ABSTRACT
Capsicum annum cultivars Yellow cayenne, Portafortuna, Idealino, Sole, Duemila, Pellegrino, Fantasia, Loco, and Effix were investigated for their antioxidant properties and foodborne pathogens inhibitory activity. The total phenols, flavonoids, anthocyanins, capsaicinoids, carotenoids, ascorbic acid, and vitamin E content were also determined. Duemila and Portafortuna cultivars showed the highest total phenol content with values of 924.3 and 935.0 mg of chlorogenic acid equivalents/100 g dried weight, respectively. Idealino pepper presented the highest capsaicin content (2932.1 μg/g dried weight). The antioxidant capacity was evaluated by using 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid test, 2,2-diphenyl-1-picrylhydrazyl test, and ferric reducing ability power assay. Both Pellegrino and Idealino samples exhibited a promising radical scavenging ability against 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid radical with IC50 values of 45.2 and 45.7 μg/mL, respectively. All the extracts were able to inhibit the growth of Staphylococcus aureus and Listeria monocytogenes investigated strains. The most active are Effix and Fantasia peppers with minimal inhibitory concentration between 12.5 and 25.0 mg/mL, respectively. Overall, these results support the use of these pepper species as dried extract in food preservation.
Introduction
The genus Capsicum (Solanaceae) comprises more than 20 species. The most common ones are C. annuum, C. baccatum, C. chinensis, C. frutescens, and C. pubescens. Capsicum species are used as spice since ancient time. It contains a wide array of phytochemicals (mainly carotenoids, phenols, and capsaicinoids) that are subject to variation as a result of the genotype, fruit maturity, agronomic practices, and processing methods. Capsanthin, capsorubin, capsanthin 5,6-epoxide, antheraxanthin, β-cryptoxanthin, β-carotene, cucurbitaxanthin, violaxanthin, and zeaxanthin are all found in the Capsicum genus. Phenols are other important constituents of peppers that exhibit several health benefits. Quercetin, luteolin, and kaempferol were the main abundant aglycones identified in pepper. The main capsaicinoids are capsaicin and dihydrocapsaicin. Other capsaicinoids found in this spice are nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin. Several research articles evidenced the nutritional value and the antioxidant and antimicrobial activities of Capsicum species.[Citation1–Citation6]
Dehydration is a process applied to spice to reduce the microbial spoilage reactions, without affecting the texture or physical properties of the food or the content in bioactive compounds.[Citation7] Several methods are used for dried peppers; however, the traditional one, and the widely used by consumers, is to hang the fruits on a thread and allow them to dry under sunlight in the open air.
The immediate reason for spice use is to enhance food palatability. More than 4500 recipes from 36 different countries in their traditional cookbooks contain the use of spice and particularly Capsicum spp. not only for its organoleptic character but also for its antibacterial properties, since member of this genus are able to kill or inhibit up to 70% of bacteria.[Citation4]
The problem of foodborne diseases, which implicate morbidity and mortality, is distributed worldwide. The contamination of a food matrix can cause a global burden of foodborne diseases in numerous countries as a consequence of food marketing globalization. Staphylococcus aureus and Listeria monocytogenes represent two of the most common foodborne pathogens. Enterotoxin-producing S. aureus strains can contaminate food by direct contact by food handlers, meat grinders, knives, storage containers, and cutting blocks. Food matrices that are more contaminated by this commensal and opportunistic pathogen are milk and dairy products, meat, poultry and eggs, salads, pastries and cakes, etc. In order to prevent the contamination, the ideal processing temperature should be below 5°C and above 60°C, respectively.[Citation8] Listeria monocytogenes contamination is one of the leading microbiological causes of food recalls, mainly of seafood, meat, poultry, and dairy products. This organism is resistant to the most common processing techniques and food storage for this reason it is a significant threat to public health.[Citation9]
During the last decades, the food industry has been increasingly incorporating food-preservative compounds since they significantly improve the color, aroma, taste and shelf life of food matrix. Comparing with synthetic food preservatives, natural preservatives, such as spices, are gaining more and more acceptance from consumers.[Citation10] These spices, besides the role of preserving agents, offer other additional nutritional benefits arising from their antioxidant power. Antioxidants are used as food additives to prevent and delay the negative effects of oxygen in food matrix.[Citation11] Moreover, several research and clinical studies evidenced that antioxidant compounds from diet show benefits in cardiovascular disease, metabolic syndrome, cancer, and neurodegenerative disease prevention.[Citation12]
The purpose of the study was threefold: (1) to examine the micronutrient content of sun-dried C. annum fruits; (2) to evaluate the antioxidant properties in terms of food preservatives and healthy food matrix; (3) to determine the antimicrobial activity against some of the most important foodborne pathogens such as Staphylococcus aureus, Listeria monocytogenes. Therefore, we focused our interest on the determination of the total anthocyanins, ascorbic acid, carotenoids, flavonoids, phenols, and vitamin E content; capsaicin and dihydrocapsaicin were also quantified. Pepper extracts were subjected to evaluation of antioxidant activity by using different in vitro assays namely 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS•+) radical, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), ferric reducing ability power (FRAP) and β-carotene bleaching test. The investigation of antimicrobial activity was performed by microdilution method.
Materials and methods
Chemicals and reagents
All chemicals and reagent were purchased from Sigma-Aldrich S.p.a. (Milan, Italy). Brain Heart Infusion Broth (BHI) was obtained from Oxoid-Thermofisher (Rodano, Italy). Analytical grade solvents were bought from VWR International s.r.l. (Milan, Italy).
Sample preparation
The nine Capsicum annum cultivars investigated in this research project were harvested from a Miceli farm (Scalea, Cosenza, Italy) and picked in in October 2013 at maturity stage from a random sample of 25 plants in order to obtain a set of representative samples. Peppers of each cultivar (500 g) were distributed as a thin layer in a stainless steel trays and dried under direct sunlight at temperatures in the range 25–38°C for 2 weeks. Dried peppers were extracted at room temperature by ethanol three times (500 mL × 48 h). reported the pepper cultivar, the color, length and width, and yield of extraction (%).
Table 1. Investigated Capsicum annum cultivars.
Total phenols, flavonoids, anthocyanins, and carotenoids content
Total phenols content of dried C. annum samples was determined using the Folin–Ciocalteau method.[Citation13] Results were expressed as chlorogenic acid equivalents in mg/100 g dried weight (DW). The flavonoids content was determined following the procedure previously reported.[Citation13] The content was expressed as quercetin equivalents in mg/100 g DW. The total carotenoids content was evaluated following the procedure previously described.[Citation13] The total carotenoids content was expressed as β-carotene equivalents in mg/100 g DW. Total monomeric anthocyanins content was determined using the pH-differential method.[Citation14] Results are expressed as mg of cyanidin 3-glucoside equivalents/100 g DW.
Determination of vitamin E content
The vitamin E content was investigated by gas chromatography-mass spectrometry (GC-MS) analyses. GC-MS analyses were carried out following the methodology applied by Loizzo et al.[Citation14] Results are expressed as mg/100 g DW.
Determination of capsaicin and dihydrocapsaicin content
The content of the two main capsaicinoids present in Capsicum species was evaluated by using the methodology previously reported.[Citation15] Results are expressed as μg/g DW.
Determination of ascorbic acid content
The content of ascorbic acid in dried pepper samples was determined according to the method of Klein and Perry[Citation16] and expressed as mg/100 g DW.
Evaluation of antioxidant activities
The antioxidant activities of C. annum dried samples were assessed by using several in vitro assays namely ABTS, DPPH, FRAP and β-carotene bleaching tests).[Citation17] ABTS and DPPH tests were used for the evaluation of the free radical scavenging ability of samples. ABTS+ radical was obtained by the reaction of ABTS solution (7 mM) with potassium persulphate (2.45 mM). The mixture was stored in the dark at room temperature for 12 h before use. Ethanol was added to radical solution up to an absorbance of 0.70 at 734 nm. In DPPH test, a mixture of 0.25 mM DPPH and sample was prepared and left at 25°C for 30 min. Absorbance was read at 517 nm. The ABTS and DPPH radicals scavenging ability was expressed as inhibitory concentration 50% (IC50) value.
In the FRAP test ethanol solutions of FeSO4 (50–500 mM), were used for obtaining the calibration curve. The absorbance of the reaction mixture was measured at 595 nm and FRAP value was expressed as mM Fe(II)/g. The last applied test was the β-carotene bleaching test in which mixture of linoleic acid, Tween 20, and β-carotene was prepared. After evaporation of solvent and dilution with water, the emulsion was added into tubes containing sample and incubated at 45°C for 60 min. The absorbance was measured at 470 nm at 30 and 60 min of incubation. Results were expressed as IC50 values. Ascorbic acid was used as positive control in ABTS and DPPH tests, BHT in FRAP assay and propyl gallate in β-carotene bleaching test.
Evaluation of antimicrobial activity
The antimicrobial activity of the dried peppers extract was evaluated on Staphylococcus aureus SA3, SA4 and SA6, on Listeria monocytogenes LM4 and LM7 and on type strain ATCC 7644. All the isolates were previously isolated from meat products and belonged to the collection of the Faculty of Bioscience, University of Teramo. Strains were cultivated twice in BHI broth overnight at 30°C, then they were washed three times in phosphate buffer saline (PBS) 50 mM, pH 7.2, afterward, the inocula were standardized at about 1 × 106 cells/mL, as described by Mazzarrino et al.[Citation18] Pepper extracts were suspended in dymethylsolfoxide (DMSO) to obtain stock concentrations of 100 mg/mL. The antibacterial activity was evaluated by microdilution method, according to Clinical Laboratory Standards Institute (CLSI) guidelines.[Citation19] Each determination was repeated three times. The minimal inhibitory concentration (MIC) was calculated as the lowest pepper extract concentration able to inhibit microbial growth in BHI broth after 48 h at 30°C.
Statistical analysis
Data were statistically analyzed by one-way analysis of variance (ANOVA) test and Dunnet’s test (p < 0.05). IC50 values were calculated by plotting the percentage of inhibition versus concentrations by using Prism GraphPad (GraphPad Software, San Diego, CA, USA). Studies of the Pearson’s correlation coefficient (r) and linear regression, assessment of repeatability, calculation of average and relative standard deviation were performed using Microsoft Excel 2010 software.
Results and discussion
Phytochemical results
reported the C. annum cultivars color, length, width, and extraction yield. All investigated peppers are red except Yellow cayenne that is yellow. Portafortuna is the longer (21.8–24.2 cm), followed by Yellow cayenne (9.2–10.3 cm), and Idealino (8.2–9.8 cm). The other peppers are 2–5 cm long. The width ranged from 0.7 to 1.9 cm.
The yield of extraction of C. annum samples ranged from 15.0 to 27.9 for Sole and Effix, respectively (). reported the ascorbic acid, anthocyanins, capsaicinoids, carotenoids, flavonoids, phenols, and vitamin E content of dried pepper extracts. Duemila and Portafortuna extracts exhibited the highest total phenols content with values of 924.3 and 935.0 mg of chlorogenic acid equivalent/100 g DW, respectively. The lowest values were recorded for the Loco, Sole, and Yellow cayenne cultivars (193.8, 141.2, and 116.9 mg of chlorogenic acid equivalents/100 g DW, respectively).
Table 2. Micronutrient content in Capsicum annum cultivars.
Effix, Pellegrino, and Portafortuna cultivars showed the highest flavonoids content (values of 213.4, 206.3, and 202.1 mg quercetin equivalents/100 g DW, respectively). Except for Fantasia peppers (71.2 mg quercetin equivalents/100 g DW), Loco, Sole, and Yellow cayenne cultivars, characterized by the lowest phenols content, showed also the lowest flavonoids content (85.5, 69.0, and 53.3 mg quercetin equivalents/100 g DW, respectively).
Pigmentation of Capsicum species is mainly related to their content in anthocyanins and carotenoids. Purple is the most frequent color observed in peppers, although it is possible to find pepper magenta and black. displays a total carotenoids content with values ranged from 14.5 to 25.2 mg/100 g DW for Yellow cayenne and Duemila cultivar, respectively. Anthocyanins content is ranged from 26.3 to 37.5 mg/100 g DW for Pellegrino and Sole, respectively. The only yellow pepper, Yellow cayenne, showed the lowest carotenoids content (14.5 mg β-carotene equivalents/100 g DW). The same statement cannot be made to the content of anthocyanins.
Vitamin E refers to a group of fat-soluble compounds namely tocopherols and tocotrienols. As a fat-soluble antioxidant, this vitamin stops the production of reactive oxygen species formed when fat undergoes oxidation. GC-MS analysis showed a vitamin E content from 18.8 to 25.3 mg/100 g DW, for Loco and Duemila pepper, respectively.
C. annuum is the major source of capsaicinoids, a member of alkaloids group responsible of the pungency sensation. The main abundant capsaicinoids are capsaicin and dihydrocapsaicin that are responsible of the 90% of pungency.[Citation20] As reported in capsaicin ranged from 344.3 to 2932.1 μg/g DW for Sole and Idealino pepper, respectively. A significant amount of capsaicin was detected also in Yellow cayenne cultivar (1147.7 μg/g DW). Regard dihydrocapsaicin the cultivar that exhibited the highest value are Pellegrino and Idealino with 1291.2 and 1053.5 μg/g DW (). A low content of dihydrocapsaicin was found in Portafortuna pepper.
Peppers had ascorbic acid content four times higher than oranges that are considered the most important source for this healthy compound. Several scientific evidences indicate that the intake of ascorbic acid is associated with a reduced risk of chronic diseases, probably due to its ability to scavenge free radicals.[Citation21] Our results demonstrated that all investigated cultivars exhibited a similar ascorbic acid content with the highest value in Pellegrino cultivar (5.4 mg/100 g DW). The great variability (similar, lower, or higher) in bioactive content of C. annum samples in relation to data reported in literature could be explained by variation of cultivar, maturity stage, use of fresh of processed pepper, way of extraction, sample preparation (whole fruits or fruits without seed), etc.
In our previous works we have investigated the total phenols, flavonoids, and capsaicinoids content of the same C. annuum cultivars fresh and after boiling and blanching.[Citation22] Comparison of data highlights that all phytochemicals increased their concentration in dried pepper samples as consequence of weight loss. The high amount of phenols and flavonoids in dried samples could be not explained by enzyme inactivation since polyphenol oxidase is still active at 55–60°C that it is a range of temperature much higher than that obtained with sun-drying process.[Citation23] According to Kozukue et al.[Citation24] and in comparison with previously published data sun-drying process cause an increase in the capsaicinoids concentration. Several works investigated the effect of drying process at different temperatures rather than the traditional sun-dried process. Ozgur et al.[Citation25] investigated the phytochemical content of green and red pepper (C. annum cv Yalova Yaglik 28) fresh and dried. A total phenol content of 89.82 mg/gallic acid equivalent (GAE) g DW was found for dried red pepper. The same pepper showed an ascorbic acid content of 108.65 mg/100 g DW.
The effect of dried temperatures (50–90°C) on the C. annuum var. Hungarian total phenols and ascorbic acid content was investigated.[Citation26] Results clearly evidenced a reduction of both phytochemicals in relation to the increase of temperature with a maximum loss at 90°C. Our data are lower than those previously reported by Hervert-Hernández et al.[Citation27] for C. annum cv Arbol, Chipote, Guajillo, and Morita that exhibited a total phenols and carotenoid content in the range from 2325.4 to 2843.5 mg/100 g DW for Guajillo and Arbol, and from 87.6 to 373.3 mg/100 g DW for Guajillo and Morita, respectively. More recently, Lutz et al.[Citation28] reported the total phenol and antocyanin content of dried fruit of C. annuum, green, and red cv. Almuden. In agreement with our results dehydrated samples are richest in both phytochemicals (6.9 versus 58.3 mg GAE/g and 7.1 versus 62.6 mg cyaniding-3-glucoside/100 g for total phenols and antocyanin in green and red pepper, respectively). The effects of the drying process on total phenol and flavonoid content were studied in Moroccan red pepper cultivar (C. annuum).[Citation29] Results evidenced that drying in a hot-air oven induced a significant loss of both total phenol and flavonoid content with particular reference to epicatechin, cyanidin-3-O-galactoside, phloridzin, and quercetin glycosides concentrations. The effect of drying temperature (60 and 70°C) and time (0–35 h) on total phenolic content of air-dried red chili spur pepper (C. annuum cv Jinda) was studied.[Citation30] A decrease in total phenolic content was observed during the early stage of drying. In particular, at day 14 of storage, those dried at 60 and 70°C exhibited a reduction in total phenols of 14.5 and 18.6%, respectively, as compared to day 0 of storage. In spite of that, prolonged heating brought about an increase in phytochemical content.
Antioxidant properties
The oxidative deterioration in food matrix is one of the major detrimental process. It conduces to the development of undesirable compounds such as organic acids, ketones, and aldehydes, which confer to the food off-flavor such as rancid odor.[Citation31] Moreover, the development of compounds with a possible toxic character reduced the nutritional quality and safety of foods.[Citation32] Based on the consumer’s demand of natural antioxidant that are considered safer, researchers address their attention on the possibility to demonstrate the antioxidant potential of some spice extract. According to the American Spice Trade Association, Capsicum is the most widely used spice. For this reason, the antioxidant potential of different Capsicum species is largely investigated.[Citation1,Citation3,Citation5,Citation13,Citation22,Citation33]
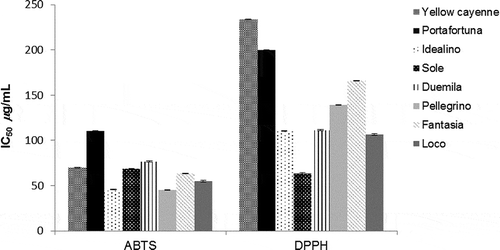
In the current experiment, all dried peppers showed a concentration–antioxidant relationship. Radical scavenging activity was assessed by ABTS and DPPH assay. Pellegrino and Idealino cultivars showed the highest radical scavenging power against ABTS+ with IC50 values of 45.2 and 45.7 μg/mL, respectively (), followed by Loco peppers (IC50 of 54.6 μg/mL). Correlation analysis revealed that, except for capsaicin and dihydrocapsaicin, all investigated compounds positively correlated with ABTS+ scavenging activity. Among phytochemicals, carotenoids and phenols exhibited the highest Pearson’s coefficient value (r = 0.7 and 0.6, respectively). In DPPH test, the most promising sample resulted Sole cultivar that exhibited an IC50 value of 63.7 μg/mL, followed by Loco, Idealino, and Duemila with IC50 values in the range 106.1–111.3 μg/mL (, ). Analysis of correlation data evidenced that carotenoids are the main responsible of the DPPH· scavenging ability (r = 0.57). In our previous works, fresh C. annuum cultivars were tested for radical scavenging ability.[Citation26] In ABTS assay, generally the IC50 values of dried peppers were lower than those reported for fresh samples, except for Fantasia, Loco, and Sole cultivars. On the contrary, fresh samples resulted more active in scavenging the DPPH· except dried Sole pepper (IC50 of 33.1 versus 63.7 μg/mL, respectively). The high radical scavenging ability of dried samples respect fresh one is in agreement with those reported by Arslan and Özcan[Citation34] that found in DPPH radical scavenging ability a percentage of 48.0 and 76.14% for fresh and dried pepper, respectively. A significant DPPH radical scavenging activity was evidenced also for dried C. annuum cv guajillo extracts (24.2%) followed by cv pasilla and cv ancho peppers (15.6 and 12.3%). The differences in the antioxidant activity can be attributed to the higher β-carotene and β-cryptoxanthin contents of guajillo peppers.[Citation35] A similar result was obtained also by Lutz et al.[Citation28] that evidenced significantly differences in the DPPH radical scavenging ability between fresh and dehydrated samples in both green and red pepper.
Table 3. Antioxidant activities of Capsicum annum cultivars.
Figure 1. Radical scavenging ability of nine dried C. annuum cultivars evaluated by ABTS and DPPH assays.
In β-carotene bleaching test, at 30 min of incubation the IC50 values of investigated peppers ranged from 6.3 to 11.6 μg/mL for Fantasia and Yellow cayenne, respectively. Values ranged from 7.9 to 31.7 μg/mL for Loco and Portafortuna pepper, respectively, were recorded at 60 min of incubation. The correlation analysis revealed that phenols positively correlated with β-carotene bleaching test (r = 0.5 and 0.6 at 30 and 60 min incubation, respectively). A positive Pearson’s correlation coefficient was found with carotenoids at 60 min incubation (r = 0.7). The comparison of data between fresh and dried samples in β-carotene bleaching test evidenced also a reduction in IC50 values for all peppers, except for Sole and Portafortuna cultivars after 30 and 60 min of incubation, respectively. Analysis of correlation data evidenced that phenols showed the highest Pearson’s coefficient value (r = 0.7 at both times of incubation).
In our previous work we have investigated the antioxidant potential of air-dried C. annuum var. acuminatum medium and big by using different in vitro methods. Medium pepper exhibited the best free radical scavenging activity against DPPH with an IC50 value of 85.3 μg/mL while big pepper showed the highest scavenging effect against ABTS (IC50 value of 16.4 μg/mL).[Citation36] Big pepper showed, also, the highest inhibition of linoleic acid oxidation (IC50 values of 1.2 and 2.9 μg/mL at 30 and 60 min of incubation, respectively). Both values are quite similar to those reported for the positive control propyl gallate (IC50 value of 1.0 μg/mL at 30 min of incubation).
During Fenton reaction iron is involved into redox processes that generates oxygen free radical.[Citation37] One of the possible approach to limit ROS is to reduce iron. Among investigated samples, Idealino and Loco showed the highest FRAP value with 43.9 and 40.5 μM Fe(II)/g), respectively. A positive Pearson’s correlation coefficient of 0.7 and 0.6 was found for capsaicin and dihydrocapsaicin, respectively. Both capsaicin that diidrocapsaicin play antioxidant activity due to the ability to donate hydrogens or reduced metals.[Citation38]
Antimicrobial activity
All the tested extracts inhibited the growth of three Staphylococcus aureus and three Listeria monocytogenes strains, with MIC lower or equal than 50 mg/mL (). The most effective extracts were those obtained from cultivars Effix and Fantasia with MIC between 12.5 and 25.0 mg/mL. Other authors investigated the antimicrobial activity of extracts obtained from different Capsicum species. Acero-Ortega et al.[Citation39] established the positive effect of three C. annuum L. varieties on the inhibition of L. monocytogenes, correlating their activity with the presence of high amounts of cinnamic and caffeic acids. Dorantes et al.[Citation40] also confirmed the antimicrobial activity of several cultivars against S. aureus and L. monocytogenes, with the latter as the most sensitive. Recently, dihydrocapsaicin exerted a selective antimicrobial activity, being more active on Gram positive bacteria, in relation to their cell wall composition and structure, mainly composed by peptidoglycan.[Citation41] Our results are in line with those observations, as the most effective extracts, Effix and Fantasia, were not particularly rich in capsaicin and dihydrocapsaicin, as Idealino, nor in phenolic substances, generally responsible of distortion and disruption in the structure, and, therefore, in the functionality of the cytoplasmic membrane.[Citation42] Because of this antimicrobial action against foodborne pathogens, the application of C. annuum extracts has already been proposed in food matrices, such as minced raw beef to control the growth of pathogenic microorganisms.[Citation43]
More recently, Gayathri et al.[Citation44] demonstrated the ability of C. chinense acetone and acetonitrile extracts to inhibit the growth of Klebsiella pneumonia and Staphylococcus aureus through agar well diffusion method.
Table 4. Antimicrobial activity of Capsicum annum cultivars against S. aureus and L. monocytogenes (MIC values expressed as mg/mL).
Conclusions
This study reported for the first time the phytochemicals content and the bioactivity of nine C. annuum cultivars namely Yellow cayenne, Portafortuna, Idealino, Sole, Duemila, Pellegrino, Fantasia, Loco, and Effix. Peppers were investigated after sun-drying process. Dehydration has been used widely used as method of food preservation. The comparison of our data with those reported in literature evidenced that the applied process did not negatively affect the phytochemicals content and consequently the bioactivity of peppers. Obtained data support the hypothesis that the synergism between flavonoids and other phenols is responsible for the antioxidant effects while the synergism of capsaicin and dihydrocapsaicin with other minor components, as already proposed by other researchers, is correlate with the antimicrobial activity. Further studies could be done in order to identify phytochemicals responsible of the antioxidant and antimicrobial activities, and to comprehend the possible application in real food matrices.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
The authors wish to thank Dr. Domenico Sturino, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, I-87036 Rende (CS), Italy for the proof-reader version of the manuscript.
References
- Bae, H.; Jayaprakasha, G.K.; Jifon, J.; Patil, B.S. Variation of Antioxidant Activity and the Levels of Bioactive Compounds in Lipophilic and Hydrophilic Extracts from Hot Pepper (Capsicum spp.) Cultivars. Food Chemistry 2012, 134, 1912–1918.
- Liao, C.H. Control of Foodborne Pathogens and Soft-Rot Bacteria on Bell Pepper by Three Strains of Bacterial Antagonists. Journal of Food Protection 2009, 72, 85–92.
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Statti, G.A.; Menichini, F. Influence of Ripening Stage on Health Benefits Properties of Capsicum Annuum var. Acuminatum L.: In Vitro Studies. Journal Medicinal Food 2008, 11, 184–189.
- Omolo, M.A.; Wong, Z.-Z.; Mergen, A.K.; Hastings, J.C.; Le, N.C.; Reiland, H.A.; Case, K.A.; Baumler, D.J. Antimicrobial Properties of Chili Peppers. Infectious Diseases and Therapy 2014, 2, 145–153.
- Pugliese, A.; O’Callaghan, Y.; Tundis, R.; Galvin, K.; Menichini, F.; O’Brien, N.; Loizzo, M.R. In Vitro Assessment of the Bioaccessibility of Carotenoids from Sun-Dried Chilli Peppers. Plant Foods for Human Nutrition 2014, 69, 8–17.
- Srinivasan, K. Antioxidant Potential of Spices and Their Active Constituents. Critical Reviews in Food Science and Nutrition 2014, 54, 352–372.
- Chenlo, F.; Chaguri, L.; Santos, F.; Moreira, R. Osmotic Dehydration/Impregnation Kinetics of Padrón Pepper (Capsicum Annuum L. Longum) with Sodium Chloride Solutions: Process Modelling and Colour Analysis. Food Science and Technology International 2006, 12, 221–227.
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus Aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. BioMed Research International 2014, Article ID 827965, 1–9.
- Jemmi, T.; Stephan, R. Listeria Monocytogenes: Food-Borne Pathogen and Hygiene Indicator. Revue Scientifique et Technique 2006, 25, 571–580.
- Nabavi, S.F.; Nabavi, S.M.; Setzer, W.N.; Nabavi, S.A.; Nabavi, S.A.; Ebrahimzadeh, M.A. Antioxidant and Antihemolytic Activity of Lipid-Soluble Bioactive Substances in Avocado Fruits. Fruits 2013, 68, 185–193.
- Bonilla, J.; Atarés, L.; Chiralt, A.; Vargas, M. Recent Patents on the Use of Antioxidant Agents in Food. Recent Patents on Food, Nutrition & Agriculture 2011, 3, 123–132.
- Paur, I.; Carlsen, M.H.; Halvorsen, B.L.; Blomhoff, R. Antioxidants in Herbs and Spices: Roles in Oxidative Stress and Redox Signaling. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd Ed, Chapter 2; Benzie, I.F.F.; Wachtel-Galor, S.; Eds.; CRC Press/Taylor and Francis: Boca Raton, FL, 2011.
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; De Luca, D.; O’Brien, N.; Menichini, F.; Tundis, R. Influence of Drying and Cooking Process on the Phytochemical Content, Antioxidant and Hypoglycemic Properties of Two Bell Capsicum Annum L. Cultivars. Food and Chemical Toxicology 2013, 53, 392–401.
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers: A Rich Source of Phytochemicals with Antioxidant and Hypoglycemic Properties. Journal of Agricultural and Food Chemistry 2016, 64, 2467–2474.
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.A.; De Cindio, B.; Houghton, P.J.; Menichini, F. The Influence of Fruit Ripening on the Phytochemical Content and Biological Activity of Capsicum Chinense Jacq. cv Habanero. Food Chemistry 2009, 114, 553–560.
- Klein, B.P.; Perry, A.K. Ascorbic Acid and Vitamin A Activity in Selected Vegetables from Different Geographical Areas of the United States. Journal of Food Science 1982, 47, 941–945.
- Loizzo, M.R.; Rashed, K.; Said, A.; Bonesi, M.; Menichini, F.; Tundis, R. Antiproliferative and Antioxidant Properties of Alhagi Maurorum Boiss (Leguminosae) Aerial Parts. Industrial Crops and Products 2014, 53, 289–295.
- Mazzarrino, G.; Paparella, A.; Chaves-López, C.; Faberi, A.; Sergi, M.; Sigismondi, C.; Compagnone, D.; Serio, A. Salmonella Enterica and Listeria Monocytogenes Inactivation Dynamics after Treatment with Selected Essential Oils. Food Control 2015, 50, 794–803.
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement M100-S21. LSI: Wayne, PA, 2011.
- Imaizumi, K.; Sato, S.; Kumazawa, M.; Arai, N.; Aritoshi, S.; Akimoto, S.; Sakakibara, Y.; Kawashima, Y.; Tachiyashiki, K. Capsaicinoids-Induced Changes of Plasma Glucose, Free Fatty Acid and Glycerol Concentrations in Rats. Journal of Toxicological Sciences 2011, 36, 109–116.
- Liu, R.H. Dietary Bioactive Compounds and Their Health Implications. Journal of Food Science 2013, 78, A18–A25.
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of Chemical Profile and Antioxidant Activity of Twenty Cultivars from Capsicum Annuum, Capsicum Baccatum, Capsicum Chacoense and Capsicum Chinense: A Comparison Between Fresh and Processed Peppers. LWT 2015, 64, 623–631.
- Madrau, M.A.; Piscopo, A.; Sanguinetti, A.M.; Caro, A.D.; Poiana, M.; Romeo, F.V.; Piga, A. Effect of Drying Temperature on Polyphenolic Content and Antioxidant Activity of Apricots. European Food Research and Technology 2009, 228, 441–448.
- Kozukue, N.; Han, J.S.; Kozukue, E.; Lee, S.J.; Kim, J.A.; Lee, K.R.; Levin, C.E.; Friedman, M. Analysis of Eight Capsaicinoids in Peppers and Pepper-Containing Foods by High-Performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry. Journal of Agricultural and Food Chemistry 2005, 53, 9172–9181.
- Ozgur, M.; Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. Functional Compounds and Antioxidant Properties of Dried Green and Red Peppers. African Journal of Agricultural Research 2011, 6(25), 5638–5644.
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemusmondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of Air-Drying Temperature on Physico-Chemical Properties, Antioxidant Capacity, Colour and Total Phenolic Content of Red Pepper (Capsicum Annuum, L. var. Hungarian). Food Chemistry 2009, 117, 647–653.
- Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. Bioactive Compounds of Four Hot Pepper Varieties (Capsicum Annuum L.), Antioxidant Capacity, and Intestinal Bioaccessibility. Journal of Agricultural and Food Chemistry 2010, 58, 3399–3406.
- Lutz, M.; Hernández, J.; Henríquez, C. Phenolic Content and Antioxidant Capacity in Fresh and Dry Fruits and Vegetables Grown in Chile, CyTA. Journal of Food 2015, 13, 541–547.
- Salima, N.; Ouatmane, A.; Latrache, H.; Ennahli, S.; Zinelabidine, L.H.; Hanine, H. Bioactive Components and Antioxidant Activity of Moroccan Paprika (Capsicum Annuum L.) Under Different Storage Time and Conditions. Turkish Journal of Agricultural and Natural Sciences 2014, 1, 734–742.
- Chittapraphai, B.; Piansri, W.; Mahawanich, T. Effect of Drying Conditions on Total Phenolic Content and Antioxidant Activity of Chili Spur Pepper. Proceedings of AFHW 2013, International Symposium on Agri-Foods for Health and Wealth, Golden Tulip Sovereign Hotel, Bangkok, Thailand, August 5–8, 2013.
- André, C.; Castanheira, I.; Cruz, J.M.; Paseiro, P.; Sanches-Silva, A. Analytical Strategies to Evaluate Antioxidants in Food: A Review. Trends Food Science Technology 2010, 21, 229–246.
- Moure, A.; Cruz, J.M.; Franco, D.; Domõânguez, J.M.; Sineiro, J.; Dominguez, H.; Núňez, M.J.; Parajó, J.C. Natural Antioxidants from Residual Sources. Food Chemistry 2001, 72, 145–171.
- Materska, M.; Perucka, I. Antioxidant Activity of the Main Phenolic Compounds Isolated from Hot Pepper Fruit (Capsicum Annuum L). Journal of Agricultural and Food Chemistry 2005, 53, 1750–1756.
- Arslan, D.; Ozcan, M.M. Dehydration of Red Bell-Pepper (Capsicum Annuum L.): Change in Drying Behavior, Colour and Antioxidant Content. Food Bioproduct Processing 2011, 89, 504–513.
- Hernández-Ortega, M.; Ortiz-Moreno, A.; Hernández-Navarro, M.D.; Chamorro-Cevallos, G.; Dorantes-Alvarez, L.; Necoechea-Mondragón, H. Antioxidant, Antinociceptive, and Anti-Inflammatory Effects of Carotenoids Extracted from Dried Pepper (Capsicum Annuum L.) Journal of Biomedicine and Biotechnology 2012, Article ID 524019, 1–10.
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; De Luca, D.; Menichini, F. Air-Dried Capsicum Annuum var. Acuminatum Medium and Big: Determination of Bioactive Constituents, Antioxidant Activity and Carbohydrate-Hydrolyzing Enzymes Inhibition. Food Research International 2012, 45, 170–176.
- Puntarulo, S. Iron, Oxidative Stress and Human Health. Molecular Aspects of Medicine 2005, 26, 299–312.
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendino, G.; Ballero, M.; Dessi, M.A. Antioxidant Activity of Capsinoids. Journal of Agricultural and Food Chemistry 2002, 50(25), 7396–7401.
- Acero-Ortega, C.; Dorantes-Alvarez, L.; Hernández-Sánchez, H.; Gutiérrez-López, G.; Aparicio, G.; Jaramillo-Flores, M. Evaluation of Phenylpropanoids in Ten Capsicum Annuum L Varieties and Their Inhibitory Effects on Listeria Monocytogenes Murray, Webb and Swann Scott A. Food Science and Technology International 2005, 11, 5–10.
- Dorantes, L.; Colmenero, R.; Hernandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of Growth of Some Foodborne Pathogenic Bacteria by Capsicum Anuum Extracts. International Journal of Food Microbiology 2000, 57, 125–128.
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Galindo Gomes, J.E.; Falcão, R.E.A.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum Frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434–5447.
- Serio, A.; Chaves-López, C.; Martuscelli, M.; Mazzarrino, G.; Di Mattia, C.; Paparella, A. Application of Central Composite Design to Evaluate the Antilisterial Activity of Hydro-Alcohol Berry Extract of Myrtus Communis L. LWT 2014, 58(1), 116–123.
- Careaga, M.; Fernández, E.; Dorantes, L.; Mota, L.; Jaramillo, M.E.; Hernandez-Sanchez, H. Antibacterial Activity of Capsicum Extract Against Salmonella Typhimurium and Pseudomonas Aeruginosa Inoculated in Raw Beef Meat. International Journal of Food Microbiology 2003, 83, 331–335.
- Gayathri, N.; Gopalakrishnan, M.; Sekar, T. Phytochemical Screening and Antimicrobial Activity of Capsicum Chinense Jacq. International Journal of Advances in Pharmaceutics 2016, 5, 12–20.
