal of Crop and Horticultural Science 2005, 33, 1–7.
- Chenard, C.A.; Zimmerman, M.B.; Smith, K.L.; Nonnie, P.F.; Wahls, T.L. New Measured Weight for One Cup Raw Kale Reduces Nutrient Intake of Individual Following the Wahls™ Diet. Procedia Food Science 2015, 4, 39–47.
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F.; Costa, H.S.; Albuquerque, T.G.; Silva, A.S.; Hayran, O.; Kocaoğlu, B. Comparison of Leafy Kale Populations from Italy, Portugal, and Turkey for Their Bioactive Compound Content: Phenolics, Glucosinolates, Carotenoids, and Chlorophylls. Journal of the Science of Food and Agriculture 2013, 93, 3478–3489.
- Kim, S.Y.; Yoon, S.; Kwon, S.M.; Park, K.S.; Kim, Y.C.L. Kale Juice Improves Coronary Artery Disease Risk Factors in Hypercholesterolemic Men. Biomedical and Environmental Sciences 2008, 21, 91–97.
- Murador, D.C.; Mercadante, A.Z.; de Rosso, V.V. Cooking Techniques Improve the Levels of Bioactive Compounds and Antioxidant Activity in Kale and Red Cabbage. Food Chemistry 2016, 196, 1101–1107.
- Korus, A.; Lisiewska, Z. Effect of Preliminary Processing and Method of Preservation on the Content of Selected Antioxidative Compounds in Kale (Brassica Oleracea L. Var. Acephela) Leaves. Food Chemistry 2011, 129, 149–154.
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. Journal of Agricultural and Food Chemistry 1996, 44(11), 3426–3431.
- Xu, F.; Zheng, Y.; Yang, Z.; Cao, S.; Shao, X.; Wang, H. Domestic Cooking Methods Affect the Nutritional Quality of Red Cabbage. Food Chemistry 2014, 161, 162–167.
- Zhang, D.; Hamauzu, Y. Phenolics, Ascorbic Acid, Carotenoids and Antioxidant Activity of Broccoli and Their Changes during Conventional and Microwave Cooking. Food Chemistry. 2004, 88, 503–509.
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fagliano, V.; Porrini, M. Effect of Different Cooking Methods on Color, Phytochemical, Concentration, and Antioxidant Capacity of Raw and Frozen Brassica Vegetables. Journal of Agricultural and Food Chemistry 2010, 58, 4310–4321.
- Jaiswal, A.K.; Gupta, S.; Abu-Ghannam, N. Kinetic Evaluation of Colour, Texture, Polyphenols and Antioxidant Capacity of Irish York Cabbage After Blanching Treatment. Food Chemistry 2012, 131, 63–72.
- İlyasoğlu, H.; Burnaz, N.A. Effect of Domestic Cooking Methods on Antioxidant Capacity of Fresh and Frozen Kale. International Journal of Food Properties 2015, 18, 1298–1305.
- Sikora, E.; Cieślik, E.; Leszczyńska, T.; Filipiak-Florkiewicz, A.; Pisulewski, P.M. The Antioxidant Activity of Selected Cruciferous Vegetables Subjected to Aquathermal Processing. Food Chemistry 2008, 107, 55–59.
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists: Washington, DC, 2003.
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiology 1949, 24(1), 1–15.
- Chan, H.T. Jr.; Cavaletto, C.G. Aseptically Packaged Papaya and Guava Puree: Changes in Chemical and Sensory Quality during Processing and Storage. Journal of Food Science 1982, 47(4), 1164–1169.
- Lee, H.S.; Coates, G.A. Vitamin C in Frozen, Fresh Squeezed, Unpasteurized, Polyethylene-Bottled Orange Juice: A Storage Study. Food Chemistry 1999, 65, 165–168.
- Park, S.; Arasu, M.V.; Jiang, N.; Choi, S.H.; Lim, Y.P.; Park, J.T.; Al-Dhabi, N.F.; Kim, S.J. Metabolite Profiling of Phenolics, Anthocyanins and Flavonols in Cabbage (Brassica Oleracea Var. Capitata). Industrial Crops and Products 2014, 60, 8–14.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolic with Phosphomolybdic -Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Coloric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic Acids, Syringaldehyde, and Juglone in Fruits of Different Cultivars of Juglans regia L. Journal of Agricultural and Food Chemistry 2005, 53, 6390–6396.
- Pyo, Y.H.; Lee, T.C.; Logendra, L.; Rosen, R.T. Antioxidant Activity and Phenolic Compounds of Swiss Chard (Beta Vulgaris Subspecies Cycla) Extracts. Food Chemistry. 2004, 85, 19–26.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Armesto, J.; Carballo, J.; Martínez, S. Physicochemical and Phytochemical Properties of Two Phenotypes of Galege Kale (Brassica Oleracea L. Var. Acephala cv. Galega). Journal of Food Biochemistry 2015, 39, 439–448.
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Velioglu, Y.S. Effects of Cooking Methods on Chlorophylls, Pheophytins and Colour of Selected Green Vegetables. International Journal of Food Science and Technology 2006, 41, 281–288.
- Humphery, A.J.; Beale. M.H. Terpens. Plant Secondary Metabolites; Croizer, A.; Clifford, M.N.; Ashihara, H.; Eds.; Blackwell Publishing Ltd.: Oxford, 2006; 47–101.
- de Sá, M.C.; Rodriguez-Amaya, D.B. Optimization of HPLC Quantification of Carotenoids in Cooked Green Vegetables-Comparison of Analytical and Calculated Data. Journal of Food Composition and Analysis 2004, 17, 37–51.
- Becerra-Moreno, A.; Alanis-Garza, P.A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Kale: An Excellent Source of Vitamin C, Pro-vitamin A, Lutein and Glucosinolates. Journal of Food 2014, 12(3), 298–303.
- Hagen, S.F.; Borge, G.I.A.; Solhaug, K.A.; Bengtsson, G.B. Effect of Cold Storage and Harvest Date on Bioactive Compounds in Curly Kale (Brassica Oleracea L. Var Acephala). Postharvest Biology and Technology 2009, 51, 36–42.
- Ewalda, C.; Fjelkner-Modig, S.; Johansson, K.; Sjöholm, I.; Akesson, B. Effect of Processing on Major Favonoids in Processed Onions, Green Beans, and Peas. Food Chemistry 1999, 64, 231–235.
- Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. Effect of Postharvest Storage and Processing on the Antioxidant Constituents (Flavonoids and Vitamin C) of Fresh-Cut Spinach. Journal of Agricultural and Food Chemistry 1999, 47, 2213–2217.
- Acosta-Estrada, B.A.; Guttiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Food, A Review. Food Chemistry 2014, 152, 46–55.
- Ayaz, F.A.; Hayırlıoğlu-Ayaz, S.; Alpay-Karaoğlu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic Acid Contents of Kale (Brassica Oleracea Var. Acephala DC.) Extracts and Their Antioxidant and Antibacterial Activities. Food Chemistry 2008, 107, 19–25.
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chemistry 2005, 93, 713–718.