ABSTRACT
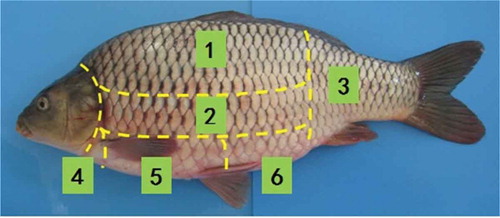
The fish body of cultured common carp (Cyprinus carpio) was divided into six sections, including the upper back, lower back, jaw, chest, belly, and tail. Differences in the physicochemical, micro-structural, and textural properties of different muscle tissues were investigated. The upper and lower back, with high content of protein, low content of fat, high water-holding capacity, and desirable textural properties, was proven to be the most valuable part of common carp from both a nutritional point-of-view and an organoleptic perspective. This could provide a theoretic basis for the comprehensive utilization of freshwater fish.
Introduction
Freshwater fish is considered as healthy food due to its high proportion of protein with a balanced amino acid profile, moderate amounts of healthy lipids though less n-3 polyunsaturated fatty acids (n-3 PUFA) than marine fish. It is also a good source of vitamins and essential minerals.[Citation1] During the past two decades, aquaculture continues to be the fastest-growing sector of aquatic animal production worldwide with an average growth rate of approximately 7.5%.[Citation2] As a result, freshwater aquaculture is gaining increasing importance in supplying sustainable and high-quality animal protein to a growing world population, especially developing countries. Common carp (Cyprinus carpio) is a commercially important fish species widely farmed in many Asian countries and also in some Central and Eastern European countries. In 2012, its production in China was estimated at 2.897 million tons and ranked third among domestic freshwater fish production.[Citation3] Although a wide variety of value-added products, such as fish fillet, surimi, fish protein hydrolysate, gelatin, are commercially available, the majority of freshwater fish are still sold as fresh whole fish, and approximate 70% of them have not received proper treatment or processing.[Citation3] Therefore, it is imperative that scalable processing technologies keep pace with the rapid growth in aquaculture production.
A comprehensive and detailed characterization of chemical composition and physiochemical properties of different muscle tissues is the prerequisite for deep processing and comprehensive utilization of freshwater fish. For mammalian animals (such as pigs, cows, goats, and poultry like chicken, turkey), the nutritional composition and muscle quality characteristics in various tissues (loin, rib, breast, liver, heart, etc.) have been thoroughly investigated.[Citation4–Citation7] Based on these valuable data, the mandatory grading standards have been established for different cuts of meat or different parts of the carcass. These standards greatly promoted the utilization of these animal materials, diversified products, bringing huge benefits to both meat processing industry and consumers. Compared with mammalian animals and poultry, fish grading system has not been well-developed. Only a few species like salmon, comprehensive meat grading systems have been built up.[Citation8] This could be due to big variations in quality attributes between individuals. Another reason could be the small size of most fish species, which causes inconvenience for grading operation. In most cases, the body of a fish is dived into head, tail, trunk, scale, bone, and guts during processing, which is not specific enough for their comprehensive processing. There is, therefore, a great need to develop more efficient separation and grading techniques. In addition, common carp is widely popular in China as fish dish and a favorite at banquets. However, it is traditionally prepared with fresh whole fish, without taking into account the composition and structural variations in different muscle tissues. The objective of this study was to analyze the physicochemical properties, texture and histological structure of different muscle tissues of common carp. The data obtained is expected to provide scientific basis for the establishment of comprehensive fish muscle grading systems.
Materials and Methods
Materials
Live farmed common carp with body weight ranging from 1.50–1.75 kg and length 44–48 cm (n = 10) were purchased from Meilinyuan Agro-product Market, Dalian, China. Upon arrival, the fish were stunned by a sharp blow to the head with a wooden stick. Immediately after death, they were manually eviscerated, rinsed with tap water and then placed in ice water for 30 min. Each fish was cut into six sections (upper back, lower back, jaw, chest, belly, and tail) along the length of the fish at a low temperature ().
Proximate Analysis
Proximate composition was determined using standard AOAC methods.[Citation9] Moisture content was measured gravimetrically by drying the sample to a constant weight in an oven at 105ºC for 16 h. The crude ash content was estimated by incineration in a muffle furnace at 550ºC for 5–6 h until the sample was completely free from carbon particles. The crude protein content was determined by Kjeldahl methods with a Kjeldahl azotometer (KDN-103F, Shanghai QianJian Instrument Company, Shanghai, China). Fat content was determined by Soxhlet method with a fat tester (SZF-06A, Shanghai XinJia Electronics Company, Shanghai, China). Total carbohydrate content was determined by phenol-sulfuric acid method. All analyses were performed in triplicate.
Color Measurement
Color, represented as CIE, L*, a*, and b* values, were measured at various locations along the fillet using a Hunterlab UltraScan PRO colorimeter (Hunter Associates Laboratory, Inc., Reston, VA, USA). Measurements were made directly on the fillets, with three color measurements on each section. L*, a*, and b* represents the whiteness, red–green axis and yellow–blue axis, respectively.[Citation10]
pH Measurement
The pH of muscle tissues was measured following a reported procedure.[Citation11] The tissue sample was homogenized with 10 volumes of cold deionized water (w/v, 4 ºC) for 2 min and kept at 4ºC for 1 h. The pH value of the homogenate was measured in triplicate using a digital pH-meter (PHS-3C, Shanghai Precision & Scientific Instrument Co., Shanghai, China). The pH meter was previously calibrated with buffer solutions of pH 7.00 ± 0.01 and 4.00 ± 0.01 at 20°C.
Myofibrillar Protein Extractability
The muscle tissue was homogenized with 10 volumes of 0.05 M KCl–20 mM Tris-maleate buffer solutions (w/v, pH 7.0) for 2 min and centrifuged at 12,000 × g for 10 min (CF16RXⅡ, Hitachi, Japan). The supernatant was discarded. The procedure was repeated to remove soluble protein. All steps were carried out at 4ºC. The precipitate was homogenized with 10 volumes of 0.6 M KCl–20 mM Tris-maleate buffer solutions (pH 7.0) for 1 min and allowed to stand at 4ºC for 1 h. The homogenate was centrifuged at 18,000 × g for 30 min and the supernatant was collected (myofibrillar protein). Protein content was measured using a Bradford protein assay kit (GenStar Biosolutions, Beijing, China).[Citation12]
Water-Holding Capacity (WHC)
Sample was cut into 1.0 cmCitation3 block, weighted and centrifuged at 5000 × g for 30 min. After centrifugation, the moisture on sample surface was wiped and the mass of sample was recorded. The WHC was calculated according to the formula given below:[Citation13]
W0 is initial moisture content of sample; ΔW is difference in moisture content of sample before and after centrifugation.
Instrumental Textural Properties Analysis
Texture profile analysis was performed in different parts of carp except the jaw using TA.XTplus texture analyzer (Stable Micro Systems, Haslemere, Surrey, UK). The sample was cut into 1.0 cmCitation3 cubic block. The operating parameters were: Model P50 head; pre-test speed 2.0 mm/s, test speed 1 mm/s and post-test speed 1 mm/s; compressed depth 60%, time interval 5.0 s, compressed times 2.
Shear force was measured in both fresh and cooked samples. Fresh sample tissue was cut into 2.0 cm × 1.0 cm × 1.0 cm block. For cooked sample, the block was packed in a plastic bag and heated at 80ºC for 1 min until the center temperature of tissue reached 60ºC. The cooked samples were then cooled down to room temperature. For shear force measurement, the texture analyzer was equipped with blade set (HDP/BS) and the test speed was 1 mm/s.
Microstructure Analysis
Muscle tissue samples from various parts of carp were fixated in 10% formaldehyde for 24 h at 4°C and dehydrated in ascending grades of alcohol (50, 60, 70, 80, 90, and 100%). The samples were cleared (made transparent) with xylene and embedded in paraffin according to the procedure described by Liu.[Citation14] The medial portion of the muscle was cut into 6 μm thickness thin slice under a dissecting microscope. The thin sections were dipped in 30% ethanol for 15 s and then immersed in 45ºC double distilled water for 20 s. They were dried in 60ºC for 30 min and then dewaxed in xylene and ethanol. The obtained specimens were stained according to Van-Gieson’s method as described by Liu,[Citation11] examined and photographed using a light microscope (BX51, Olympus Corporation, Tokyo, Japan) and an image analysis system (Image Pro-Express 6.0, Olympus).
Fat Distribution
The fat distribution was analyzed according to the method of Liu.[Citation14] For fat tissue samples, the frozen sample was cut into 15 µm thickness slice and then fixed with 10% formaldehyde for 1 min. The thin sections were immersed in 50% ethanol for 5 min, stained with Sudan IV for 5 min, and then cleaned with 70% ethanol and double distilled water. The specimens were dipped into hematoxylin for 5 min, carefully rinsed with distilled water, and then examined and photographed as described above.
Statistical Analysis
Data were examined by analysis of variance (ANOVA) and comparison of means was done by Least Significance Difference (LSD) test. Significance was defined at the 5% level (p < 0.05). All statistical analysis was performed by using statistical package SPSS 19.0 (SPSS, Chicago, IL, USA).
Results and Discussion
Proximate Composition
The proximate compositions of different parts of carp are shown in . The upper back, lower back and tail portions had higher moisture (77–79%) content than jaw, chest, and belly. The moisture content in upper backs, lower backs, and tail is similar to the results from previous study.[Citation15] The percentage concentration of ash in different parts from carp muscles were 3.22–5.26% (dry weight), were within the range for raw flesh of marine fish (0.6–1.5%) as reported earlier by Venugopal.[Citation16] Upper back had the highest ash content (5.26%), followed by lower back (4.84%), and tail (4.71%), respectively. Fish are good sources of several essential mineral macroelements (sodium, potassium, magnesium, calcium, iron, phosphorus) and microelements (iodine, fluorine, selenium, manganese, cobalt). Protein is one of the most important nutrients in fish. The crude protein content in fish muscle is usually in the range of 11–28% (wet weight).[Citation16] An earlier study reported a protein content of 16.1% in the dorsal muscle of common carp.[Citation15] In this study, the mean protein content in different muscle tissues ranged from 47.3 to 76.4% (dry weight), If converted into wet weight, it was 15.2 to 16.8%, which is comparable to previous published data.
Table 1. Proximate composition of muscles from six portions of farmed common carp (Cyprinus carpio).
Lipid is another important nutrient in fish. Fish species can be divided into four classes on the basis of their lipid content: lean (<2%), low fat (2–4%), medium fat (4–8%), and high fat (>8%) fish.[Citation17] Based on this classification, common carp could be characterized as low fat fish. The lipid content in fish muscle is highly variable and is dependent on season, geographical origin, fish diet, age, and reproductive status.[Citation17,Citation18] It also varies with anatomical location of muscle. Common carp contains moderate amount of healthy fatty acids (mainly 20:5n-3 and 22:6n-3).[Citation19] As shown in , lipids were not uniformly distributed throughout the muscle tissues of common carp. The chest and belly sections contained significantly higher levels of lipid than the upper and lower backs. A similar trend was also observed in the study of Mráz.[Citation1] The lipid content in upper and lower back muscles was 16.5 and 17.9%, respectively; however, in belly and chest muscles (45.1–46.2%), many studies have shown that lipids in chest and belly are comprised predominantly triacylglycerols with a high level of monounsaturated fatty acids (MUFA) and low level of n-3 PUFA, which is not favorable for human health. On the contrary, the leanest part, the dorsal white muscles were rich in n-3 PUFA and therefore were nutritionally more beneficial than the other portions.[Citation1,Citation19] In accordance with the study by Venugopal,[Citation16] a significant inverse relationship was observed between lipid and moisture content in upper and lower backs, chest, and belly.
Carbohydrate is generally present in very low level (less than 0.5%) in fish muscle. The carbohydrate content found in our study (1.22–2.55%; ) is similar to the result from previous study.[Citation15] The highest mean concentration of carbohydrate was found in jaw whereas significantly lower carbohydrate content was detected in chest and upper back portions.
In summary, the upper, lower backs and tail sections were high in moisture, protein, and ash content while the chest and belly were characterized by high lipid content. The jaw was somehow in between for all these components.
Physicochemical Properties
Myofibrillar Protein Extractability
Myofibril proteins account for 60–80% of total protein content and is considered as the most important edible part of fish muscle.[Citation20] It is well known that myofibrillar proteins are the major components responsible for the textural properties of fish muscle. High content of myofibrillar protein will give high elasticity and tenderness to fish muscle. In addition, the three-dimensional network of myofibrillar proteins provides the major binding sites for water. As shown in , the highest myofibrillar protein extractability was found in upper back muscle (1.6%) while the lowest was recorded in belly (0.9%). Therefore, the back portions are the most valuable part of common carp from both a nutritional point of view and an organoleptic perspective.
Table 2. Physicochemical properties of muscles from six portions of farmed common carp (Cyprinus carpio).
WHC
The WHC of muscle tissues has a profound impact on quality attributes like tenderness, juiciness, and flavor, which ultimately determines their commercial value and consumer acceptance. As shown in , WHC in upper back, lower back, tail, and jaw were similar and higher than those in chest and belly. Myofibril proteins are the most important proteins in the binding of the water. The majority of the water (around 85%) in muscular tissue is held within the myofibrillar protein network, and only 15% is located in extramyofibrillar spaces (extra-myofibrillar).[Citation21] The ionic strength, pH, and temperature of muscle protein are the important factors that influence its structural features and thus the WHC.[Citation22] Since there is no significant difference in pH value among all the tested muscle tissues (), the slightly higher WHC of the back muscle could be partly explained by the higher content of myofibrillar protein. The initial muscle pH of most freshwater fish immediately after death was in the range of 6.0–6.2.[Citation15] The results from our study showed a higher pH value. This might be explained by the difference in harvest seasons.
Color Measurement
Color is considered to be one of the most important sensory attributes because it directly influences the consumer’s perception of the quality of fish and fish products. There are many factors that influence the color of fish muscle, such as fish species, harvest season, maturation, chemical composition (water, lipid, and protein content), type and amount of heme proteins, slaughtering methods, and storage conditions.[Citation23,Citation24] Among all these factors, the concentration and chemical state of myoglobin has a significant impact on the overall color of fish muscle. Color, represented as CIE, L*, a*, and b* values, at different locations along the fish fillet are shown in . The L* value, which is an index of visual lightness, was relatively and slightly higher in chest than in other parts. This could be partly explained by the higher content of free water. The WHC of chest was found to be the lowest in our study, and this means that the chest has the weakest ability of binding water. So, the content of free water in the part of chest was relatively higher. The more content of free water among the muscle tissues, the more reflectance in the surface of muscle, and so the lightness will be higher.[Citation25] The values of a* and b* were very low in all the tested tissues. These two values were generally higher for jaw, chest, and belly than those for tail, upper, and lower back portions. The reasons for the variations are not fully understood and need further research.
Textural Properties
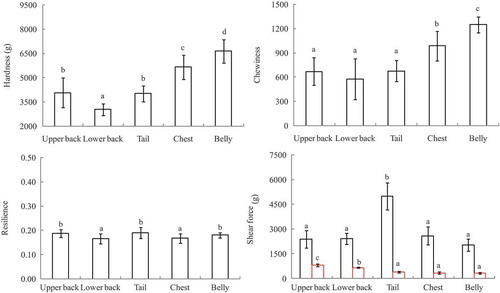
shows variations in the textural properties between different parts of common carp. Except for upper back and tail, significant differences in hardness were observed for the other parts of the fish. The highest value was recorded for belly, whereas the lowest for the lower back. Hardness of fish muscle is influenced by many factors such as density of muscle fiber, fiber length (or fiber diameter), intermyofibrillary spaces and gaps.[Citation26,Citation27] The chemical composition also varies along the fillet and will affect muscle texture properties. Hatae reported that the shorter the muscle fibers, the greater the hardness of the muscle.[Citation26] A study on the textural characteristics of raw Atlantic salmon (Salmo salar) fillets showed that both the hardness and shear force increased from head to tail.[Citation28] Lipid content was distributed unevenly along fish fillets, being one of the key factors that affect muscle texture properties. A significant negative correlation between lipid content and hardness has been observed in several previous studies.[Citation29] Chewiness is a secondary parameter derived from multiplying hardness by cohesiveness and springiness.[Citation30] Chewiness value was higher in belly (12.51 N) than in chest section (9.89 N), both of which were significantly higher than those of the other three parts (upper back, lower back and tail; ). No significant difference could be detected between these three locations on the fillet. In contrast to chewiness, the tail section exhibited the highest resilience value, followed by upper back and belly. The lowest resilience values were recorded in lower back and chest. Resilience describes the ability of the muscle to recover from deformation and offer resistance to subsequent deformation, thus reflecting the elasticity of the muscle.[Citation31] The lower resilience values in lower back and chest indicated that these two regions were less elastic and more rigid than the other parts of the fish. It is also worth noting that resilience properties showed much less variation between different anatomical locations compared to the other textural parameters. This is in agreement with a previous study showing that springiness only gave limited information and did not distinguish texture difference among different body parts of Atlantic salmon (Salmo salar).[Citation28] As shown in , shear force value was significantly higher in the tail section (49.96 N) than in other locations of the fish, whereas the shear force of the other parts was generally not significantly different from each other. This coincides with results reported by Montero and Jonsson[Citation28] who observed that the shear strength values were significantly higher near the tail region of both trout and Atlantic salmon fillets, owing to a higher proportion of insoluble collagen. In addition, muscle fiber dimensions and arrangement vary along the location of the fish and have a great impact on the textural properties.[Citation32,Citation33] The muscle near the tail region is composed mainly of muscle fibers with relatively smaller diameter compared to other parts of the fish, leading to a firmer texture.[Citation34] For shear force after cooking, all the tested parts exhibited significantly lower shear force after heat treatment, being about 20–30% of their fresh counterparts. The upper and lower backs had significantly higher values than the other three portions.
Microstructure
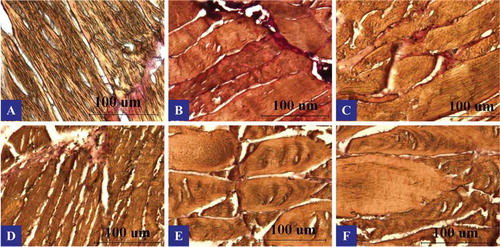
shows the representative light microscopic observations on longitudinal sections of common carp muscles. The muscle fibers were stained with Van Gieson dye which stains myofibrils yellow and connective tissue red. The muscle fibers of common carp are comprised of densely packed contracting subunits called myofibrils, which fill up most of the fiber volume. Each myofibril contains bundles of highly organized longitudinal myofilaments-thick and thin myofilaments. A thin layer of connective tissue, known as the endomysium, surrounds each of these muscle fibers.[Citation35] In the present study, muscle fibers of upper back showed a typical polygonal shape and were tightly attached to one another. The individual fiber, fiber bundles, as well as the global spatial structure were clearly distinguishable (). Lower back fibers were slightly thicker and shorter than those of upper back. In the tail region, larger gaps could be observed between the fiber bundles. The chest and belly sections exhibited similar structural features. The individual fiber was shorter and fiber bundles were relatively loose in arrangement. The fiber clusters in fish jaw were relatively thin, with uniform spacing between clusters.
Fat Distribution Pattern
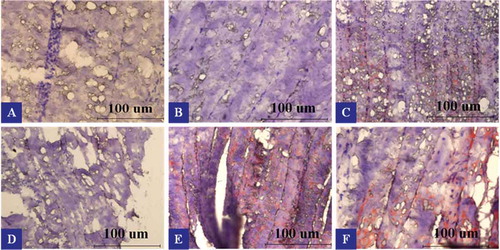
depicts the fat distribution patterns in the six portions of common carp. The muscle tissues were stained with Sudan IV dye which stains adipose tissue orange, fat droplets red and nucleus blue or purple. Intense redness was observed on the surface of upper and lower backs, especially the lower back. This could be attributed to higher fat deposition in the subcutaneous adipose tissue. In contrast, almost no fat depots could be detected in the middle part of upper and lower backs. There is some red area in the innermost part of both portions. The red areas declined gradually from surface to inner part of the tail and jaw sections. Chest and belly contained large amount of red area throughout all parts of them, the redness being the most intense near the surface region of belly flap. Many studies have shown that the distribution of lipids in fish tissues varies widely with species, type of muscle, and portion of muscle. The principal sites of fat deposition are in subcutaneous tissue, belly flap, muscle tissue, liver, mesenteric tissue, and in the head.[Citation36] A visible trend of increasing lipid content along the dorsal-to-ventral direction has been observed in the studies on several seawater and freshwater fish species, including rainbow trout,[Citation37] European sea bass, gilthead sea bream,[Citation38] and Asian catfish (Pangasius bocourti).[Citation19,Citation39] Mráz[Citation19] investigated the lipid distribution in different muscle tissues of pond-cultivated common carp in South Bohemia. The highest lipid contents were observed in abdominal wall (30.2 ± 7.8%). In contrast, dorsal white muscle had the lowest lipid content (0.95 ± 0.14%). The red muscle was also found to be of high lipid content (16.7 ± 5.0%). Our observations are, therefore, consistent with these previous studies.
Conclusions
The nutritional composition, physiochemical properties and structural characteristics varied widely among different anatomical locations of common carp. The upper and lower backs were high in protein and low in fat. They also possessed desirable color and textural properties such as moderate hardness and chewiness as well as good WHC. Therefore, they are the most valuable parts of common carp from both a nutritional point-of-view and an organoleptic perspective. On the other hand, the chest and belly sections had much higher lipid content and relatively high hardness and chewiness values. In order to maximize the nutritional and sensory qualities of processed carp products, further investigations are required to develop scalable processing technologies and optimize process parameters for different types of muscle tissues.
Funding
This work was supported by the National Key Technology Research and Development Program of China during the 12th Five-Year Plan (No. 2014BAD04B09).
Additional information
Funding
References
- Mráz, J.; Pickova, J. Differences Between Lipid Content and Composition of Different Parts of Fillets from Crossbred Farmed Carp (Cyprinus Carpio). Fish Physiology and Biochemistry 2009, 35, 615–623.
- FAO. The State of World Fisheries and Aquaculture. 2012. In FAO Fisheries and Aquaculture Department, Rome, 2012; 29.
- FBAM. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2013.
- Banskalieva, V.; Sahlu, T.; Goetsch, A.L. Fatty Acid Composition of Goat Muscles and Fat Depots: A Review. Small Ruminant Research 2000, 37, 255–268.
- Intarapichet, K.O.; Suksombat, W.; Maikhunthod, B. Chemical Compositions, Fatty Acid, Collagen and Cholesterol Contents of Thai Hybrid Native and Broiler Chicken Meats. The Journal of Poultry Science 2008, 45, 7–14.
- Nuernberg, K.; Fischer, K.; Nuernberg, G.; Kuechenmeister, U.; Klosowska, D.; Eliminowska-Wenda, G.; Fiedler, I.; Ender, K. Effects of Dietary Olive and Linseed Oil on Lipid Composition, Meat Quality, Sensory Characteristics and Muscle Structure in Pigs. Meat Science 2005, 70, 63–74.
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat Deposition, Fatty Acid Composition and Meat Quality: A Review. Meat Science 2008, 78, 343–358.
- https://salmon.fromnorway.com/preparing-and-cooking/filleting-and-cuts/ (accessed on 21 October 2016).
- AOAC. Official Methods of Analysis, 17th Ed; Association of Official Analytical Chemists: Washington, DC, 2002.
- Skipnes, D.; Johnsen, S.O.; Skara, T.; Sivertsvik, M.; Lekang, O. Optimization of Heat Processing of Farmed Atlantic Cod (Gadus Morhua) Muscle With Respect to Cook Loss, Water Holding Capacity, Color, and Texture. Journal of Aquatic Food Product Technology 2011, 20, 331–340.
- Zhu, S.; Luo, Y.; Hong, H.; Feng, L.; Shen, H. Correlation Between Electrical Conductivity of the Gutted Fish Body and the Quality of Bighead Carp (Aristichthys Nobilis) Heads Stored at 0 and 3℃. Food and Bioprocess Technology 2013, 6, 3068–3075.
- Bradford, M.N. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochmiestry 1976, 72, 248–254.
- Skipnes, D.; Østby, M.L.; Hendrickx, M.E. A Method for Characterising Cook Loss and Water Holding Capacity in Heat Treated Cod (Gadus Morhua) Muscle. Journal of Food Engineering 2007, 80, 1078–1085.
- Liu, S. Practical Biohistology Technology (in Chinese), 1st Ed; Science Press: Beijing, China, 2006; 86–87.
- Xia, W.; Luo, Y.; Xiong, S.; Xu, Y. Preservation and Processing Technology for Fresh Water Fish; China Agricultural Press (in Chinese): Beijing, China, 2004; 30–31.
- Venugopal, V.; Shahidi, F. Structure and Composition of Fish Muscle. Food Reviews International 1996, 2, 175–197.
- Ackman, R.G. Seafood Lipids and Fatty Acids. Food Reviews International 1989, 6, 617–646.
- Lunn, J.; Theobald, H.E. The Health Effects of Dietary Unsaturated Fatty Acids. Nutrition Bulletin 2006, 31, 178–224.
- Mráz, J. Lipid Quality of Common Carp (Cyprinus Carpio) in Pond Culture. Ph.D. Dissertation, Swedish University of Agricultural Sciences, Uppsala, Sweden, January 2011, 12.
- Delbarre-Ladrat, C.; Chéret, R.; Taylor, R.; Verrez-Bagnis, V. Trends in Postmortem Aging in Fish: Understanding of Proteolysis and Disorganization of the Myofibrillar Structure. Critical Reviews in Food Science and Nutrition 2006, 5, 409–421.
- Huff, L.; Elisabeth, E.; Steven, M. Mechanisms of Water-Holding Capacity of Meat: The Role of Postmortem Biochemical and Structural Changes. Meat Science 2005, 1, 194–204.
- Ofstad, R.; Egelandsdal, B.; Kidman, S.; Myklebust, R.; Olsen, R.L.; Hermansson, A. Liquid Loss as Effected by Post Mortem Ultrastructural Changes in Fish Muscle: Cod (Gadus Morhual) and Salmon (Salmo Salar). Journal of the Science of Food and Agriculture 1996, 3, 301–312.
- Stien, L.H.; Hirmas, E.; Bjørnevik, M.; Karlsen, Ø.; Nortvedt, R.; Rørå, A.M.B.; Sunde, J.; Kiessling, A. The Effects of Stress and Storage Temperature on the Color and Texture of Pre-Rigor Filleted Farmed Cod (Gadus Morhua L.). Aquaculture Research 2005, 36, 1197–1206.
- Roth, B.; Foss, A.; Imsland, A.K. Relationship Between Muscle pH and Flesh Color of Atlantic Halibut. Journal of Food Science 2009, 74, 123–S125.
- Ocan-higuera, V.M.; Marquez-rios, E.; Canizales-davila, M.; Castillo-Yáñez, F.J.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; García-Orozco, K.D.; Graciano-Verdugo, A.Z. Postmortem Changes in Cazon Fish Muscle Stored on Ice. Food Chemistry 2009, 116, 933–938.
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Role of Muscle Fibres in Contributing Firmness of Cooked Fish. Journal of Food Science 1990, 55, 693–696.
- Lin, W.L.; Zeng, Q.X.; Zhu, Z.W.; Song, G.S. Relation Between Protein Characteristics and TPA Texture Characteristics of Crisp Grass Carp (Ctenopharyngodon Idellus C. et V</i>) and Grass Carp (Ctenopharyngodon Idellus). Journal of Texture Studies 2012, 43, 1–11.
- Sigurgisladottir, S.; Hafsteinsson, H.; Jonsson, A.; Lie, Ø.; Nortvedt, R.; Thomassen, M.; Torrissen, O. Textural Properties of Raw Salmon Fillets as Related to Sampling Method. Journal of Food Science 1999, 64, 99–104.
- Nielsen, D.; Hyldig, G.; Nielsen, J. Liquid Holding Capacity and Instrumental and Sensory Texture Properties of Herring Related to Biological and Chemical Parameters. Journal of Texture Studies 2005, 36, 119–138.
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd Ed; Academic Press: San Diego, CA, 2002; 184.
- Veland, J.O.; Torrissen, O.J. The Texture of Atlantic Salmon (Salmo Salar) Muscle as Measured Instrumentally Using TPA and Warner-Brazler Shear Test. Journal of the Science of Food and Agriculture 1999, 79, 1737–1746.
- Montero, P.; Borderias, J. Distribution and Hardness of Muscle Connective Tissue in Hake (Merluccius Merluccius L.) and Trout (Salmo Irideus Gibb). Lebesnmittel Untersuchung and Forschung 1989, 189, 530–533.
- Jonsson, A.; Sigurgisladottir, S.; Hafsteinsson, H.; Kristbergsson, K. Textural Properties of Raw Atlantic Salmon (Salmo Salar) Fillets Measured by Different Methods in Comparison to Expressible Moisture. Aquaculture Nutrition 2001, 7, 81–90.
- Casas, C.; Martinez, O.; Guillen, M.D.; Pin, C.; Salmeron, J. Textural Properties of Raw Atlantic Salmon (Salmo Salar) at Three Points Along the Fillet, Determined by Different Methods. Food Control 2006, 17, 511–515.
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and Physical Changes of Grass Carp (Ctenopharyngodon Idella) Fillets Stored at –3 and 0°C. Food Chemistry 2013, 140, 105–114.
- Zhou, S.; Ackman, R.G.; Morrison, C. Storage of Lipids in the Myosepta of Atlantic Salmon (Salmo Salar). Fish Physiology and Biochemistry 1995, 14, 171–178.
- Fjellanger, K.; Obach, A.; Rosenlund, G. Proximate Analysis of Fish with Special Emphasis on Fat. In Kestin, S.C.; Warriss, P.D.; Eds.; Farmed Fish Quality; Blackwell Science: Oxford, UK, 2001; 307–317.
- Testi, S.; Bonaldo, A.; Gatta, P.P.; Badiani, A. Nutritional traits of dorsal and ventral fillets from three farmed fish species. Food Chemistry, 2006, 98:104–111.
- Thammapat, P.; Raviyan, P.; Siriamornpun, S. Proximate and Fatty Acids Composition of the Muscles and Viscera of Asian Catfish (Pangasius Bocourti). Food Chemistry 2010, 122, 223–227.