ABSTRACT
The conditions of roasting are the key step in manufacturing Sesame (Sesamum indicum L.) oil. Roasting sesame seeds changed its oil, moisture, and ash content. In oils from unroasted and roasted seeds, nine fatty acids analyzed by gas chromatography-mass spectrometry. The fatty acids composition were changed in both oils. Oleic and linoleic changed in large amount, but changes for other fatty acids were small. 1H and 13C NMR analysis showed the frequency changes for both oils, the roasting condition caused to shift the frequency to lower degree.
Introduction
Sesame (Sesamum indicum L.) is a member of the Pedaliaceae family.[Citation1] It is an economically important oil seed crop which is widely cultivated in many parts of the world, primarily in tropical and subtropical areas.[Citation2] It has been used as a food source extensively for thousands of years, then later as significance for edible oil, paste, cake, confectionary purposes, and flour.[Citation2–Citation6] The extensive usage of sesame may be due to its stable oil content, nutritious protein, and savory nutty roasted flavor.[Citation7,Citation8] The chemical composition of sesame shows that the seed is an important source of oils (44–58%), proteins (18–25%), carbohydrates (11–13.5%), and ash (±5%).[Citation1,Citation5,Citation9] Its oil has a mild taste and high unsaturated fatty acids, about 85%.[Citation10,Citation11] It is known that sesame oil is resisted to oxidative deterioration compared to other edible oils,[Citation10] because it is naturally contains antioxidants such as tocopherols and lignans.[Citation2,Citation3,Citation12–Citation14] High contents of essential linoleic and linolenic acids are another merit of sesame oil as a food source.[Citation15]
Roasting is a significant step in the processing of some types of foods like coffee, peanuts, and beans, which results in important physical, chemical, structural, and organoleptic changes.[Citation16–Citation19] It is also the basic operation in the processing of sesame products.[Citation7,Citation10] Roasting of the sesame enhances its flavor, color, and texture and this ultimately increases the overall palatability.[Citation7,Citation20] The conventional production of sesame oil involves cleaning, roasting, grinding, cooking, and pressing, but not refining.[Citation21]
The roasting process introduces chemical composition changes in the sesame seeds and oil. It is reported that the oxidative stability of sesame oil depends on the roasting temperature.[Citation21–Citation23] At higher roasting temperatures a strong favor is obtained, but the quality of the oil may be reduced.[Citation21,Citation24] Also the concentration of hydrocarbons varies between roasted and unroasted seed; the lower molecular weight hydrocarbons (C12–C19) are decreased, whereas the higher molecular weight hydrocarbons (C20–C32) and squalene are increased as a percent of the hydrocarbon fraction.[Citation20] The percentage of unsaponifiable matter is also slightly increased after the roasting process.[Citation13] The object of this study was therefore to compare the fatty acid profile between oils from unroasted and roasted brown sesame seeds from Kurdistan Region, Iraq using gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR).
Materials and method
Materials
Brown sesame seeds were purchased from a local market in Rania City, Kurdistan Region, Iraq. All chemicals and solvents used were either analytical or high-performance liquid chromatography (HPLC) grade. The chemicals n-butanol, chloroform, n-hexane, and methanol were obtained from Fisher (UK). Free fatty acid standards (~99% purity) of palmitic, palmitoleic, stearic, oleic, vaccenic, linoleic, α-linolenic, and eicosenoic acid were purchased form Sigma-Aldrich (UK).
Roasting and extraction oil
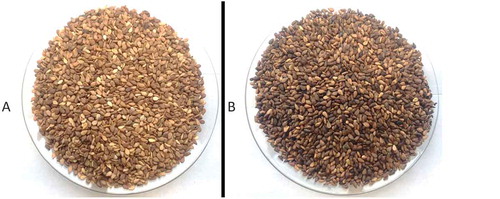
The seeds were cleaned manually to remove dust, dirt, stones, chaff, immature and broken seeds; and stored in sealed plastic bags at room temperature (25°C) to preserve the quality. Sesame seeds (500 g) were roasted (30 min) at 200°C on a hot plate () then milled to 1–3 mm.
Figure 1. Sesame seeds from Kurdistan Region–Iraq; A: unroasted brown sesame seeds, B: roasted brown sesame seeds at 200°C for 30 min on a hot plate.
Ground seeds (100 g) were extracted in a conical flask (250 mL) with n-hexane (250 mL) and stirring at room temperature for 3 h. The solution was filtered, reserving the filtrate and further extracted successively in n-hexane (3 × 200 mL). All n-hexane filtrates were combined and then filtered under gravity (Whatman 150) to remove any solid particles. The n-hexane was dried over magnesium sulphate (MgSO4) and filtered. Finally, the n-hexane was removed by rotary evaporator (Buchi R-114 Rotavapor, Switzerland) and water bath (Buchi B-481 water bath, Switzerland), at 40ºC. Residual solvent was removed under vacuum until a constant weight was obtained. The final product was golden yellow oil.
Transesterification and analysis of sesame oils
All standards and sesame oils were trans-esterified to produce fatty acid methyl esters (FAMEs). Aliquots of oil (2.0 g) were dissolved in sodium methoxide (8 mmol, 20 mL) and heated under reflux for 20 min. Deionized H2O (50 mL) was added, and the solution extracted successively with n-hexane (3 × 100 mL). The organic extracts were dried with MgSO4, then filtered and evaporated at 40°C. The trans-esterified samples and standards were diluted 1:1000 with n-hexane, then injected in the GC-MS device.
FAMEs of both unroasted and roasted sesame oil and standards were analyzed using a GC-MS Thermofisher Trace 1300 GC fitted with a DB-5 column (L 30 m, ID 0.25 mm, DF 0.25 mm). The injector type was a programmed temperature vaporizer, operating in split/splitless mode, with an injection volume of 1 μL at 250ºC, with a split flow of 20 mL min −Citation1. The initial temperature was set at 150ºC, which was held for 2 min. The temperature of oven was increased at a rate of 5ºC/min until final temperature, 260ºC and then held for 5 min. The detector was a Thermofisher ITQ900. Electron impact (70 eV) ionization was used for fragmentation at a rate of two scans per second. All processing and analysis was carried out on Thermo Xcalibur, v 2.2.
NMR analysis
Sesame oil (100.0 mg) was dissolved in 0.6 mL deuterated chloroform (CDCl3) and Citation1H and Citation13C NMR spectra were obtained on the Ultra Shield TM Plus 400—Bruker 400 MHz spectrometer at 25°C. The Citation1H NMR was performed at an operating frequency of 400 MHz and Citation13C NMR was at 100 MHz in NMR tube (5 mL). There was a relaxation time of 2 s for Citation1H NMR and 4 s for Citation13C NMR operations. All processing and data analysis was performed on MestReNova LITE v 5.2.5.
Moisture content and ash
The moisture contents of unroasted and roasted sesame seeds were determined according to Kahyaoglu and Kaya.[Citation7] Five grams of seeds were heated to constant weigh at 105°C. Ash contents of unroasted and roasted sesame seeds were determined.[Citation7] The sesame seeds were dried, and 5.0 g placed in a porcelain crucible and heated under normal atmospheric conditions to 500ºC for 4 h in a muffle furnace (Carbolite–ELF11/148, England). The samples were cooled in a desiccator for 1 h, then the ash left in the crucible was weighed to determine percentage ash content.
Statistical analysis
There were three independent measurements for all observations with mean values ± standard deviations reported for each case. Analysis of variance (ANOVA) was performed on the data at the level of p < 0.05 to evaluate the significance of differences between treatment means.
Result and discussion
While sesame oil was extracted from 100 g of both unroasted and roasted brown sesame seeds, different oil contents were derived from the two treatments. The mean oil yields for unroasted and roasted seeds were 45.7 and 51.1 g, respectively (). This was consistent with other studies that roasting resulted in an increase in oil yield because it enhanced protein denaturation which in turn improved lipid extractability.[Citation13] However moisture content and ash content were negatively affected by roasting. Moisture content decreased from 4.9 g in unroasted seeds to 3.8 g in roasted seeds and ash content decreased from 5.9 g in unroasted seeds to 5.8 g in roasted seeds, in 100 g of raw seeds. Other studies showed that, as the roasting temperature and time were increased, the moisture content of sesame seeds decreased for all roasting methods. The seeds become more fractured with roasting and take on more crispy texture.[Citation7] Meanwhile roasting caused an increased in the percentage of unsaponifiable matter from 1.2 to 1.3 g, in 100 g of raw seeds.
Table 1. Chemical composition of unroasted and roasted brown sesame seeds, in 100 g raw seeds matter.
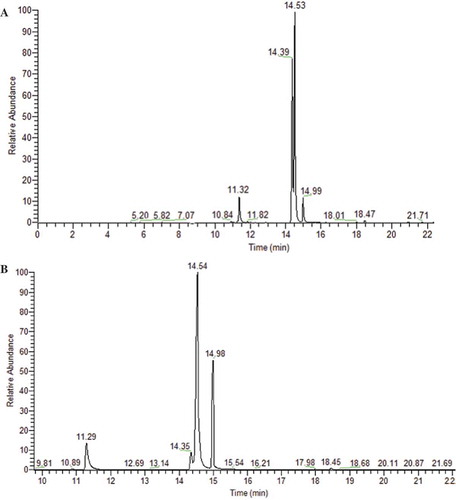
The analysis of sesame oils by GC-MS needed derivatization to improve their volatility and the frequency of resolution. It could be obtained by transesterification using an alkyl ester. In the GC chromatogram (), nine fatty acids were determined in both sesame oils. The relative fatty acid content for both oils was listed in (). The chain lengths of fatty acids included C14, C16, C18, C20, and C22. The saturated fatty acids (SFA) present were myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), arachidic acid (C20:0), and eicosadienoic acid (C22: 0). Unroasted sesame oil was comprised of 12.8% SFAs, while roasted sesame oil contained 15.9% SFAs. Roasting increased the oil content of SFAs. In both oils palmitic acid was the common fatty acid, followed by stearic acid, while the other SFAs were present in trace amounts. The monounsaturated fatty acids (MUFAs) palmitoleic acid (C16:1n-9) and oleic acid (C18:1n-9) were detected in both sesame oils. Linoleic acid (C18:2n-9, 12) and α-linolenic acid (C18:3n-9, 12, 15) were present as polyunsaturated fatty acids (PUFAs). The PUFA content was negatively affected by roasting; linoleic acid decreased from 46.1% in unroasted oil to 24.8% in roasted oil, and α-linolenic acid decreased from 0.4 to 0.2%.
Table 2. Fatty acid composition (%) of unroasted and roasted brown sesame seeds oil.
Figure 2. Chromatograms of GC-MS (Thermofisher) of FAMEs sesame oil; A: chromatogram of GC-MS of unroasted sesame seeds oil, B: chromatogram of GC-MS of roasted sesame seeds oil.
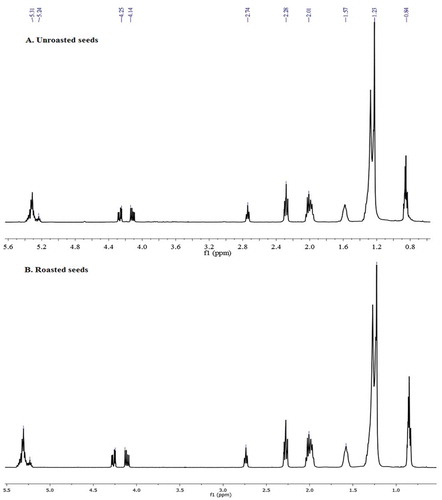
There have been reports on the analysis of oils by Citation1H NMR. To determine oil composition olefinic carbons, ω-3 methylene carbons, allylic carbons, and β-methylene carbons were used. The Citation13C NMR was based on determination of the resonance intensities of carbon-13 nuclei of triglyceride fatty acids.[Citation25] Citation1H NMR indicates the approximate relative composition of FAs in the oils.[Citation26] To determine any changes in the oil composition between unroasted and roasted sesame we may evaluate the correlation in their olefinic region (). In the Citation1H NMR spectrum of unroasted sesame oil (), the integration of the olefinic region showed a multiplet at
Table 3. Chemical shifts and assignments of the characteristic resonances in the 1H NMR spectra of unroasted and roasted crude sesame seed oil, (400 MHz in CDCl3).
Figure 3. The Citation1H NMR spectra of unroasted and roasted crude sesame seed oil at 400 MHz in CDCl3; A: Citation1H NMR spectrum of crude unroasted sesame oil, B: Citation1H NMR spectrum of crude roasted sesame oil.
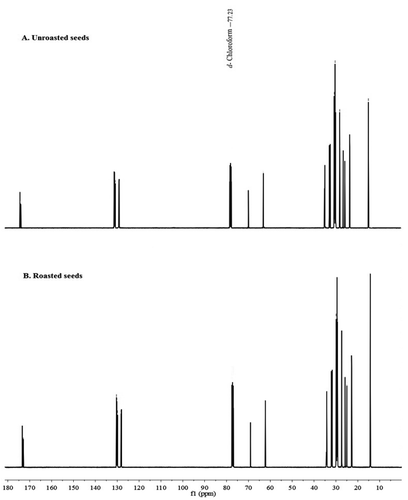
The Citation13C NMR resonances of the oils could be grouped into four well-defined spectral regions: carbonyl carbons ranging from 173.3 to 172.8 ppm; unsaturated carbons ranging from 132.1 to 126.8 ppm; glycerol carbons ranging from 69.1 to 61.6 ppm; and aliphatic carbons ranging from 34.5 to 13.9 ppm. All assayed oils differed in quantitative chain composition rather than in qualitative acid profile; therefore, exhibiting analogous signals, although with varying intensities which were characteristic for each oil.[Citation28,Citation29] All chemical shifts of Citation13C NMR of sesame oil were listed ().
Table 4. 13C NMR assignments of unroasted and roasted crude Sesame oil at 100 MHz in CDCl3. Two signals for the same olefinic environment indicate an equivalent resonance between two different olefinic carbons. Two signals for the same environment are splits in the triacylglycerol signal between the sn-1,3 or sn-2 fatty acid chains.
In Citation13C NMR, the carbonyl carbons of oil triglycerides appeared as two sets of resonances, the high frequency set at 1(3)-glycerol positions, whereas the low frequency was at the 2-glycerol position chains, as shown in .[Citation29] In particular, the signals from carbonyl carbons of 1(3)-chains were shifted by about 0.42 ppm for unroasted and 0.41 ppm for roasted seeds, at higher frequency from those of carbonyls of 2-chains.[Citation28,Citation30]
Figure 4. Citation13C NMR spectra of unroasted and roasted crude sesame oil at 100 MHz in CDCl3; A: Citation13C NMR spectrum of unroasted crude sesame oil, B: Citation13C NMR spectrum of roasted crude sesame oil, showing the Citation13C assignment in .
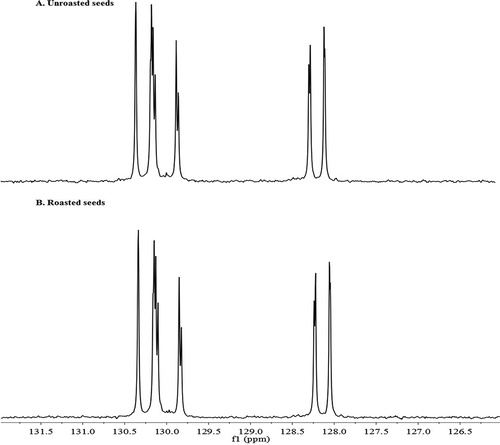
Citation13C NMR also showed the unsaturated region of fatty acid carbons between 127.79–131.81 ppm (). For each double bond of UFAs, symmetrical pairs of signals can be seen for each side. In both oils the chemical shifts were changed but the difference between the peaks had not changed that much, therefore the roasting condition caused to shift the frequency to lower degree. For instance the differences between carbon shifts of linoleyl chains in unroasted seeds were 0.17 ppm for C-10 to C-12 and for C-9 and C-13 was 0.45 ppm, also for oleyl chains were changed by 0.33 ppm for C-9 to C-10. However in roasted seeds the chemical shifts for linoleyl chains were 0.17 ppm for C-10 to C-12 and for C-9 and C-13 was 0.45 ppm, and for oleyl chains were changed by 0.31 ppm for C-9 to C-10. Also in Citation13C NMR the chemical shifts showing the presence of α-linolenic acid (C18:3n-9, 12, 15), as Citation1H NMR shifting of the terminal methyl at (δ 0.95 ppm, t) confirmed that there is ω3-fatty acid, but it is in trace amount.[Citation26] The presence of the glycerol backbone of oil resulted in 1- and 2-carbon split signal for carbons in the olefinic (alkene) region (); therefore, each unsaturated carbon is split according to glycerol position. The distance between the pair of signals depends on its distance from the C1 environment. The non-equivalence of chemical shifts of olefinic carbons has been studied in a series of MUFAs with different number of C–C bonds separating the ester group and the double-bond. The relevant feature of these chemical shifts is that the carbons of a double-bond pair are shifted in opposite directions, it means the unsaturated carbon nearest to the chain ester group is shifted to lower frequency from the unsaturated carbon closest to the chain methyl end. The chemical shift differences which increase as the double bond moves toward the carboxyl group. The chemical shift differences which increase as the double-bond moves toward the carboxyl group, were proved to arise from a linear electric field effect. Also, it was possible to determine the proportion of UFAs at one or two positions.[Citation28]
Figure 5. The olefinic region 127–132 ppm of 100 MHz Citation13C NMR spectra of crude sesame oil; A: Citation13C NMR spectrum of olefinic region of crude unroasted sesame seeds oil, B: Citation13C NMR spectrum the olefinic region of crude roasted sesame seeds oil.
The glycerol carbons resonate in the spectral region from 62.26 to 69.07 ppm for unroasted seeds and for roasted seeds was 62.25 to 69.05 ppm. The chemical shift assignments were based on the assumption that acylglycerol symmetry or asymmetry determines the number of resonances and their relative intensities. The resonances of methylene and methyl carbons of saturated and unsaturated acyl chains are reported. The C-16–C-18 carbons, which were assigned using additively relationship which predicts the Citation13C chemical shifts of n-alkanes, appear at chemical shifts centered at 31.70, 22.86, and 14.28 ppm for roasted seeds also 31.70, 22.75, and 14.27 ppm for unroasted seeds. Within each set of signals, saturated, oleyl, and linoleyl chains resonate from higher to lower frequency in this order. However, an influence of the chain position on glycerol backbone was not detected.
Conclusion
Sesame (Sesamum indicum L) is an oil seed herbaceous crop that is of great economic importance due to its highly stable oil and nutritious content. The manufacture of high quality sesame oil requires that the maintenance of the optimum roasting conditions. The roasting process affected the physiochemical properties of sesame seeds and oil, measurably in oil content, moisture, ash, and unsaponifiable matter in 100 g of seeds. The oil content increased by roasting from 49.1 and 51.1 g for unroasted and roasted seeds, respectively. The moisture and ash content were decreased, the moisture content in unroasted seeds was 4.9 g decreased to 3.8 g also ash content decreased from 5.9 to 5.8 g. Unsaponifiables matters had increased slightly. The oils from unroasted and roasted seeds were analysed by GC-MS and NMR. By GC-MS analysis in both oils those fatty acids were analyzed; myristic, palmitic, palmitoleic, stearic, oleic, linoleic, α-linolenic, arachidic, and eicosadienoic acid. The fatty acid compositions of oils were changed by roasting. Oleic acid increased in high amount, it was 40.3% in unroasted seeds increased 58.7% in roasted seeds. Also, palmitic acid increased from 5.6 to 7.1% and stearic acid increased from 5.3 to 6.8%. But, linoleic acids decreased significantly, from 46.1 to 24.8% in unroasted and roasted seeds, respectively. The other fatty acids were changed in low amount. Both Citation1H and Citation13C NMR analysis showed frequency changes during determination of oils. Overall, the roasting process caused to shift the frequency to lower degree. For all individual fatty acids the frequencies were changed during measurements. Finally, the procedures and laboratory analysis fall within the general patterns for other contents and general organic compounds of sesame seeds. It is apparent that roasting of sesame seeds had potential changes, further studies are needed to explore such combination effects and further investigations should focus on the changes of biological active content of sesame seeds.
Acknowledgments
The authors would like to thank technical staff of Chemistry Department at University of Raparin.
Funding
This work was financed with grants from the HCDP program in the Ministry of Higher Education and Scientific Research of Kurdistan – Iraq, No.: JUOR15.
Additional information
Funding
References
- Kang, M.H.; Choi, J.S.; Ha, T.Y. Chemical Properties of Sesame Seed Cultivated in Korea and China. Food Science Biotechnology 2003, 12, 621–624.
- Wen-Huey, W. The Contents of Lignans in Commercial Sesame Oils of Taiwan and Their Changes During Heating. Food Chemistry 2007, 104, 341–344.
- Rangkadilok, N.; Pholphana, N.; Mahidol, C.; Wongyai, W.; Saengsooksree, K.; Nookabkaew, S.; Satayavivad. J. Variation of Sesamin, Sesamolin and Tocopherols in Sesame (Sesamum Indicum L.) Seeds and Oil Products in Thailand. Food Chemistry 2010, 122, 724–730.
- Abou-Gharbia, H.A.; Shehata, A.A.Y.; Shahidi, F. Effect of Processing on Oxidative Stability and Lipid Classes of Sesame Oil. Food Research International 2000, 33, 331–340.
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Attia, H. Quality Characteristics of Sesame Seeds and by-Products. Food Chemistry 2007, 103, 641–650.
- Sawaya, W.N.; Ayaz, M.; Khalil, J.K.; Al-Shalhat, A.F. Chemical Composition and Nutritional Quality of Tehineh (Sesame Butter). Food Chemistry 1985, 18, 35–45.
- Kahyaoglu, T.; Kaya. S. Modeling of Moisture, Color and Texture Changes in Sesame Seeds During the Conventional Roasting. Journal of Food Engineering 2006, 75, 167–177.
- Yoshida, H.; Tanaka, M.; Tomiyama, Y.; Mizushina, Y. Regional Distribution in the Fatty Acids of Triacylglycerols and Phospholipids of Sesame Seeds (Sesamum Indicum). Journal of Food Lipids 2007, 14, 189–201.
- Kang, C.H.; Park, J.K.; Park, J.U.; Chun, S.S.; Lee, S.C.; Ha, J.U.; Hwang, Y.I. Comparative Studies on the Fatty Acid Composition of Korean and Chinese Sesame Oils and Adulterated Sesame Oils with Commercial Edible Oils. Journal of the Korean Society of Food Science and Nutrition 2002, 31, 17–20.
- Gow-Chin, Y.; Shyi-Liang, S. Oxidative Stability of Sesame Oil Prepared from Sesame Seed with Different Roasting Temperatures. Food Chemistry 1989, 31, 215–224.
- Mondal, N.; Bhat, V.K.; Srivastava, S.P. Variation in Fatty Acid Composition in Indian Germplasm of Sesame. Journal of the American Oil Chemists’ Society 2010, 87, 1263–1269.
- Ahmad, S.; Yousuf, S.; Ishrat, T.; Khan, M.B.; Bhatia, K. Fazli, I.S.; Khan, J.S.; Ansari, N.H.; Islam, F.L. Effect of Dietary Sesame Oil as Antioxidant on Brain Hippocampus of Rat in Focal Cerebral Ischemia. Life Sciences 2006, 79, 1921–1928.
- Mohamed, H.M.A.; Awatif, I.I. The Use of Sesame Oil Unsaponifiable Matter as a Natural Antioxidant. Food Chemistry 1998, 62, 269–276.
- Kiso, Y. Antioxidative Roles of Sesamin, a Functional Lignan in Sesame Seed, and Its Effect on Lipid- and Alcohol-Metabolism in the Liver: A DNA Microarray Study. BioFactors 2004, 21, 191–196.
- Park, Y.W.; Chang, P.; Lee, J. Application of Triacylglycerol and Fatty Acid Analyses to Discriminate Blended Sesame Oil with Soybean Oil. Food Chemistry 2010, 123, 377–383.
- Pittia, P.; Rosa, M.D.; Lerici, C.R. Textural Changes of Coffee Beans as Affected by Roasting Conditions. LWT–Food Science Technology 2001, 34, 168–171.
- Cammerer, B.; Kroh. L.W. Shelf Life of Linseeds and Peanuts in Relation to Roasting. LWT–Food Science Technology 2009, 42, 545–549.
- Ozdemir, M.; Devres, O. Analysis of Color Development During Roasting of Hazelnuts Using Response Surface Methodology. Journal of Food Engineering 2000, 45, 17–24.
- Anjum, F.; Anwar, F.; Jamil, A.; Iqbal, M. Microwave Roasting Effects on the Physico-Chemical Composition and Oxidative Stability of Sunflower Seed Oil. Journal of the American Oil Chemists’ Society 2006, 83, 777–784.
- Yoshida, H. Composition and Quality Characteristics of Sesame Seed (Sesamum Indicum) Oil Roasted at Different Temperatures in an Electric Oven. Journal of the Science of Food and Agriculture 2006, 65, 331–336.
- Bozkurt, H. Comparison of the Effects of Sesame and Thymbra Spicata Oil During the Manufacturing of Turkish Dry-Fermented Sausage. Food Control 2007, 18, 149–156.
- Yoshida, H.; Takagi, S. Microwave Roasting and Positional Distribution of Fatty Acids of Phospholipids in Soybeans (Glycine Max L.), Journal of the American Oil Chemists’ Society 1997, 74, 915–921.
- Takagi, S.; Ienaga, H.; Tsuchiya, C.; Yoshida, H. Microwave Roasting Effects on the Composition of Tocopherols and Acyl Lipids Within Each Structural Part and Section of a Soya Bean. Journal of the Science of Food and Agriculture 1999, 79, 1155–1162.
- Lee, E.; Choe, E. Changes in Oxidation-Derived off-Flavor Compounds of Roasted Sesame Oil During Accelerated Storage in the Dark. Biocatalysis and Agricultural Biotechnology 2012, 1, 89–93.
- Siddiqui, N.; Sim, J.; Christopher, J.L.; Toms, H.; Richard, A.I.; Grootveld, M. Multicomponent Analysis of Encapsulated Marine Oil Supplements Using High-Resolution 1-H and 13-C NMR Techniques. Journal of Lipid Research 2003, 44, 2406–2427.
- Miyake, Y.; Yokomizo, K.; Matsuzaki, N. Determination of Unsaturated Fatty Acid Composition by High-Resolution Nuclear. Magnetic Resonance Spectroscopy 1998, 75, 1091–1094.
- Igarashi, T.; Aursand, M.; Hirata, Y.; Gribbestad, I.S.; Wada, S.; Nonaka, M. Non Destructive Quantitative Determination of Decosahexaenoic Acid and N-3 Fatty Acids in Fish Oils By High-Resolution Nuclear Magnetic Resonance Spectroscopy. Journal of the American Oil Chemists’ Society 2000, 77, 737–748.
- Sega, A.; Zanardi, I.; Chiasserini, L.; Gabbrielli, A.; Bocci, V.; Travagli, V. Properties of Sesame Oil by Detailed 1H and 13C NMR Assignments Before and After Ozonation and Their Correlation with Iodine Value, Peroxide Value, and Viscosity Measurements. Chemistry and Physics of Lipids 2010, 163, 148–156.
- Wollenberg, K.F. Quantitative High Resolution 13C NMR of the Olefinic and Carbonyl Carbons of Edible Vegetable Oils. Journal of the American Oil Chemists’ Society 1990, 67, 487–494.
- Vlahov, G. Regiospecific Analysis of Natural Mixtures of Triglycerides Using Quantitative 13C Nuclear Magnetic Resonance of Acyl Chain Carbonyl Carbons. Magnetic Resonance in Chemistry 1998, 36, 359–362.
