ABSTRACT
Recently a great deal of interest has been developed towards natural bioactive components from seaweeds as functional food ingredients. Antioxidant, anti-inflammatory, antidiabetic, and antihypertensive activities of ethyl acetate:methanol and chloroform solvent fractions of the brown seaweed Sargassum wightii were evaluated using different in vitro systems. The ethyl acetate:methanol fraction registered greater Fe2+ ion chelating ability (IC50 0.52 mg/ml), and were effective in stabilizing the 2,2-azino-bis-3-ethylbenzothiozoline-6-sulfonic acid (IC50 0.82 mg/mL), and 1,1-diphenyl-2-picryl hydrazil radicals (IC50 0.32 mg/mL) than those derived from chloroform solvent fractions. The chloroform solvent fraction showed greater angiotensin converting enzyme-I inhibitory activity (IC50 0.084 mg/mL), while the ethyl acetate:methanol fraction exhibited greater anti-COX-1, 2, and 5-LOX (IC50 0.03-0.05 mg/mL) and DPP-4 inhibitory (IC50 ~ 0.013 mg/mL) properties. A significant co-linearity was recorded between target bioactive properties and the electronegative groups appeared in the downfield space of the nuclear magnetic resonance spectra of the ethyl acetate:methanol and chloroform solvent fractions from S. wightii.
Introduction
Reactive oxygen species (ROS), which are generated in living organisms during various metabolic processes, achieved an immense attention during the last two decades in medical field. Excessive amounts of ROS may be harmful because they can initiate biomolecular oxidations, which lead to the cell injury and death, and create oxidative stress.[Citation1] The oxidative stress results in numerous diseases and disorders, such as, myocardial infarction, diabetes, inflammation, and hypertension. Increased oxidative stress is also a widely accepted participant in the development and progression of diabetes and its complications.[Citation2] One of the long-term complications of type-2 diabetes is high blood pressure, or hypertension, and one of the most important intermediary factors for controlling hypertension is the action of the angiotensin converting enzyme (ACE).[Citation3] Inhibition of ACE is considered as a useful therapeutic approach in the treatment of high blood pressure.
Many synthetic drugs have been developed to remediate oxidative stress. However, the factors, such as greater cost, lack of availability and side effects of these, large populations cannot afford to get benefit from these drugs. In this context natural antioxidants have prominent effect because they are free from side effects, less expensive, and abundantly available. Dietary ingestion of seaweeds has been shown to decrease blood pressure in humans and also have strong antioxidant properties,[Citation4] antimicrobial activity,[Citation5] and antiviral properties.[Citation6] Seaweeds have long been part of the traditional diet of coastal communities. It is widely consumed in East Asia, particularly in Japan, China, and Korea. Studies demonstrated that in Japan the rate of mortality is less, and, hence, their life expectancies are very high. It is reasonable to evaluate this result, because the Japanese are regular consumers of seaweeds.
Seaweed derived functional food ingredients have potential pharmaceutical advantages, and is endowed with pluralities of bioactivities against different diseases. The rich diversity of brown seaweeds belonging to the genus Sargassum speaks to an untapped reservoir of their bioactive properties with significant pharmaceutical and biomedical utilization. The present work anticipated to assess the in vitro antioxidant, anti-inflammatory, antidiabetic, and antihypertensive activities of ethyl acetate:methanol (EM) and chloroform (CHCl3) fractions of the brown seaweed Sargassum wightii. Spectroscopic characterization of the different solvent fractions from S. wightii was studied to understand different types of carbons, protons, and to predict various functional groups, which are responsible to induce the target bioactive properties. The bioactivities of the various solvent fractions derived from the seaweed species as directed by various in vitro bioassays were correlated with the presence of different types of functional groups responsible for the target bioactivities.
Materials and methods
Seaweed material and description of study area
The brown seaweed S. wightii was collected from Gulf of Mannar of Mandapam region located between 8º48’ N, 78º9’ E and 9º14’ N, 79º14’E on the Southeast coast of India. The seaweed samples were shade-dried and powdered after washing thoroughly in fresh water to remove salt and other unwanted materials before being stored in airtight containers for further study.
Preparation of solvent fractions of S. wightii
The ground and shade-dried seaweed samples (1 kg) were extracted with n-hexane (600 mL × 2), at room temperature for 24 h to eliminate the pigments. The residue was thereafter sequentially extracted with EM (1:1 v/v, 500 mL × 3), at room temperature for 12 h followed by soxhlet extraction on a water bath at 70°C. The extracts were pooled and dried over anhydrous Na2SO4 (30 g) before being filtered (Whatman No. 1) and evaporated (50°C) under reduced pressure using rotary evaporator (Heidolph, Germany) under vacuum to dryness to furnish a dark green viscous mass of EM solvent fraction (42 g). The residue was further extracted using chloroform (600 mL × 2) at 65°C, and the filtrate was dried and thereafter concentrated under reduced pressure to yield the CHCl3 fraction (35 g) of S. wightii.
Chemicals and reagents
All solvents used were of analytical grade (E-Merck, Darmstadt, Germany). All other chemicals and reagents were of analytical, spectroscopic, or chromatographic reagent grade and were obtained from E-Merck (Darmstadt, Germany) and Sigma-Aldrich Chemical Co. Inc. (St. Louis, MO). DPP-IV from porcine kidney, gly-pro-p-nitroanilide, diprotein-A (Ile-Pro-Ile), ACE (from rabbit lung), N-(3-[2-furyl] acryloyl)-Phe-Gly-Gly (FAPGG), lipoxidase extra pure (LOX-5), cyclooxygenase-I (from sheep, COX-I), cycloxygenase-II (human recombinant, COX-II), α-amylase (from porcine pancreas), and α-glucosidase (from yeast) were procured from Sigma-Aldrich Chemical Co. Inc.
Instrumentation
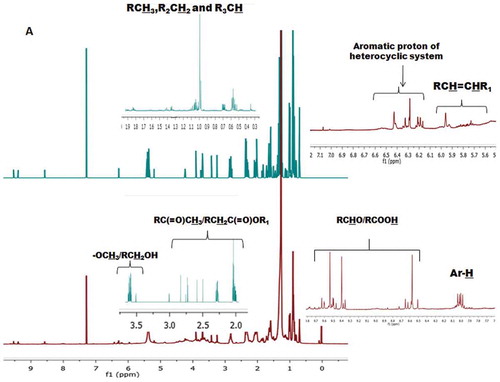
Ultraviolet (UV) spectra were obtained on a Varian Cary 50 UV-VIS spectrometer (Varian Cary, USA). Fourier transform infrared spectrometer (FTIR) spectra under KBr pellets were recorded in a Perkin–Elmer Series 400 FTIR spectrophotometer equipped with an air-cooled deuterated triglycine sulfate (DTGS) detector scanning between 4000 and 400 cm−1. The sample solvent fractions (10 mg) were mixed with KBr (100 mg), and subjected to a pressure of about 5×106 Pa in an evacuated die to produce a clear transparent disc of 13 mm diameter and 1 mm thickness. For each spectrum, 32 scans were co-added at a spectral resolution of 4 cm. The frequencies for all sharp bands were accurate to 0.01 cm. All the spectral values were expressed in (%) transmittance. 1H and 13C-NMR spectra were recorded on a Bruker AVANCE III 500 MHz (AV 500) spectrometer (Bruker, Karlsruhe, Germany) in CDCl3 as aprotic solvent at ambient temperature with tetramethylsilane (TMS; Cortec, Paris, France) as the internal standard (δ 0 ppm) equipped with 5 mm probes. The number of attached protons for the 13C-NMR signals was determined from DEPT experiments. Chemical shift (δ) values are expressed in parts per million (ppm), and referenced to the residual solvent signals of CDCl3. The 1H-NMR spectra were subsequently integrated to get the number of protons in specific regions. The 1H-NMR spectra were divided into five distinct regions, which included aliphatic (RCH3, R2CH2and R3CH; δ0.1-2.0), acetyl (RC(=O)CH3)/functionalized hydride group of alkyl alkanoates (RCH2C(=O)OR1)/substituted alkyl amines (R2NCH3; δ2-2.5), methoxy(-OCH3)/alkyl halide (RCH2-X)/functionalized hydride H of the substituted alkanol (RCH2OH; δ 2.8-3.5), olefinic (RCH=CHR1)/protons of the parent hydride group of alkyl alkanoates (RCH2C(=O)OCH3; δ 4-6), and aromatic protons (Ar-H; δ 6.5-8.5). A peak between δ 9 and 10 in the 1H-NMR spectra indicates the presence of the aldehyde proton. The protons at the defined regions of the 1H-NMR spectra were integrated to get the number of protons in specific regions ().
Table 1. Type and integral values of protons obtained from the 1H-NMR of the EM and CHCl3 fractions of seaweed S. wightii.
To record the 13C-NMR data, the EM and CHCl3 fractions of S. wightii (10–20 mg) were dissolved in CDCl3 (80 µL) and a 5 mm tube was used. This spectrum was recorded at 125 MHz and room temperature, using a spectral width of 26.3 kHz, an acquisition time of 0.56 s, and a relaxation delay of 0.6 s for 5376 scans. The 13C-NMR spectra were labelled to get the different types of carbons in specific regions characteristic of aliphatic (δ 10–40), alkoxy carbon (RCH2OR1; δ 45–70), carbon atom attached with the parent hydride group of alkyl alkanoates (RCH2C(=O)OCH3; δ 70-80), olefinic carbon atoms (δ110–140), aryl (δ 140–160), alkanoate carbon atom of esters (RC(=O)OR1; δ 160–180), and carbonyl carbon (δ 180–10).
Total phenolic content
Total phenolic content was determined using Folin–Ciocalteu colorimetric method.[Citation7] Briefly, the EM and CHCl3 fractions (5 mg/mL in MeOH) were mixed with Folin–Ciocalteu reagent (0.25 mL) and methanol (2.25 mL) before being incubated at room temperature for 8 min. Sodium carbonate solution (1 mL, 7.5%, w/v) was thereafter added to the mixture, before being incubated for 120 min at 25°C. The absorbance, relative to that of the blank (MeOH), was measured at 756 nm using a UV-Vis spectrophotometer (Varian Cary 50, USA) by using gallic acid as standard. The results were expressed as milligram of gallic acid equivalents (mg GAE)/g of the solvent fractions.
1,1-Diphenyl-2-Picryl Hydrazil (DPPH) and 2,2-Azino-bis-3-Ethylbenzothiozoline-6-Sulfonic Acid (ABTS+) radical scavenging activities
DPPH radical scavenging activity was described by the method reported earlier.[Citation7] In brief, DPPH (100 µM) was dissolved in methanol to prepare the stock solution. The EM and CHCl3 fractions (1 mL each in methanol) were mixed with DPPH solution (1 mL) before being vortexed and kept in the dark at room temperature for 10 min. The decrease in absorbance of the mixture was measured at 517 nm against a reagent blank by using a UV-Vis spectrophotometer. To determine the ABTS radical scavenging activity,[Citation7] ABTS was dissolved in deionized water to a concentration of 7 µM. This was then mixed with potassium persulfate (K2S2O8, 2.45 µM) before being kept in dark at room temperature for 12–16 h. The ABTS radical solution was diluted with MeOH to an absorbance of 0.70 at 734 nm. About 3 mL of this diluted ABTS.+ solution was mixed with 30 µL EM and CHCl3 fractions, and the absorbance were recorded after 6 min at 734 nm. The percentage of DPPH and ABTS+ scavenging activities were calculated by the following formula:
where A0 is the absorbance of control and A1 is the absorbance of the sample. The plots of scavenging activity on DPPH/ABTS+ radical were recorded and the IC50 value (mg/mL) was then calculated.
Hydrogen peroxide (H2O2) scavenging activities
Hydrogen peroxide (H2O2) scavenging activity was measured according to the reported literature.[Citation8] Briefly, a solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). The EM and CHCl3 fractions of S. wightii in different concentrations (0.1–1.0 mg/mL) were prepared in MeOH (3.0 mL), and were added to hydrogen peroxide solution (0.6 mL, 40 mM). Absorbance of the solution was taken at 230 nm after 10 min against a blank solution, containing phosphate buffer without H2O2. The percentage scavenging of H2O2 was calculated as: % H2O2 scavenging activity = [(A0 – A1) /A0] × 100, where A0 is the absorbance of the control and A1 is the absorbance in the presence of the samples. The plot of scavenging activity on H2O2 was recorded and the IC50 value (mg/mL) was calculated.
Fe2+ ion chelating activities
The Fe2+ ion chelating activities of solvent fractions considered under the present study were measured as described earlier.[Citation8] Briefly, the EM and CHCl3 fractions (0.1, 0.2, 0.4, and 0.6 mg/mL) were added to a solution of FeSO4 (1 mL, 0.125 mM) and ferrozine (1.0 mL, 0.3125 mM). The mixture was vortexed and allowed to equilibrate for 10 min at room temperature for 10 min, before measuring the absorbance at 562 nm. The ability of the fractions to chelate Fe2+ was calculated relative to the control (consisting of Fe and ferrozine only) using the equation: % Fe2+ ion chelating ability = (A0 – A1) × 100 /A0, where A0 is the absorbance of control and A1 is the absorbance of sample. The concentration of fractions required to get 50% Fe2+ ion chelating activity, called effective concentration (IC50) was calculated.
Lipid peroxidation inhibition activity in model system: Thiobarbituric Acid-Reactive Species (TBARS) formation inhibitory activity
The ability of the solvent fractions to arrest lipid peroxidation was assessed by TBARS assay, and expressed as mM of malondialdehyde (MDA) equivalent compounds formed per kg sample (MDAEQ/kg sample).[Citation9] The model system used for this assay was lyophilized green mussel (Perna viridis L.) as a lipid source. The solvent fractions (1 mL; 2 mg/mL) were preincubated with P. viridis meat (10 mg) for 10 min. The incubation was stopped by the addition of cold acetic acid (2 mL, 20 % v/v; pH 3.6), and the MDA formation was followed by the addition of TBA (2 mL, 0.78% w/v in acetic acid). The mixtures were incubated at 95°C for 45 min, cooled to room temperature before being centrifuged (8000 rpm, 10 min). The absorbance was measured at 532 nm. TBARS activity was expressed as mM of MDA equivalent compounds formed per kg sample (MDAEQ/kg sample) related to the control (lyophilized green mussel) undergoing maximum lipid peroxidation on the same assay conditions.
In vitro anti-hypertensive activities
Anti-hypertensive activity was measured by the ability of solvent fractions to inhibit ACE-I as described earlier.[Citation10] In brief, the commercial ACE-I (20 μL, 1 U/mL, from rabbit lung) was mixed with EM and CHCl3 fractions (200 μg), and the contents were added with N-[3-(2-furyl) acryloyl]-L-phenylalanylglycylglycine (FAPGG, 1 mL, 0.5 mM) dissolved in 50 mM Tris-HCl buffer (pH 7.5) containing 0.3 M NaCl. The decreased absorbance at 345 nm was recorded within a 1.5 min span at room temperature, and was expressed as ΔAbsample/min. Distilled water was used as the blank, and expressed as Δ Abblank/min. The ACE inhibition (%) was calculated as follows: [1 – (Δ Absample/min ÷ Δ Abblank/min)] × 100. The results were expressed as IC50, the concentration at which it inhibits 50% of the enzyme activity.
In vitro anti-inflammatory activities
The in vitro anti-inflammatory activities of the EM and CHCl3 fractions of S. wightii have been carried out in this study using cyclooxygenase (COX-1 and COX-2) inhibition assays by 2,7-dichlorofluorescein method.[Citation11] In brief, leuco-2, 7-dichlorofluorescein diacetate (5 mg) was hydrolyzed at room temperature in NaOH (1 M, 50 μL) for 10 min, and HCl (1 M, 30 μL) was added to neutralize the excess of NaOH before the resulting 1-dichlorofluorescein (1-DCF) was diluted in Tris HCl-buffer (0.1 M, pH 8). COX enzyme (COX-1 and COX-2) was diluted in 0.1 M Tris-buffer (pH 8), so that a known aliquot gave an absorbance change of 0.05/min in the reaction. The EM and CHCl3 solvent fractions (or the equivalent volume of MeOH, 20 μL) were preincubated with the enzymes at room temperature for 5 min in the presence of hematin. Premixed phenol, 1-DCF, and arachidonic acid were added to the enzyme mixture to begin the reaction and to give a final reaction mixture of arachidonic acid (50 μM), phenol (500 μM), 1-DCF (20 μM), and hematin (1 μM) in 1 mL final volume of 0.1 M Tris-buffer (pH 8). The reaction was recorded spectrophotometrically over 1 min at 502 nm. A blank reaction mixture (without enzyme) was analyzed in the spectrophotometer reference cell against each test reaction to account for any non-enzymatic activity attributed to the test sample. The IC50 values (mg/mL), which is the concentration of the EM and CHCl3 fractions of S. wightii that inhibit 50% of cyclooxygenases, were calculated from the non-linear regression curve.
The 5-lipoxygenase (5-LOX) inhibition assay was performed according to the earlier literature.[Citation12] Briefly, an aliquot of the stock solution (50 μL, in DMSO and tween 20 mixture; 29:1, w/w) of each sample were added with the pre-warmed potassium phosphate buffer (0.1 M, 2.95 mL, pH 6.3) and linoleic acid solution (48 µL) before being placed in a cuvette (3 mL). Thereafter, ice-cold buffer (potassium phosphate; 12 μL) was mixed with 5-LOX (100 U). The mixture was then transferred to the cuvette, before being placed into the spectrophotometer, and the absorbance was recorded at 234 nm. The IC50 value (mg/mL) was calculated from the non-linear regression curve as described in the earlier section.
In vitro antidiabetic activities
Antidiabetic activities of the EM and CHCl3 fractions of S. wightii were determined by inhibition of α-amylase, α-glucosidase, and dipeptidyl peptidase-4 (DPP-4) enzymes.[Citation13] For the inhibition of α-amylase, EM and CHCl3 fractions (500 μL) were added to phosphate buffer (500 μL, 0.20 mM, pH 6.9) containing α-amylase (0.5 mg/mL) solution and were incubated at 25°C for 10 min. Thereafter starch solution (500 μL, 1% w/v in 0.02 M sodium phosphate buffer pH 6.9) was added to the contents, and the reaction mixtures were incubated at 25°C for 10 min. The reaction was stopped with 3, 5 dinitrosalicylic acid reagent (1.0 mL) under heating for 5 min before being cooled at room temperature. The reaction mixture was then diluted with distilled water (10 mL), and the absorbance was measured at 540 nm. The control represents 100% α-amylase activity, and was conducted in similar way by replacing the sample fractions with the vehicle. The plot of inhibition of α-amylase activity was recorded and the IC50 value (mg/mL) was calculated.
For α-glucosidase assay, the solvent fractions (EM and CHCl3) under study were prepared in Tris-HCl buffer (500 μl, 0.2M, pH 8) in different concentrations, and were added to the enzyme solution (1 U/mL prepared in 0. 2M Tris-HCl, pH-8). The reaction mixture was pre-incubated for 5 min at 37°C. Starch solution (500 μL, 2%, w/v) was added and incubated for 10 min at 37°C. The reaction was stopped with 3, 5 dinitrosalicylic acid reagent (1 mL) under heating for 2 min in a boiling water bath before being cooled at room temperature. The reaction mixture was then diluted with distilled water (9 mL), and the absorbance was measured at 540 nm. The IC50 value (mg/mL), which is the concentration of the sample fractions that inhibit 50% of the α-glucosidase activity, was calculated from the non-linear regression curve.
For the inhibition of DPP-4 enzyme, the EM and CHCl3 fractions of S. wightii were prepared in different concentrations in Tris-HCl buffer (50 mM, 7.5 pH). About 350 μL of the fractions were mixed with DPP-4 enzyme (15 µL, 0.05 U/mL) prepared in Tris-HCl buffer (100 mM, pH 8). The reaction mixture was pre-incubated for 10 min at 37°C, and added with the substrate (gly-pro-p-nitroanilide, 50 µL, 0.2 M in Tris HCl buffer). The reaction mixture was incubated for 30 min at 37°C, and the reaction was terminated by the addition of glacial acetic acid (25 µL of 25%). The absorbance of the reaction mixture was measured at 405 nm. The IC50 value (mg/mL), which is the concentration of the EM and CHCl3 fractions of S. wightii that inhibit 50 % of the DPP-4, was calculated from the non-linear regression curve. Diprotein-A (Ile-Pro-Ile) was used as reference inhibitor standard for the method.
Statistical analysis
One-way analysis of variance (ANOVA) was carried out with the Statistical Program for Social Sciences 13.0 (SPSS, USA, ver. 13.0, SPSS Inc., Quarry Bay, HK) through ANOVA and Duncan analysis, to assess for any significant differences between the means. The significant differences were represented as p < 0.05 and the values were given as mean of triplicates ± standard deviation. The mean variance in the data set was detected using principal component analysis (PCA). The selected variables for PCA were the antioxidant, antidiabetic, anti-inflammatory, and antihypertensive activities exhibited by EM and CHCl3 crude solvent fractions of the seaweed. Pearson correlation analyses were performed to evaluate the relationship between different activities.
Results and discussion
Antioxidant compounds play immense role against inflammation, type-2 diabetes, hypertension, atherosclerosis, and other life-style diseases,[Citation14] which explain their potential use in the pharmaceutical and functional food industry. The present study demonstrated the potential of EM and CHCl3 fractions of the brown seaweed S. wightii as potential sources of antioxidative, antidiabetic, anti-inflammatory, and antihypertensive activities. The EM fraction of S. wightii showed significantly greater total phenolics (2.51 mg GAE/g, 20 mg/L, p < 0.05) than those in CHCl3 fraction (2.04 mg GAE/g). The total yield of the EM fraction also showed greater yield (4.2 g/100 g dry sample) than the CHCl3 fraction (3.5 g/100 g dry sample), which demonstrated the commercial feasibility to isolate phenolics from the EM fraction of the seaweed. Notably, the phenolic compounds were reported to possess radical scavenging properties, as reported in other marine organisms and seaweeds.[Citation15] As reported earlier,[Citation16] the present work likewise observed a significant correlation between the phenolic contents and the antioxidant activities signifying the role of phenolics as antioxidants. The free radical scavenging activities of EM and CHCl3 fractions and also that of synthetic antioxidants were evaluated through their ability to quench the free radical. The EM fraction of S. wightii registered significantly greater Fe2+ ion chelating ability (IC50 0.52 mg/mL), and were effective in stabilizing the ABTS+ (IC50 0.82 mg/mL) and DPPH radicals (IC50 0.32 mg/mL) than those recorded in the CHCl3 fraction (IC50 1.45, 1.35, and 0.37 mg/mL, respectively). Likewise, the EM solvent fraction of S. wightii was significantly more effective in neutralizing H2O2 (IC50 0.19 mg/mL) than the CHCl3 fraction (IC50 0.36 mg/mL). No significant differences in TBARS (MDA) formation inhibitory capacities of the EM and CHCl3 fractions of S. wightii were apparent (51-54 mM MDAEQ/kg; ).
Table 2. Phenolic content, antioxidant, anti-inflammatory, antidiabetic, and ACE inhibitory activities of the EM and CHCl3 fractions of the seaweed S. wightii.
The greater Fe2+ ion chelating ability probably relates to the vicinity of phenolic moieties, which bond with Fe2+, subsequently diminishing the redox potential and balance out the oxidized type of Fe2+. It was reported that brown seaweeds possessed potentially high Fe2+ chelating ability,[Citation17] which was due to the presence of phenolic compounds such as phlorotanins. It is significant to note that the metal chelating potency of phenolic compounds are dependent upon their unique phenolic structure, and the number and location of –OH groups.[Citation18] The double bond, in conjugation with an oxo group in polyphenols as well as other functional groups, might help to bind transition metal ions, such as Fe and Cu. Significant positive correlation between the phenolic contents with ABTS+ and DPPH radical scavenging activities of the EM solvent fraction derived from S. wightii were demonstrated, which substantiated that the polyphenols might play a vital role in scavenging free radicals. The greater DPPH and ABTS+ activities might additionally be explained because of the compounds with multiple –OH groups and/or center of unsaturation, which enabled them to donate a proton to free radical by hydrogen atom transfer (HAT) to deactivate the free radicals. Likewise, a significant co-linearity was found between the phenolic contents of the EM fraction and H2O2 scavenging activity that demonstrated that the polyphenolic compounds are responsible for antioxidative properties. Similar results were apparent in the earlier studies reporting the significantly greater H2O2 scavenging activities (IC50 0.009 mg/mL) of the EtOAc-MeOH fraction from Ecklonia cava indicating the potential of hydrophilic total phenolics to impart H2O2 scavenging activity.[Citation19] A negative correlation between the total phenolic contents and TBARS activity apparently demonstrated the presence of non-phenolic compounds responsible for lipid peroxidation inhibitory properties of the seaweed solvent fractions.
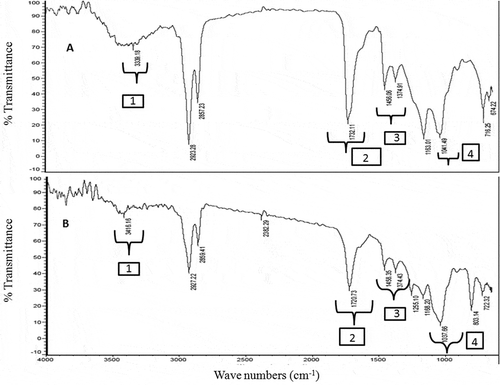
The FTIR spectrum was used to identify the functional groups present in the solvent fractions of S. wightii (). The band appeared at 3339 and 3416 cm−1 might be due to the O-H stretching vibration of phenols and/or alcohols and/or the N-H stretching vibration corresponds to amide groups. The intense peaks at 1732 and 1721 cm−1 were attributed due to the C=O stretching vibration of aldehydes or saturated aliphatic groups. A particularly intense signal was recorded at 1456 cm–1, which is particular to the C-C stretching frequency of the aryl ring framework. The intense absorption bands in the 1037–1041 cm−1 region of the FTIR spectra of the EM-MeOH and CHCl3 fractions showed the vicinity of C=C stretching vibration because of the olefinic groups. By comparing the FTIR spectra of both the solvent fractions, it is reasonable to locate greater number of polar functional groups in EM fraction than those in the CHCl3 fraction. The IR signal at 3339 cm−1 appeared in the EM spectra was of medium intensity, while the peak appeared at 3416 cm−1 in the CHCl3 fraction was weak. The prominent presence of the polar functionalities, such as, >C=O groups in the EM solvent fraction was confirmed by the strong signal appeared at 1732 cm−1. But these signals were appeared as medium intense (at 1721 cm−1) in the spectrum of CHCl3 fraction.
Figure 1. FT-IR spectra of A: EM and B: CHCl3 solvent fractions of S. wightii. The functional groups representing the distinct regions of the IR spectra were illustrated as (1) OHν of phenols and/or alcohols or N-Hν of amide groups, (2) >C=Oν of aldehydes or saturated aliphatic groups, (3) C-Cν of the aryl ring framework, and (4) C=Cν of olefinic groups. Stretching vibration has been indicated by “ν” as subscript to the functional group.
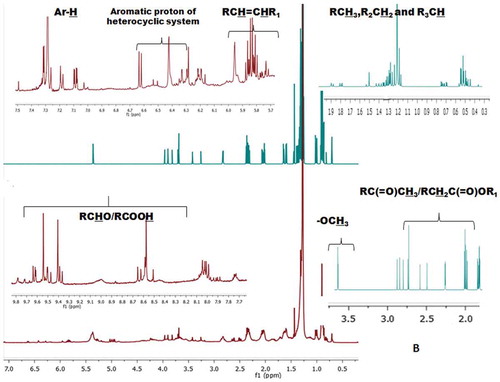
The 1H-NMR conjugated with 13C-NMR spectra of the solvent fractions were taken into consideration for the elucidation of possible functional groups, types of bonds and characteristic proton/carbon atoms associated with different magnetic environments of the compounds (). The 1H-NMR spectra of CHCl3 fraction extracted from S. wightii showed greater proton integrals (~687) at δ 0–2, which indicate the presence of methyl (CH3-C), methylene (RCH2-), and methine (-CH-) protons associated with saturated hydrocarbons (). EM-MeOH fraction was found to exhibit lesser proton integral (~579) at this region compared to CHCl3 fraction (, and ). The number of protons at δ 2–2.5, presumably of allylic or acetyl substitution, which were found to be greater in EM-MeOH fraction (proton integral of 101), while CHCl3 fraction exhibited lesser proton integral (proton integral of 66) at this region (). Singlet peak at δ 3.25 and δ 3.68 appeared in the spectra of EM and CHCl3 solvent fractions might be due to the methoxy protons (R-OCH3). The proton integral at the olefinic region (δ 4.5–6) of CHCl3 fraction was greater (~80) compared to EM fraction (~74; ). Protons appeared at δ 6.5–8.5 are typical of the aryl or furyl (aromatic) ring system ( and ). Proton integral at this region was found to be greater in EM (proton integral of ~3), while very weak proton signals were observed for CHCl3 fraction at this region. The aldehydic protons appeared at δ 8.5–10 in the spectra of CHCl3 fraction.
Figure 2b. CHCl3 solvent fractions of S. wightii. The protons at the defined regions of the 1H-NMR spectra were integrated to get the number of protons in specific regions.
The structural attributions of the EM and CHCl3 fractions of S. wightii were confirmed by the analysis of the corresponding 13C-NMR spectra. The signal intensity and number of carbon atoms belonging to saturated hydrocarbons (δ 10–40) were greater in the CHCl3 solvent fraction than EM (). The number of –C-H- (methine) signals at δ 30–40 were also found to be greater in CHCl3 fraction than EM. The sp2-hybridized olefinic carbon combine at the region between δ 110–140 were found to be greater in CHCl3 fraction. Prominent peaks were observed in the carbonyl region (δ 180–200) of the CHCl3 fraction. This can be derived from ester (R-C=O-O-R’) or aldehydic/ketonic carbons.
Figure 2c. The stacked plot representing the 13C-NMR spectra of EM and CHCl3 fractions of S. wightii. The functional groups representing the distinct regions of the 1H- and 13C-NMR spectra were labeled.
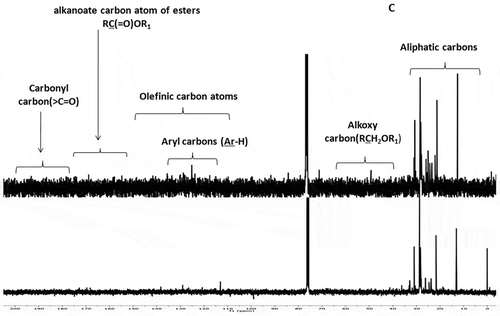
Spectroscopic analysis such as 1H-NMR, 13C-NMR, and FT-IR of the EM and CHCl3 fractions of S. wightii provided an idea to understand and compare the pattern of signals and there by assigning of different types of protons and carbons. This assessment can give information about the functional groups associated with the fractions derived from S. weightii, which in turn provide a useful tool for the prediction of biological activities associated with these solvent fractions. The total number of protons at the region depicting the saturated hydrocarbons (δ 0.1–2) was fundamentally lesser (total proton integral of about 578.9) in the EM fraction of S. wightii than the CHCl3 fraction derived from the seaweed species (~687.2). The signals at δ 1.2–1.5 in the CHCl3 fraction could be disclosed due to the methylene envelope [–(CH2)n] in the long alkyl chain possibly connected with sterols/fatty acids or lipidic structures. The overlapped >CH2 bis-allylic protons (-CH=CH-CH2-CH=CH-) might display their signals at δ 2.5–3 (m), and were found to be more noteworthy in the CHCl3 fraction of S. wightii. These bis-allylic peaks indicate the prevalence of the unsaturated fatty acids. The 1H-NMR spectra of EM and CHCl3 fractions acquired well-resolved, deshielded signals at about δ 4.5–6, which presumably showed the vicinity of the olefinic substituents. The strong signals in the 1H-NMR spectrum of the EM fraction at δ 6.5–8.5 (proton integral of 2.9) could be ascribed to the vicinity of protons because of the aromatic groups. Interestingly, extremely frail proton signals at δ 2–2.5 for the CHCl3 fraction (a total proton integral of 65.5) were noted, which apparently demonstrated the absence of the functional groups belonging to RC(=O)CH3/RCH2C(=O)OR1 in more prominent intensities. The EM fraction displayed very intense signals at this region, which ascribed to be because of the vicinity of electronegative auxochromes, for example, acetyl or functionalized hydride group of alkyl alkanoates. These electron withdrawing groups can prevent lipid peroxidation by inhibiting the generation of free radicals. The 1H-NMR spectra of the CHCl3 fraction recorded the presence of greater proton integrals at δ 4.5–6 due to the olefinic protons or those associated with the parent hydride group of alkyl alkanoates (RCH2C(=O)OCH3). The olefinic groups in the CHCl3 fraction might be due to the presence of terpenoids, lipids or unsaturated fatty acids. In both the spectra, olefinic protons appeared at highly deshielded region may be due to the attachment of these protons with a highly conjugated system or some electronegative functionalities. A distinct proton integral was found at δ 6–7 in the spectra of both fractions. These highly deshielded peaks can be derived from the aromatic protons associated with heterocyclic moieties. In general, the EM solvent fraction derived from S. wightii showed greater proton integrals than CHCl3 fraction when these potentially electronegative regions were taken into consideration. This assumption was further validated by the presence of bands corresponding to olefinic and carbonyl groups in the FTIR and 13C-NMR spectra.
Characteristic groups or functionalities predicted by 1H-NMR spectra were further validated by 13C-NMR chemical shifts. The upfield region of the 13C-NMR spectrum demonstrated the presence of saturated hydrocarbons (δ 35–10), which might be because of the fatty acids (or fatty acid derivatives) in the CHCl3 fraction of S. wightii. A lesser number of these –CH3 and –CH2- signals in the EM fraction of S. wightii demonstrated the absence of fatty acid or lipidic components. The sp2-hybridized olefinic carbon atoms showed prominent peaks at δ 130–140 in the CHCl3 fraction, which apparently demonstrated the presence of unsaturation, probably belonging to fatty acid moieties. Interestingly, the EM fraction did not show the presence of the intense olefinic system (at about δ 130).
The EM fraction of S. wightii exhibited significantly greater in vitro anti-inflammatory properties as determined by COX-1 and COX-2 inhibitory activities (IC50 0.03–0.05 mg/mL; p < 0.05) than those extracted by CHCl3 (IC50 0.09–0.17 mg/mL) and commercial synthetic non-steroidal anti-inflammatory drug aspirin (IC50 0.83–0.86 mg/mL). It is significant to note that EM fraction of S. wightii also exhibited greater 5-LOX inhibitory activity (IC50 0.03 mg/mL) than synthetic anti-inflammatory drug aspirin (IC50 value of 0.86 mg/mL). The EM solvent fraction was found to have greater number of electronegative functional groups present in the downfield space of the 1H-NMR and 13C-NMR spectra. These electronegative/hydrophilic groups prevent abstraction of hydrogen from arachidanoic acid in COX-1, and thus prevent prostaglandin synthesis. Electronegative groups can potentially coordinated with the COX active site by ion pairing, thereby preventing the synthesis of prostaglandins. The up-field region of 13C-NMR spectra indicating the presence of saturated hydrocarbons (δ 35–10) in both the EM and CHCl3 solvent fractions and greater proton integral at δ 0–1.2 in the 1H-NMR spectra are good indications of non-polar hydrophobic groups, which account for more acceptable hydrophilic-lipophilic balance (HLB) and better binding of active site of enzyme and the inhibitors.
The CHCl3 fraction derived from S. wightii displayed significantly greater antihypertensive properties as dictated by angiotensin-I converting enzyme inhibitory activity (IC50 0.084 mg/mL) than EM fraction (IC50 0.11 mg/mL; ). It is of note that the anti-ACE-I property of the CHCl3 fraction showed no significant difference with the standard antihypertensive drug captopril (IC50 0.077 mg/mL; p < 0.05). The greater inhibitory activity can be explained by the fact that the CHCl3 fraction has greater proton integral due to saturated hydrocarbons (at δ 0.1–2), which might form the part of long chain fatty acids, phospholipids or glycolipids. A considerable co-linearity was found to exist between the olefinic groups and the ACE-inhibitory activity. A positive correlation might suggest an interaction depending on the polarizability of the bioactive leads present in the solvent fractions. It is of note that the proton integral of the olefinic protons (at δ 4.5–6) in the CHCl3 fraction derived from S. wightii was about 79.6, which possibly interact with the contacting polar space in the enzyme (ACE) thereby inhibiting the enzyme responsible for hypertensive activity. It can, therefore, be inferred that the electronic descriptors along with hydrophobic variables might significantly contribute toward the greater anti-ACE activity of the solvent fraction derived from the experimental seaweed species.
The EM and CHCl3 fractions of S. wightii exhibited greater α-glucosidase inhibitory activity (IC50 values of 0.005 and 0.069 mg/mL, respectively) than the positive control (acarbose, IC50 0.2 mg/mL). Likewise the α-amylase inhibitory activity of EM was found to be significantly greater (0.08 mg/mL) than the CHCl3 fraction (IC50 0.14 mg/mL) and the standard, acarbose (IC50 0.21 mg/mL). DPP-4 inhibitory activity of the EM solvent fraction was found to be significantly greater (IC50 0.013 mg/mL) than the CHCl3 fraction of S. wightii (IC50 0.28 mg/mL; p < 0.05). As described above, the EM and CHCl3 fractions contained high levels of phenolic compounds. Hence, the high levels of phenolic compounds contained in the solvent fractions of S. wightii are most likely responsible for the strong antidiabetic activity. The α-amylase and α-glucosidase enzyme inhibition results in a delayed glucose absorption and thus lowering of postprandial hyperglycemia. Acarbose, a pseudo tetrasaccharide inhibitor of the α-amylase family, in which the cyclitol group resembles the presumed oxocarbenium ion-like transition state expected for glycoside hydrolysis.[Citation20] Cyclitol unit was exposed to the solvent region, and the hydroxyl groups interacted with water molecules via hydrogen bonds. So the active components in the solvent fractions derived from the seaweed compete with the substrate for binding to the active site of the enzyme, thereby preventing the breaking down of oligosaccharides to disaccharides. Previous studies on α-amylase and α-glucosidase inhibitors suggested that several potential inhibitors belong to phenolic class had features of inhibiting α-amylase and α-glucosidase activities.[Citation21] The polyphenolic compounds have been shown to inhibit the activities of digestive enzymes due to their ability to bind with proteins. Presence of signals at δ 160–180 in the 13C-NMR spectra of both the solvent fractions are a good indication about the acyl carbonyl carbon [R-(C=O)X]. These acyl halides can effectively inhibit α-glucosidase enzyme.[Citation22] Matsui et al.[Citation22] found that α-glucosidase inhibition of anthocyanins was due to the acylated structure.
The DPP-4 inhibitors were reported to possess an electophilic functional group, which can interact with the hydroxyl of the catalytic serine630 in the active binding site of the DPP-IV, the prolyloligopeptidase enzyme. The EtOAc-MeOH and CHCl3 fractions of S. wightii recorded the presence of carbonyl groups at δ 180–200 in the 13C-NMR spectra, and greater proton integral at δ 2–2.7 were attributed to the presence of protons attached to carbonyl group (R-CH2-C=O or R2CHC=O). These electrophilic functionalities can bind with the active site of DPP-4 enzyme. Dietary intake of phenolics might prove to be important for alternative diabetes treatments or reduction of the risk of the disease,[Citation23] which substantiate the present study.
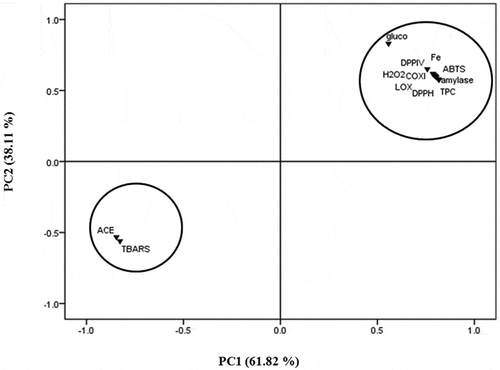
The relationships between different bioactivities of the EM and CHCl3 fractions derived from S. wightii were statistically analyzed using principle component analysis (PCA; ). The loading of first and second principle components (PC1 and PC2) were accounted for 61.82 and 38.11% of the variance. PC1 was mainly influenced by TPC, DPPH, ABTS, and α-amylase (). On the other hand, H2O2 scavenging, Fe2+ ion chelating, 5-LOX, COX-1, COX-2, α-glucosidase, and DPP-IV were mainly contributed to PC2. The high negative loading of TBARS and ACE with PC1, indicated their negative correlation with other components, such as, antioxidant, anti-inflammatory, and antidiabetic properties. This data further demonstrated the fact that the antihypertensive activities were related to the presence of compounds with relatively hydrophobic moieties. The similarity in the greater loading of total phenolic compounds, antioxidant, anti-inflammatory, and antidiabetic property indicated that these bioactivities were closely related. The greater loading of TPC in PC1 proves that phenolic compounds present in the seaweed S. wightii are good antioxidants, and were attributed for anti-inflammatory and antidiabetic properties.
Conclusions
The waters of the Gulf of Mannar of south eastern coast of the Indian subcontinent have abundant resources of brown seaweed belonging to S. wightii, which have proven to produce an effective antioxidant defense system due to their adverse habitat. Antioxidant, antidiabetic, anti-inflammatory, and antihypertensive potential of EM and CHCl3 fractions of S. wightii were evaluated using different in vitro systems. The EM fraction registered greater antioxidative properties, and the activities showed significant positive correlation with the total phenolic contents and Fe2+ chelating activity. This demonstrated the presence of phenolic compounds responsible for metal (Fe2+) ion chelation by phenoxyl radical and free hydrogen on -OH group. The utilities of spectroscopic tools for analyzing the signature peaks and relative abundance of the vital functional groups present in the solvent fractions, and to furnish with essential rules regarding the presence of these functional groups responsible for bioactivities have been illustrated. The CHCl3 fraction derived from S. wightii exhibited significantly greater antihypertensive properties than those extracted by the EM fraction, which apparently indicated that these antioxidants might have significant role in deterring hypertensive modulators in the system. The target bioactive properties of the EM fraction derived from S. wightii were found to be directly proportional with the abundance of the electronegative groups appeared in the downfield space of the NMR spectra. This study demonstrated the application of S. wightii as prospective source of bioactive pharmacophores for use as functional food supplements to prevent the occurrence of ROS-induced diseases, such as, hypertension, diabetes, and inflammatory disorders.
Nomenclature
| COX-1 | = | Cycloxygenase-1 |
| COX-2 | = | cycloxygenase-2 |
| 5-LOX | = | 5-liopxygenase |
| DPP-4 | = | dipeptidyl peptidase-4 |
| ACE | = | angiotensin-I converting enzyme |
| NMR | = | nuclear magnetic resonance |
| ABTS | = | 2, 2′-azino-bis-3 ethylbenzothiozoline-6-sulfonic acid diammonium salt |
| DPPH | = | 1, 1-diphenyl-2-picryl-hydrazil |
| PCA | = | principle component analysis |
| TBARS | = | thiobarbituric acid reactive species |
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors thank the Director, Indian Council of Agricultural Research-Central Marine Fisheries Research Institute (ICAR-CMFRI), for his guidance and support. Thanks are due to the Head, Marine Biotechnology Division for facilitating the research works.
Funding
This work was supported by the funding under the Science & Engineering Research Board (SERB) Scheme (Grant number SR/S1/OC-96/2012 SERB) from Department of Science and Technology, Ministry of Science and Technology, New Delhi, India. Anusree Maneesh acknowledges ICAR, for the award of a scholarship. Fasina Makkar would like to thank the Department of Science and Technology for fellowship.
Additional information
Funding
References
- Hold, G.L.; El-Omar, M.E. Genetic Aspects of Inflammation and Cancer. Journal of Biochemistry 2008, 410(2), 225–235.
- Baynes, J.W.; Thorpe, S.R. Role of Oxidative Stress in Diabetic Complications: A New Perspective on an Old Paradigm. Diabetes 1999, 48, 1–9.
- Hernadez-Ledesma, B.; Martin-Alvarez, P.J.; Pueyo, E. Assessment of Spectrophotometric Method for Determination of Angiotensin Converting Enzyme Activity: Influence of Inhibition Type. Journal of Agricultural Food Chemistry 2003, 51, 4175–4179.
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with Free Radical Scavenging Properties from Marine Macroalga Ulva Fasciata Delile. Food Chemistry 2010, 122(1), 8–16.
- Valdebenito, H.; Bittner, M.; Sammes, P.G.; Silva, M.; Watson, W.H. A Compound with Antimicrobial Activity Isolated from the Red Seaweed Laurencia Chilensis. Phytochemistry 1982, 21(6), 1456–1457.
- Soares, A.R.; Abrantes, J.L.; Souza, T.M.L.; Fontes, C.F.L.; Pereira, R.C.; Frugulhetti, I.C.P.P.; Teixeira, V.L. Antiviral Effect Of Meroditerpenes Isolated from Stypopodium Zonale (Dictyotaceae) Against Human Immunodeficiency Virus Type 1 (HIV-1) and Herpes Simplex Virus (HSV-1). Planta Medica 2007, 73, 1221–1224.
- Wojdyło, A.; Oszmianski, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chemistry 2007, 105, 940–949.
- Gu¨lcin, I.; Oktay, M.; Kirecci, E.; Ku¨frevioglu, O.I. Screening of Antioxidant and Antimicrobial Activities of Anise (Pimpinella Anisum L.) Seed Extracts. Food Chemistry 2003, 83, 371–382.
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of Different Methods for Testing Antioxidative Activity of Oregano Essential Oil. Food Chemistry 2004, 85, 633–640.
- Holmquist, B.; Bunning, P.; Riordan, J.F. A Continuous Spectrophotometric Assay for Angiotensin Converting Enzyme. Analytical Biochemistry 1979, 95, 540–548.
- Larsen, L.N.; Dahl, E.; Bremer, J. Peroxidative Oxidation of Leucodichlorofluorescein by Prostaglandin H Synthase in Prostaglandin Biosynthesis from Polyunsaturated Fatty Acids. Biochimica et Biophysica Acta 1996, 1299, 47–53.
- Baylac, S.; Racine, P. Inhibition of 5-Lipoxygenase by Essential Oils and Other Natural Fragrant Extracts. International Journal of Aromatherapy 2003, 13, 138–142.
- Hamdan, I.I.; Afifi, F.U. Studies on the in Vitro and in Vivo Hypoglycemic Activities of Some Medicinal Plants Used in Treatment of Diabetes in Jordanian Traditional Medicine. Journal of Ethanopharmacology 2004, 93, 117–121.
- Kohen, R.; Nyska, A. Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Method for Their Quantification. Toxicologic Pathology 2002, 30, 620–650.
- Escrig, J.A.; Rincon, M.; Pulido, R.; Calixto, S.F. Guava Fruit (Psidium Guajava L) as a New Source of Antioxidant Dietary Fibre. Journal of Agricultural and Food Chemistry 2001, 49, 5489–5493.
- Karawita, R.; Siriwardhana, N.; Lee, K.W.; Heo, M.S.; Yeo, I.K.; Lee, Y.D.; Jeon, Y.J. Reactive Oxygen Species Scavenging, Metal Chelating, Reducing Power and Lipid Peroxidation Inhibition Properties of Different Solvent Fractions from Hizikia Fusiformis. European Journal of Food Research and Technology 2004, 220, 363–371.
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant Activity of Three Edible Seaweeds from Two Areas in South East Asia. Food Science and Technology International 2008, 41(6), 1067–1072.
- Santoso, J.; Yoshie-Stark, Y.; Suzuki, T. Antioxidant Activity of Methanol Extracts from Indonesian Seaweeds in an Oil Emulsion Model. Fisheries Science 2004, 70(1), 183–188.
- Senevirathne, M.; Kim, S.H.; Siriwardhana, N.; Ha, J.H.; Lee, K.W.; Jeon, Y.J. Antioxidant Potential of Ecklonia Cava on Reactive Oxygen Species Scavenging, Metal Chelating, Reducing Power and Lipid Peroxidation Inhibition. International Journal of Food Science and Technology 2006, 12, 27–38.
- Truscheit, E.; Frommer, W.; Junge, B.; Mu¨ller, L.; Schmidt, D.D.; Wingender, W. Chemistry and Biochemistry of Microbial α-Glucosidase Inhibitors. Angewandte Chemie-International 1981, 20, 744–761.
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. Evaluation of Pepper (Capsicum Annuum) for Management of Diabetes and Hypertension. Journal of Food Biochemistry 2007, 31, 370–385.
- Matsui, T.; Ueda, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase Inhibitory Action of Natural Acylated Anthocyanins. 2. α-Glucosidase Inhibiton by Isolated Acylated Anthocyanins. Journal of Agricultural Food Chemistry 2001, 49, 1952–1956.
- Ahmed, O.M.; Moneim, A.A.; Yazid, I.A.; Mahmoud, A.M. Antihyperglicemic Anti Hyperlipidemic and Antioxidant Effects and the Probable Mechanisms of Action of Ruta Graveolens Infusion and Rutin in Nicotinamide-Streptozotocin-Induced Diabetic Rats. Diabetologia Croatica 2010, 39, 15–35.