ABSTRACT
Acid-swollen collagen fibers and nano-hydroxyapatite were used to develop a biodegradable film. At 4 wt% of hydroxyapatite or below, collagen fiber formed acceptably homogeneous films, with hydroxyapatite dependent increase in opacity and color. Hydroxyapatite addition significantly improved tensile strength of the composite films along with an observable decrease in elongation. Furthermore, the film’s water vapor and oxygen barrier property were enhanced. Additionally, thermal stability of the films was improved. Scanning electron microscope images showed adding hydroxyapatite caused the composite films a coarser and uneven surface. Collagen fiber-hydroxyapatite composite film provides the potential to apply as a novel material for food packaging.
Introduction
In order to alleviate the increasingly serious environmental pollution and food safety concerns caused by petroleum-based polymeric materials, biodegradable and biocompatible materials have drawn more and more attention, and a vast variety of biomaterials, such as starch, fiber,[Citation1,Citation2] proteins,[Citation3,Citation4] lipids,[Citation5] and gelatin,[Citation6,Citation7] have been widely researched and developed. Collagen, the most abundant and widespread protein in animals, was considered one of the most useful biomaterials.[Citation8,Citation9] Physiologically, by hydrogen bond, aldol condensation, and interchain bifunctions, four to eight collagen molecules aggregate through fibrillogenesis into microfibrilscan and further into collagen fibrils with the diameter ranging from 10 to 500 nm.[Citation10] With further self-aggregation and intermolecular crosslinks, collagen-based collagen fibrils show extra strength and stability.[Citation11] A number of collagen fibrils assemble into collagen fibers and construct a strong network to provide supporting and protecting for animal tissues. Under certain external conditions, these collagen fibers can be isolated from animals and show perfect film-forming characteristic, such as strong mechanical strength, swelling features and insolubility in water. Due to its superior mechanical properties, biodegradable, non-toxic, and good biocompatibility, collagen is widely used in the bio-medical, food, cosmetic industries.[Citation12] Moreover, the full transition of low-valued collagen into useful material also is promoted by the abundance of collagen sources mainly from animal byproducts and leather, as well as increasing demand for efficient treating them from environment pressure.[Citation13,Citation14] The use of collagen based material in industry for bioengineering, cosmetics, pharmaceuticals, and food is expanding.[Citation15] As a good example for protein film, collagen casing has been successfully commercialized in the food industry.[Citation16] Some composited collagen-based films and stuffs have been researched in food fields recently,[Citation14] even with special properties such as antibacterial property.[Citation17]
Hydroxyapatite (HA, Ca10(PO4)6(OH)2), the main inorganic component of teeth and bones, with poor degradability and solubility, is an attractive material for bone replacement, dental defect filling, bone tissue engineering, plastic surgery, and drug delivery applications. It has become one of the most investigated biomaterials due to its degree of safety, excellent biocompatibility, and bioactivity.[Citation12,Citation18] Nano-hydroxyapatite (n-HA), no more than 100 nm in size, with high specific surface area and energy, has better biological performance.[Citation12] It is reported that collagen and HA coordinate well in enriching the calcium amount in bone[Citation19] and the dissimilar organic and inorganicnanophases have specific spatial relations with respect to each other.[Citation20] Additionally, the calcium-phosphorus ratio of HA is 1.67, approximately to 2.0, which is ready to be assimilate by the body. However, given that the unique characters of HA, many investigations used HA as an efficient reinforcement agent have be done to improve properties of natural polymers such as chitosan,[Citation21] alginate,[Citation22] and collagen[Citation19,Citation20,Citation23] for fabricating desired composite materials, mainly in biomaterials and medical fields. To the best of our knowledge, however, little information about the reinforcement of HA on collagen fiber-based film is available for food packaging application.
The objective of this study was to develop a novel collagen-HA composite films, focusing on the impact of HA on film properties. Collagen fibers were prepared from bovine hide by acid swelling and various amounts of HA were added to form the composite films. Following this, the optical, mechanical, barrier properties, microstructures, as well as thermal stability of the films were investigated for overall estimation the enhancing of HA on the performances of collagen fiber based film.
Material and Methods
Chemicals
Liming bovine hide was kindly offered by Long-bao Collagencasing Co., Ltd. (Zibo, China). n-HA (purity ≥ 97%, particle size < 100 nm) was purchased from Aladdin Industry Company (Shanghai, China). Glycerin and hydrochloric acid were obtained from Sigma-Aldrich (Shanghai, China). All commercial chemicals were all analytical grade and used without further purification.
Collagen Fiber and Film Preparation
Bovine hides were cut into pieces (4 × 4 cm) and washed by running water until the pH reached about 7.0. Then they were immersed in hydrochloric acid solution (pH = 2) for 36 h to swell completely.[Citation24] After that, the swollen bovine hides were shredded to a size of approximately 5 cmCitation3 followed by reeling off collagen fibers from them. In order not to make collagen denaturation, the whole process should be conducted below 25°C. Prepared fibers were storage at 4°C for next test.
Films were prepared by casting and dehydration of 30 g of the film forming solution containing 0.6 g of collagen fiber (dry basis). In detail, a determined amount of collagen fibers and glycerol (30 g/100 g of collagen) were added into distilled water, then the pH was adjusted to 2.0 by adding glacial acetic acid[Citation14] to make collagen fiber swell into homogeneous colloidal solution, followed by a homogenization in a magnetic stirrer for 1 h. Afterward, different ratios at 0, 1, 2, and 4 wt% of HA (m/m) were added into the solution. After stirring for 30 min, the mixed solution was immediately cast onto pre-leveled Plexiglas plates (12 × 12 cm) and dried in an oven with air renewal and circulation for approximately 8 h at 30°C. After that, the films were conditioned in desiccators for 5 days at 25°C and 51% relative humidity (RH). The samples of prepared collagen fiber and collagen fiber-HA films were shown in .
Film Thickness
The thickness of films was measured with a hand-held digital micrometer (Mitutoyo No. 293-766, Tokyo, Japan) with a precision of 0.001 mm. The value reported was the mean of 10 random measurements.
Mechanical Properties
The mechanical properties of the films were measured by a texture analyzer (Stable Micro Systems Ltd, UK), according to the published literatures,[Citation25–Citation27] with minor modifications. The samples were cut into the rectangular test strips (70 × 25 mm), and both sides were fixed on the probe. The cross-head speed was set at 2 mm/s and the initial length was set to 30 mm. Tensile strength (TS) was calculated by dividing the maximum load at break by the area of cross-section and expressed in MPa. Elongation at break (EAB, %) was expressed as:
where L0 (mm) was the initial length of the film and L1 (mm) was its length at break.
Water Vapor Permeability (WVP)
The WVP of the collagen fiber-HA films was measured using a method reported in literature.[Citation25,Citation28] The composite films were cut into 4 cm in diameter, and the film was used to seal a testing cup containing anhydrous calcium chloride. The films were allowed to equilibrate for 1 h before the cells were initially weighed. Thereafter, the weight of the cup was measured intermittently at intervals of 24 h, up to 96 h. The WVP of the films was calculated as follows:
where WVP indicates WVP (×10−Citation11 g m/Pa mCitation2 s), and W, L,T, A, and P indicates the increased cup weight (g), thickness of film (m), measuring time (s), measuring area (mCitation2), difference in pressure between outside the cup and inside the cup (Pa), respectively.
Water Solubility
The solubility in water for the collagen fiber-HA films was determined as described previously[Citation26] with some modifications. In short, randomly selected samples cut from each film were dried in an oven at 105°C. Achieving constant weight, the initial dry weight of samples was recorded. After that, they were immersed in the test beakers with 20 mL distilled water. Samples were placed at room temperature with periodical gentle agitation for 24 h. The insoluble films were removed and dried again in an oven at 105°C until they reached a constant weight. The solubility (%) of the samples was calculated as follows:
where w1 (g) was the initial dry weight and w2 (g) was the final dry weight of films
Opacity Properties and Color Measurement
The opacity of the films were determined by a previous method,[Citation26] by measuring their light absorption at wavelength of 600 nm with a Genesys 10s ultraviolet-visible (UV-Vis) spectrophotometer (Thermo Scientific, America). The film samples were cut into strips (1 × 4 cm) and placed to one side of the cuvette with the reference of air. Opacity of films was expressed as absorbance value per thickness unit. Each sample was tested in triplicate. The color of sample surface was analyzed using a colorimeter (CR-700d Minolta Chroma Meter; Konica Minolta Sensing Inc., Tokyo, Japan) according to the method proposed previously[Citation4,Citation26] with minor modifications. Briefly, samples cut into 50 × 50 mm were placed onto a white standard plate, and values (L*, a*, and b*) were measured. The L*, a*, and b* values for the standard plate were L* = 93.68, a* = –0.08, and b* = –6.13. For each sample, five measurements were taken at different locations.
Oxygen Permeability Test
The oxygen permeability of collagen fiber-HA film was expressed by the peroxide value (POV) of oil indirectly with the published method.[Citation29,Citation30] Collagen fiber-HA films were cut into 40 × 40 mm, and the films were used to seal beakers filled with 5 g corn oil; one beaker filled with corn oil was sealed with nothing as a control group. Then, the beakers covered with films were placed in an oven at 50°C for 7 days and 14 days, respectively. Next, 1.5 g of oil was taken out and dissolved in 30 mL of solvent (chloroform: acetic acid, 2:3, v/v), followed by an addition of 1 mL saturated potassium iodide. After 3 min of standing in darkness, 100 mL of distilled water and 1 mL of starch solution (1 g/100 mL) were introduced. Then 0.01 M Na2S2O3 was used to titrate the solution until colorless. Each sample was measured three times. POVs were calculated as follows:
where V, B, F, mol/L(N), and W indicate the titration amount of sample, the titration amount of blank, the titer of 0.01 mol equiv/L Na2S2O3, the normality of Na2S2O3, and the sample weight (g), respectively.
Differential Scanning Calorimetry (DSC)
The thermal properties of composite films were characterized using a DSC TA-60WS (Shimadzu, Japan) according to the method proposed before.[Citation16,Citation23] Prior to measurement, samples were conditioned at 25°C and 0% RH for 2 weeks in a desiccator. Three milligrams of films were sampled and placed in DSC crucibles. Heating was set at a rate of 10°C/min in the temperature ranging from 25 to 200°C. Nitrogen gas at a flow rate of 20 cmCitation3/min was used to purge the sample chamber. The thermal figures were analyzed by universal analysis 2000 (TA instrument, USA).
Scanning Electron Microscope (SEM)
Microstructures of the composite film were observed using a SEM (SU1510, Hitachi, Japan) following the method in literature.[Citation7,Citation28] Prior to scanning, the samples were fixed to the stage with double-sided adhesive tape, sprayed with a layer of gold in a vacuum room. Then samples were transferred to the cold stage of the SEM chamber and observed at an acceleration voltage of 20 kV and a magnification of 1000× and 2000×.
Statistical Analysis
All data were expressed as the mean ± SD. Analysis of variance (ANOVA) was used for analysis of the test results at the significance level of p < 0.05 using SPSS 18.0 statistical software (SPSS Inc, Chicago, IL).
Results and Discussion
Morphology of Collagen Fiber and Film
For estimation its possible dissolution in collagen syrup (pH = 2.0) and in resulting film, we conducted a test of n-HA solubility in hydrochloric acid solution (pH = 2.0) for 24 h. The results showed that the solubility of n-HA in hydrochloric acid solution was poor (approximately 6.93%), which is acceptable for its reinforcement for collagen film.
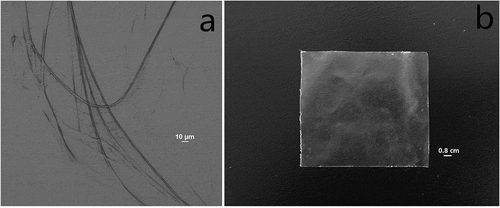
The morphology of prepared collagen fiber and collagen fiber based film was shown in . As shown in a, wetted collagen fiber presented a length of beyond 1 cm, and a width of ~5 µm, suggesting a fairly high-aspect ratio fiber was obtained from swollen bovine hide. With casting and drying, these flexible fibers formed a transparent film through crosslinking and netting, with a little uneven surface (b).
A preliminary test showed that when the additive amount of HA was beyond 4 wt%, the collagen fiber would strongly aggregate together in film-forming solution, and could not form uniform film. Therefore, the amount of HA was limited from 0 to 4 wt% in this study.
Thickness and Optical Properties
Thickness, opacity and color parameters of collagen fiber-HA composite film were demonstrated in . It was observed that the addition of HA caused no significant difference in film thickness. However, the opacity of collagen fiber–HA composite film increased significantly (p < 0.05) as the increasing amount of HA added. Moreover, the absolute values of L*, a* and b* increased gradually, indicating the incorporation of HA intensified the color of collagen fiber film. Opacity, being closely associated to film appearance and film color,[Citation31] has a key role for food coating or food packaging.[Citation32] Opacity is involved in various film properties including thickness, color, and matrix status. Here, the increasing opacity might be attributed to the opaque granules and the intensified color, wherein, a number of n-HA particles accumulated together.
Table 1. Thickness, optical properties, and color values of collagen fiber-based films reinforced with hydroxyapatite at various concentrations (n ≥ 3).
Mechanical Properties
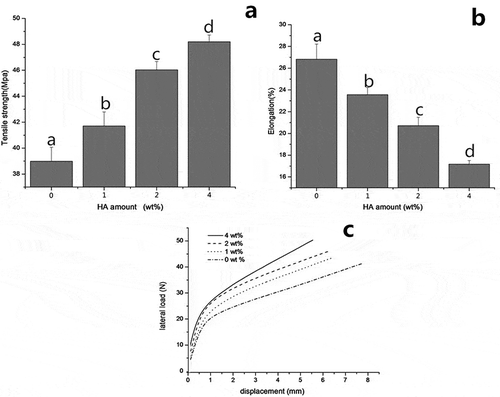
presents the TS, EAB and load-displacement curve of composite films, respectively. As shown in , the TS of collagen fiber-HA films gradually increased from 38.98 to 48.20 MPa with the increasing amount of HA. However, EAB was decreased obviously (p < 0.05). This phenomenon might be attributed to the brittleness and stiffness of HA.[Citation19,Citation33] On one hand, the presence of HA makes collagen fiber film more resistant to crush; on the other hand, it can restrict the collagen fiber film to extend. Compared with starch-based films[Citation26] and gelatin films,[Citation28] the TS of collagen fiber film is eight times and four times higher respectively, showing its super strength nature. Moreover, as clearly demonstrated in c, at a consist displacement; the lateral force was directly proportional to the additive amount of HA in a particular area. However, under a certain lateral force, the displacements of composite are in inverse proportion to HA content. These differences in the load-displacement curves indicated the strong impact of HA on the mechanical properties of collagen fiber films, which were coincided with the TS and EAB results in and .
WVP and Water Solubility
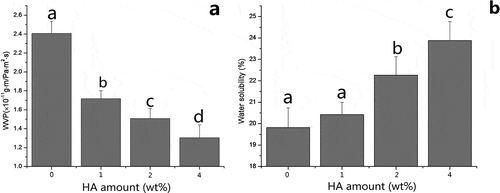
As shown in , WVP of composite films reduced from 2.41 × 10−Citation11 g·m/Pa·mCitation2·s to 1.30 × 10−Citation11 g·m/Pa·mCitation2·s as HA increased from 0 to 4 wt%. It is notable that the addition of HA significantly enhanced the water vapor barrier of collagen fiber-HA composite film (p < 0.05), indicating that the presence of HA could help resist the transfer of water moisture. Compared with gelatin-laponite composite film,[Citation28] the WVP of collagen fiber-HA composite film reduced a lot, and this is likely caused by the difference of materials. However, it is similar to methylcellulose/stearic acid blending film,[Citation5] which varies between 0.775 × 10−Citation11 g·m/Pa·mCitation2·s and 1.7 × 10−Citation11 g·m/Pa·mCitation2·s. It is believed that the presence of HA facilitated the minimization of the space and porosity in the film matrix, and tightened the network structure, restricting the transport of water vapor. [Citation7] Furthermore, the more periphrastic routes in composite film matrix could also cause a longer distance for the water vapor molecules to go through.[Citation34] Additionally, shows the water solubility of collagen fiber–HA composite films. The water solubility of pure collagen fiber film was 19.82% which was much lower than collagen powder films,[Citation14] starch/gelatin film.[Citation6] The low film insolubility can be attributed to unique nature of collagen fiber, which is insolubility and only swollen in acid or alkaline conditions. This desirable specialty obviously can make up the disadvantage of poor water resistance of common food films and provides the potential used in high humidity status. Additionally, It was observed that the low concentration of HA (1 wt%) did not change the water solubility of films significantly (p > 0.05), however, the additive amount of HA at both 2 and 4 wt% augmented the water solubility undesirably (p < 0.05). And this might be caused by the embedding of n-HA into collagen fiber that damaged the original interaction of collagen chains, obstructing the formation of network structure, as shown in SEM images of films in .
Oxygen Permeability
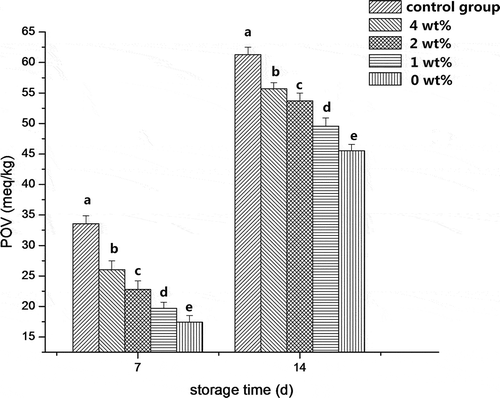
The POV of corn oil stored for 7 and 14 days was presented in , and corn oil unsealed was set as the control group. Oil POV sealed with films significantly was significantly decreased (p < 0.05) compared to the control group (unsealed corn oil) during the storage period. Moreover, the POV of corn oil decreased as the HA concentration increased from 0 to 4 wt%, suggesting that collagen fiber-HA composite film had excellent oxygen barrier property, which was closely associated with HA concentration. HA, as a good filler with a nano-scale size and high specific surface area, can fill in the network structure and partly minimized the space and porosity of the film layer and make the films more compact just mentioned above in the WVP section, benefiting the retard of the movement of gas across the films and consequently improve the gas permeability. Similarly, ZnO nanoparticles[Citation35] added into polyvinyl alcohol-based composite film led to an obvious decreased in the oxygen permeability.
Thermal Stability of Films
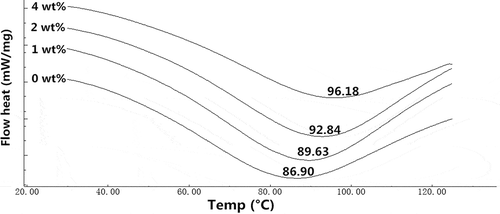
presents the heat endothermic processes of the collagen fiber-HA films. It is observed that the peak temperatures of samples climbed from 86.90 to 96.18°C as the content of HA increased from 0 to 4 wt%. This might be attributed to the assumption that the presence of HA accelerates a densely compact structure as shown in , thus it required a higher energy for the collagen to degrade. Furthermore, it was reported that inorganic component would hamper and retard the decomposition of the polymers, especially the inorganic matters with good thermal stability.[Citation21,Citation36] Further, the excellent thermal stability of HA could also contribute to a higher Tm in the DSC curves. Thermal stability is an essential parameter for the application of edible films, since food packaging materials may undergo heating during the manufacture and consumption of products. The improved thermal stability with HA can benefit the collagen based films in food packaging application. And the present results are similar to the previous research where n-HA affected thermal behavior of HA/alginate hybrid composites [Citation21]poly(caprolactone)/chitosan blends.[Citation23]
Microstructures of Films
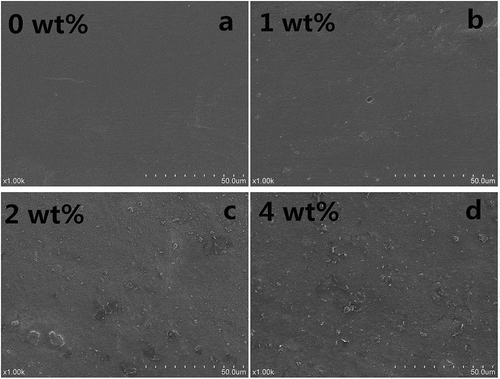
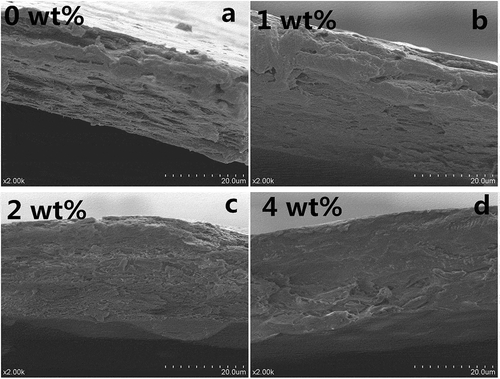
and show the SEM images of surfaces and cross-sections of collagen fiber-HA composite films, respectively. As shown in , with the increase of HA, the surfaces of collagen fiber-HA composite film become coarser and more uneven. The roughness might be attributed to that accumulation of part HAs on the surface of collagen fiber-HA composite films in crystal. For the cross-section of collagen fiber-HA composite films, a clear and visible change was presented in . Compared with pure collagen fiber film shown in , the composite films became compact and tidy, and the apertures in the cross-sections disappeared with increasing concentration of HA. The changes in microstructure of the collagen-HA films induced by HA addition are highly in consistent with those film properties mentioned above in this work, including mechanical, water, and oxygen barrier. Indeed, the intrinsic physicochemical properties of collagen fibers such as particle shape and size, can result in the slack and rough structure.[Citation14] In this study, the presence of HA facilitated obviously the minimization of the space of porosity in the film matrix and improved film structure. On one hand, HA, as the filler, tightened the molecule structure, made the structure of collagen seem more compact; on the other hand, some functional groups, such as amines, carboxyls, and hydroxyls, facilitate the enhancement of the interfacial bonding between HA and collagen.[Citation19,Citation22]
Conclusion
A novel acid-swollen collagen fiber-HA composite film was prepared successfully using casting method in this study, with an efficient enhancing of film performance with HA arranging from 0 to 4 wt%. The incorporation of HA into collagen film gave rise to a series of changes, including a loss in opacity, a gain in film gloss and so on, while it has no obvious effects on the film thickness. The addition of HA caused a significant increase in TS while an observable reduction in elongation. Furthermore, water vapor and oxygen barriers of collagen fiber film were also significantly improved. Additionally, an elevated thermal stability for collagen fiber-HA composite films was obtained. With further observation of SEM images, it was notable that HA made film uneven surface and compact structure due to its embedding in film matrix, which partly contributed to performance enhancing of HA. In a word, collagen fiber-HA composite films can be potentially applied as new food packaging and preservation materials for maintaining quality of food products in the food industry.
Acknowledgments
Financial support was kindly supplied by grants from Special Fund for Agro-scientific Research in the Public Interest of China (No. 201303082) and National High Technology Research and Development Program of China (No.2013AA102204).
References
- Bonilla, J.; Vargas, M.; Atarés, L.; Chiralt, A. Physical Properties of Chitosan-Basil Essential Oil Edible Films as Affected by Oil Content and Homogenization Conditions. Procedia Food Science 2011, 1, 50–56.
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative Study of Processing Methods for Starch/Gelatin Films. Carbohydrate Polymers 2013, 95, 681–689.
- Nie, X.; Gong, Y.; Wang, N.; Meng, X. Preparation and Characterization of Edible Myofibrillar Protein-Based Film Incorporated with Grape Seed Procyanidins and Green Tea Polyphenol. LWT–Food Science and Technology 2015, 64, 1042–1046.
- Galus, S.; Kadzińska, J. Whey Protein Edible Films Modified with Almond and Walnut Oils. Food Hydrocolloids 2016, 52, 78–86.
- Zhong, T.; Huang, R.; Sui, S.; Lian, Z.; Sun, X.; Wan, A.; Li, H. Effects of Ultrasound Treatment on Lipid Self-Association and Properties of Methylcellulose/Stearic Acid Blending Films. Carbohydrate Polymers 2015, 131, 415–423.
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible Films and Coatings Based on Starch/Gelatin: Film Properties and Effect of Coatings on Quality of Refrigerated Red Crimson Grapes. Postharvest Biology and Technology 2015, 109, 57–64.
- Wang, Y.; Liu, A.; Ye, R.; Wang, W.; X. Li. Transglutaminase-Induced Crosslinking of Gelatin-Calcium Carbonate Composite Films. Food Chemistry 2015, 166, 414–422.
- Gelse, K. Collagens—Structure, Function, and Biosynthesis. Advanced Drug Delivery Reviews 2003, 55, 1531–1546.
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. International Journal of Pharmaceutics 2001, 221, 1–22.
- Friess, W., Collagen—Biomaterial for Drug Delivery. European Journal of Pharmaceutics and Biopharmaceutics 1998, 45, 113–136.
- Palokangas, H.; Kovanen, V.; Duncan, A.; Robins, S.P. Age-Related Changes in the Concentration of Hydroxypyridinium Crosslinks in Functionally Different Skeletal Muscles Matrix 1992, 12, 291–296.
- Wang, J.; Zhou, Z.; Zhang, Z.; Du, B.; Zhang, Z.; Wang, Q.; Yuan, P.; Liu, L.; Zhang, Q. Biomimetic Synthesis of Platelet-Shaped Hydroxyapatite Mesocrystals in a Collagen Mimetic Peptide–PEG Hybrid Hydrogel. Materials Letters 2015, 159, 150–153.
- Ollé, L.; Sorolla, S.; Casas, C.; Bacardit, A. Developing of a Dehydration Process for Bovine Leather to Obtain a New Collagenous Material. Journal of Cleaner Production 2013, 51, 177–183.
- Wolf, K.L.; Sobral, P.J.A.; Telis, V.R.N. Physicochemical Characterization of Collagen Fibers and Collagen Powder for Self-Composite Film Production. Food Hydrocolloids 2009, 23, 1886–1894.
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and Characterization of Collagen from the Outer Skin of Squid (Doryteuthis Singhalensis). Food Hydrocolloids 2015, 43, 708–716.
- Wang, W.; Zhang, Y.; Ye, R.; Ni, Y. Physical Crosslinkings of Edible Collagen Casing. Internatioal Journal of Biological Macromolecules 2015, 81, 920–925.
- Wang, L.F.; Rhim, J.W. Preparation and Application of Agar/Alginate/Collagen Ternary Blend Functional Food Packaging Films. International Journal of Biological Macromolecules 2015, 80, 460–468.
- Viswanath, B.; Ravishankar, N. Controlled Synthesis of Plate-Shaped Hydroxyapatite and Implications for the Morphology of the Apatite Phase in Bone. Biomaterials 2008, 29, 4855–4863.
- Chao, S.C.; Wang, M.J.; Pai, N.S.; Yen, S.K. Preparation and Characterization of Gelatin–Hydroxyapatite Composite Microspheres for Hard Tissue Repair. Materials Science and Engineering: C 2015, 57, 113–122.
- Zhai, Y.; Cui, F.Z.; Wang, Y. Formation of Nano-Hydroxyapatite on Recombinant Human-Like Collagen Fibrils. Current Applied Physics 2005, 5, 429–432.
- Ito, M.; Hidaka, Y.; Nakajima, M.; Yagasaki, H.; Kafrawy, A.H. Effect of Hydroxyapatite Content on Physical Properties and Connective Tissue Reactions to a Chitosan-Hydroxyapatite Composite Membrane. Journal of Biomedical Materials Research 1999, 45, 204–208.
- Tampieri., A.; Sandri, M.; Landi, E.; Celotti, G.; Roveri, N.; Mattioli-Belmonte, M. HA/Alginate Hybrid Composites Prepared Through Bio-Inspired Nucleation. Acta Biomaterialia 2005, 1, 343–351.
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Akhlaghi, S.; Hedenqvist, M.S. Effect of Hydroxyapatite Nano-Particles on Morphology, Rheology and Thermal Behavior of Poly(Caprolactone)/Chitosan Blends. Materials Science and Engineering: C 2016, 59, 580–589.
- Oechsle, A.M.; Wittmann, X.; Gibis, M.; Kohlus, R.; Weiss, J. Collagen Entanglement Influenced by the Addition of Acids. European Polymer Journal 2014, 58, 144–156.
- Rivero, S.; García, M.A.; Pinotti, A. Correlations Between Structural, Barrier, Thermal and Mechanical Properties of Plasticized Gelatin Films. Innovative Food Science & Emerging Technologies 2010, 11, 369–375.
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, Barrier and Morphological Properties of Pea Starch and Peanut Protein Isolate Blend Films. Carbohydrate Polymers 2013, 98, 630–637.
- Cano, A.; Jimenez, A.; Chafer, M.; Gonzalez, C; Chiralt, A. Effect of Amylose: AmylopectinRatio and Rice Bran Addition on Starch Films Properties. Carbohydrate Polymers 2014, 111, 543–555.
- Li, X.; Liu, A.; Ye, R.; Wang, Y.; Wang, W. Fabrication of Gelatin–Laponite Composite Films: Effect of the Concentration of Laponite on Physical Properties and the Freshness of Meat During Storage. Food Hydrocolloids 2015, 44, 390–398.
- Jung, D.C.; Lee, S.Y.; Yoon, J.H.; Hong, K.P.; Kang, Y.S.; Park, S.R.; Park, S.K.; Ha, S.D.; Kim, G.H.; Bae, D.H. Inhibition of Pork and Fish Oxidation by a Novel Plastic Film Coated with Horseradish Extract. LWT–Food Science and Technology 2009, 42, 856–861.
- Feng, T.; Ye, R.; Zhuang, H.; Rong, Z.; Fang, Z.; Wang, Y.; Gu, Z.; Jin, Z. Physicochemical Properties and Sensory Evaluation of Mesona Blumes Gum/Rice Starch Mixed Gels as Fat-Substitutes in Chinese Cantonese-Style Sausage. Food Research International 2013, 50, 85–93.
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.M.V.; Silva, L.B. Mechanical Properties and Water Vapour Permeability of Hydrolysed Collagen–Cocoa Butter Edible Films Plasticised with Sucrose. Food Hydrocolloids 2013, 30, 625–631.
- Gontard, N.; Guilbert, S.; Cuq, J.L. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties Using Response Surface Methodology. Journal of Food Science 1992, 57, 190–195.
- Savino, K.; Yates, M.Z. Thermal Stability of Electrochemical–Hydrothermal Hydroxyapatite Coatings. Ceramics International 2015, 41, 8568–8577.
- Siripatrawan, U.; Harte, B.R. Physical Properties and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocolloids 2010, 24, 770–775.
- Li, X.H.; Xing, Y.G.; Li, W.L.; Jiang, Y.H.; Ding, Y.L. Antibacterial and Physical Properties of Poly (Vinyl Chloride)-Based Film Coated with ZnO Nanoparticles. Food Science and Technology International 2010, 16, 225–232.
- Bigi, A.; Ripamonti, A.; Cojazzi, G.; Pizzuto, G.; Roveri, N.; Koch, M.H.J. Structural Analysis of Turkey Tendon Collagen upon Removal of the Inorganic Phase. International Journal of Biological Macromolecules 1991, 13, 110–114.