ABSTRACT
The effect of active chlorine concentrations (1–5%) on the properties of jackfruit seed starch was investigated. Both the carbonyl and carboxyl contents of the oxidized starches generally increased with progressive increases in the active chlorine concentration. No evidences of alteration in the morphology and X-ray diffraction pattern were observed after oxidation, while the decrease in relative crystallinity was found. The swelling power of the oxidized starch tended to decrease with the active chlorine levels, particularly at a low level of active chlorine (1–3%). No significant differences in the peak temperature (Tp) and the end temperature (Te) were found between the native and the oxidized starches, while the oxidized starches had lower onset temperatures (To). Parameters, such as pasting temperature, peak viscosity, and setback, decreased with the concentration of active chlorine. In addition, lower syneresis and turbidity were found in all oxidized starches during refrigerated storage compared to the native starch.
Introduction
Starches are ubiquitous macromolecules synthesized by plants and have wide applications in both food and non-food products. In food, starch is used as a major ingredient as well as a minor ingredient imparting some specific functions. Some of these functions include thickening, coating, gelling, adhesion, and encapsulation. Starch is a raw material with several industrial applications, so there has been a growing demand for new starch sources. Jackfruit seed can be a good alternative starch source.[Citation1] However, the native starch has very limited industrial applications due to its low solubility in water, restricted swelling power, poor granule dispersibility, high gelatinization temperatures, and high syneresis.[Citation1,Citation2] Many reports revealed that the amylose content of the jackfruit seed starch ranged from 24–53%.[Citation1–Citation8] Starch-based food products made from high amylose starch normally undergo textural changes related to amylose retrogradation and show syneresis during low-temperature storage. These changes attributed to starch retrogradation may make such product unacceptable to consumer. Thus, chemical and physical modification may be necessary to improve the functionality of starches for industrial applications. Chemical modification can involve the introduction of functional groups into the starch molecule, such as through acetylation and oxidation. Modification of starch by oxidation imparts extended shelf life and cold storage stability to starch-based food products.[Citation9,Citation10] Currently hypochorite oxidation is the most common method for the production of oxidized starches on an industrial scale. The oxidation reaction is usually performed in a mild to moderate alkaline solution in order to obtain the yield of carboxyl groups. Sodium and calcium hypochlorite are usually used as the oxidizing agents.[Citation11] Oxidized starches are widely used in the food, paper, laundry, the finishing of building materials and textile industries. Their use in the food industry is increasing because of their low viscosity, high stability, clarity, and film-forming and binding properties. Over the years, the modification of starch by oxidation has been applied to many starches including corn starch,[Citation11–Citation16] banana starch,[Citation9] cassava starch,[Citation17] bean starch,[Citation10,Citation18–Citation20] barley starch,[Citation13] cocoyam starch,[Citation21] and pine nut starch.[Citation22] However, there is currently no documentation available on the modification of the jackfruit seed starch by oxidation. Therefore, the objective of this research was to evaluate the effect of various sodium hypochlorite concentrations on several physicochemical properties and morphological characteristics of the oxidized jackfruit seed starches.
Materials and Methods
Fruit Seed Sample
Jackfruit seeds (Artocarpus heterophyllus L., Sri Bun Jong cultivar) were collected from ripe fruit (approximately 20–23°Brix) grown in the Southern part of Thailand. The fruits were washed with tap water, peeled off, and the seeds were manually separated from the pulp. The mucilage peel of the seeds was removed manually. The seeds were stored at –20°C for further starch isolation.
Starch Isolation
Starch isolation was carried out by using the method of Eggleston et al.[Citation23] with some modifications. Jackfruit seeds were peeled off manually. Lye-peeling was used to remove spermoderms by soaking the jackfruit seeds in 0.5% NaOH solution for 30 min and rinsing with distilled water. The cotyledons were washed several times with distilled water to completely remove any traces of alkali on the seed surfaces. They were then wet ground in a blender (Philips juicer mixer grinder HL1632) with an extractive solvent (0.05 N NaOH; 1:5, seed to solvent) for 2 min and then stirred slowly for 2 h at ambient temperature. The resulting suspension was centrifuged (Sorvall, model RC5C Plus) at 1000 g for 10 min. The sediment was re-suspended in two volumes of distilled water and sieved through 120 mesh, until the washing water was clear. The starch suspension was then neutralized to pH 7.0 and the sediment starch was dried overnight in a hot oven at 40°C. It was then ground to pass through a 100 mesh sieve and stored at room temperature in sealed plastic containers. The native jackfruit seed starch was analyzed for moisture, protein, lipid, ash, crude fiber,[Citation24] and amylose content.[Citation25] The protein content was calculated from g/100 g N × 6.25. Standard potato amylose (Sigma Chemical, St. Louis, MO, USA) was used as the reference for the amylose content analysis.
Modification of Jackfruit Seed Starch by Oxidation
The starch oxidation procedure followed the method of Kuakpetoon and Wang[Citation11] with some modifications. NaOCl was purchased from J.T. Baker Chemical Co. (Phillipsburg, NJ, USA). The certain concentration of active chlorine in NaOCl determined by titration with sodium thiosulfate was 6% (g/100 g).[Citation26] First, a 40% (w/w) starch slurry (total weight of 1125 g) was prepared in a reaction vessel equipped with a heating mantle. The starch slurry was mechanically stirred and maintained at 35°C, and the pH was adjusted to 9.5 with 2 M NaOH. Sodium hypochlorite (NaOCl) with 1% active chlorine (w/w; 75 g) was slowly added into the starch slurry within 30 min while maintaining the pH at 9.5 with 1 M H2SO4 and a temperature of 35°C. After the addition of the NaOCl, the starch slurry was maintained at the same pH and temperature for an additional 50 min with stirring. The slurry was then neutralized to pH 7.0 with 1 M H2SO4, filtered through suction (Whatman filter #4), washed with deionized water, and dried in an oven at 40°C for 48 h. The same procedure was applied to the jackfruit seed starch with different active chlorine concentrations (AC; 2, 3, 4, and 5% w/w).
Determination of Carboxyl Content
The carboxyl content of the oxidized starches was determined according to the modified procedure of Chattopadhyay et al.[Citation27] Starch (2 g, dry basis) was added with 25 mL of 0.1 M HCl and stirred using magnetic stirrer for 30 min. The slurry was vacuum-filtered through a 150-mL medium porosity fritted glass funnel, and a fine stream of deionized water from a wash bottle was used to quantitatively transfer the sample from the beaker. The sample was washed with 400 mL of deionized water in order to completely remove the chloride ion. The starch cake was then quantitatively transferred to a 500-mL beaker with the aid of deionized water, and the slurry was diluted to 300 mL. The starch slurry was heated in a boiling water bath with continuous stirring for 15 min to ensure complete gelatinization. The hot starch solution was adjusted to 450 mL with boiling deionized water and immediately titrated to pH 8.3 with standardized 0.01 M NaOH with stirring. Unmodified starch was used as the blank for the oxidized starches to correct for inherent acidic substances. Instead of stirring with 0.1 M HCl, 2 g of unmodified starch was stirred with 25 mL of deionized water. The carboxyl group content was calculated as follows:
Determination of Carbonyl Content
The carbonyl content of the oxidized starches was determined by following the titrimetric method of Smith.[Citation28] Starch (4 g, dry basis) was suspended in 100 mL of distilled water. The suspension was gelatinized in a boiling water bath for 20 min, cooled to 40°C, adjusted to pH 3.2 with 0.1 M HCl, and 15 mL of hydroxylamine reagent was added. The flask was stopped and placed in a 40°C water bath for 4 h with slow shaking. The excess hydroxylamine was determined by rapidly titrating the reaction mixture to pH 3.2 with standardized 0.1 M HCl. A blank determination with only hydroxylamine reagent was performed in the same manner. The hydroxylamine reagent was prepared by first dissolving 25 g of hydroxylamine hydrochloride in 100 mL of 0.5 M NaOH before the final volume was adjusted to 500 mL with distilled water. The carbonyl content was calculated as follows:
Determination of Color
Color evaluation was done by Univeral Colorimeter (Hunter Lab, USA) and expressed in term of L*, a*, and b*. The whiteness value was obtained by the following equation:[Citation29]
Morphological Characteristics and Granule Size Distribution of Starch Granules
The morphology of the starch granules (native and oxidized starches) was examined using a scanning electron microscope (SEM; JEOL JSM-5600LV microscope, JEOL, England). The distribution of the particle size of the native and oxidized jackfruit seed starch was analyzed using a laser particle size analyzer (Beckman Coulter LS 230).
X-Ray Diffraction
The diffraction patterns of the starches were obtained by using an X-ray diffractometer (Philips brand), which operated at 35 kW (15 mA) using a copper target. The scanning range was 5–40° in the scale of the angle 2θ. The percentage of relative crystallinity was calculated as the percentage of the peak area related to the total area of diffraction in the diffractograms.
Swelling Power and Solubility
Swelling power and solubility were determined following the method proposed by Lawal and Adebowale[Citation19] with some modifications. A starch suspension (0.7%, w/v, dry basis) was prepared in a centrifuge tube and heated for 30 min at 50°C with stirring every 5 min. The sample preparation at 60, 70, 80, and 90°C was similar. The samples were then cooled to room temperature before centrifuging at 1000 g for 15 min. The supernatant was separated from the swollen granules and dried. The swelling power was calculated as gram of swollen starch per gram of dry sample, and the solubility was the percentage of the dried supernatant to the original dried sample.
Thermal Properties
Thermal properties were studied by using a differential scanning calorimeter (DSC; 7, Perkin Elmer, Norwalk, CT, USA), using 1:3 (w/w) starch-water mixtures. The samples were hermetically sealed in a pre-weighed aluminum pan at room temperature and re-weighed in a microbalance. After sealing the pan and leaving to equilibrate for approximately 12 h, the samples were heated from 30–100°C at 10°C/min. An empty pan was used as a reference. The temperatures of the characteristic transitions, onset temperature (To), peak temperature (Tp) and end temperature (Te) were recorded and the temperature ranges (Te-To, ΔT) were calculated. The enthalpy (ΔH) of the transition was expressed as J/g on a dry weight basis.
Pasting Properties
A rapid visco analyzer (RVA; RVA4D, Newport Scientific, Warriewood, Australia) was employed to measure the pasting properties of starch (3 g, dry basis, 28 g total weight). After the starch and water were introduced to the sample holder, the paddle was inserted and the mixture was shaken up and down ten times to eliminate lumps. The sample was then inserted into the RVA, which was used along with the accompanying software. The stirring speed was 960 rpm for the first 10 s, then 160 rpm for the remainder of the test. The standard profile was the “12.30 min” test. The initial temperature was 50°C (0–1 min) which was then increased to 95°C (ramp time 3 min 45 s). The temperature of the sample was held for 2 min 30 s before cooling to 50°C (ramp time 3 min 45 s) and then held at this temperature (1 min 30 s).
Gelation Capacity
Samples of starch, 2–12% (w/v, dry basis) were prepared in test tubes with distilled water (5 mL). The starch suspensions were mixed with Variwhirl mixer for 5 min. The test tubes were heated for 30 min at 80°C in a water bath, followed by rapid cooling under running cold tap water. The test tubes were further cooled at 4°C for 2 h. The least gelation concentration (LGC) was determined as that concentration when the sample from the inverted test tube did not fall down or slip.[Citation19]
Turbidity
The starch gel turbidity was measured as described by Sandhu et al.[Citation16] An aqueous suspension of starch (1%, w/v, dry basis) was heated in a water bath at 90°C for 1 h with constant stirring. The suspension was cooled at ambient temperature and stored at 4°C for 7 days. The transmittance (%) at 650 nm was measured on days 1, 3, 5, and 7 using a UV-visible spectrophotometer (Shimadzu, Japan).
Syneresis: Stability to Refrigeration
The stability of the gel was evaluated by the degree of syneresis at refrigeration conditions as proposed by Simsek et al.[Citation20] with some modifications. A starch suspension (6%, w/v, dry basis) was heated up to 90°C for 1 h, cooled to 50°C and then kept at this temperature for 15 min. Aliquots of 50 mL were placed in centrifuge tubes and conditioned at 4°C for 7 days. The samples were centrifuged at 3000 rpm for 20 min on days 1, 3, 5, and 7. The extent of syneresis was expressed as the ratio of the weight of the liquid decanted to the total weight of the paste.
Statistical Analysis
Analytical determinations for the samples were performed at least triplicate. Analysis of variance (ANOVA) was used to compare sample means at 95% confidence level (p < 0.05) using SPSS software. The tests of significant difference between means were done using Duncan’s multiple range test.
Results and Discussion
Proximate Composition of the Native Jackfruit Seed Starch
The proximate composition of the native jackfruit seed starch is evaluated. The moisture content of the starch was low (10.12%) and within the acceptable range for marketing and storage. Other components such as proteins (0.72%), lipids (0.34%), crude fiber (0.19%), and ash (0.21%) were presented in the starch as minor constituents. In addition, the amylose content of the jackfruit seed starch (50.45%) was higher compared to the normal range (18–30%) found in other starches.
Carbonyl and Carboxyl Content
During the modification by oxidation, the hydroxyl groups of starch molecules, primarily at the C-2, C-3, and C-6 positions, are transformed to carbonyl group and then to carboxyl group. The degree of oxidation could be indicated by the carbonyl group and carboxyl group of the oxidized starch.[Citation11,Citation12] The carbonyl and carboxyl content of the native and the modified jackfruit seed starches are shown in . It can be seen that both the carbonyl and carboxyl contents of the oxidized starches generally increased with progressive increases in the concentration of the active chlorine. The carboxyl content of the oxidized starches increased at a much faster rate than the carbonyl content. This could possibly be because as the level of active chlorine increases, more hydroxyl groups are being converted to carbonyl groups and promptly oxidized to carboxyl groups. This may then result in a higher carboxyl content formed in the oxidized jackfruit seed starch. Oxidation normally occurs in the alkaline condition with hypochlorite, which enhances the production of a carboxyl group. Factors such as types of starch, starch granule structure, oxidizing agent, and oxidation condition (such as concentration of oxidizing agent and reaction time) might affect the formation of carbonyl and carboxyl.[Citation9] Sangseethong et al.[Citation17] reported that carbonyl group was the primary functional group produced in the peroxide-oxidized starches, whereas the amount of carboxyl group formed was minor. In addition, the carbonyl and carboxyl group levels in the oxidized jackfruit seed starches tended to be higher than those reported for banana, normal corn, and waxy corn starches.[Citation9,Citation12] The occurrence of oxidation was mainly in the amorphous lamellae of the semi-crystalline growth rings in starch granules as reported by previous studies.[Citation9,Citation12] The amorphous lamellae mainly consists of amylose and the regions around the branches in amylopectin. The jackfruit seed starch contained a higher amylose content than in the banana and corn starches. Thus, the current results suggest that amylose was more susceptible to oxidation than was amylopectin, which may be due to the more accessible nature and linear structure of amylose. In addition, the granule size of starch may influence the level of oxidation. The granule size of the native jackfruit seed starch (7 µm) is smaller than those of the native banana (20–60 µm) and corn (>20 µm) starches as reported by Zhang et al.[Citation30] and Dhital et al.,[Citation31] respectively. Smaller the granule size fraction, the higher degree of oxidation, which is due to the larger specific surface area of the smaller size granule fractions. These results indicated that the jackfruit seed starch was more susceptible to oxidation than the corn and banana starches.
Table 1. Carbonyl content, carboxyl content, color, crystallinity, and average granule size of the jackfruit seed starches modified by oxidation.
Starch Color
The data for the color parameters is also shown in . All starch samples had a high degree of whiteness, expressed by high L* value and low a* and b* values, thus confirming the high purity of the starches. The whiteness of all starch samples was higher than 90. Wang et al.[Citation32] estimated that a value higher than 90 gives a satisfactory whiteness for the starch purity. The L* value and the whiteness of the native jackfruit seed starch was lower than those of the oxidized jackfruit seed starches while the b* and a* values were higher (p < 0.05). The results indicated that oxidation could improve the whiteness of starch. In addition, an increase in the active chlorine concentration caused an increase in the whiteness of the starch. This result might be because some pigments and proteins are first oxidized before the glucose units, thus changing the structures of these compounds and producing a whiter starch.[Citation9,Citation10] These results are in agreement with the findings reported by Sanchez-Rivera et al.[Citation9] and Vanier et al.[Citation10] They demonstrated that the whiteness of the hypochlorite-oxidized banana starches and bean starches increased when the AC are increased. They noted that the whiteness was close to 100, which is the maximum value for this parameter and indicates a white material. The high degree of whiteness of modified starches can provide many opportunities for application in food processing and industries.
Morphological Characteristics and Granule Size Distributions of Starch Granules
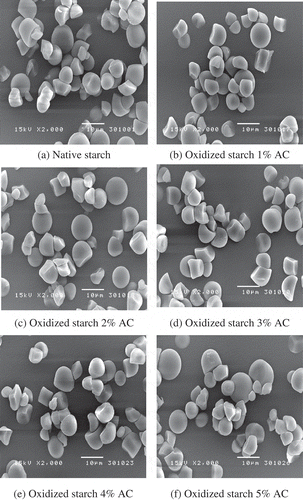
shows the scanning electron micrographs of the native and the oxidized jackfruit seed starch granules. The shapes of the jackfruit seed starch were irregular and round to bell or semi-oval in shape. This result is in agreement with the work of Tongdang,[Citation1] Dutta et al.,[Citation4] and Rengsutthi and Charoenrein.[Citation7] In addition, the native starch granules showed smooth and minimal damage, suggesting that the method of extraction and drying did not cause significant damage to starch. All the oxidized starches did not show appreciable differences when compared with their native counterparts. After oxidation, no change in surface and shape characteristics of the granules was also reported for barley starch,[Citation13] corn starch,[Citation13] and banana starch.[Citation9] However, the bean starch granules oxidized with 1.5% active chlorine had imperfections on their external structures, and the surface of these granules was rougher than the surface of the native starch granules.[Citation10] In addition, the modification of Sword starch by the oxidation method also causes rupturing of the starch granules, producing cavities in the center of some of the granules.[Citation18] These different observations might be due to the different levels of modification and preparation methods as well as to the granular architecture and fragility of the starch granules. According to the analysis of granule size distribution, the granule size of the jackfruit seed starch ranged from 0.04 to 30.07 µm with an average size of 7.02 µm. Lindeboom et al.[Citation33] classified starch granule sizes as: large (>25 µm), medium (10–25 µm), small (5–10 µm), and very small (<5 µm). Thus, the granule size of the native jackfruit seed starch could be classified as small. This study agreed well with previous work in which the size of the starch extracted from jackfruit seed was 7.75 µm on average.[Citation1] A slight increase in average granule sizes of the oxidized jackfruit seed starches was observed after oxidation as shown in . The reason for this could be due to the incorporation of carboxyl group in the adjacent chains may promote the expansion of starch granule by electrical repulsive force.
X-Ray Diffraction Pattern and Degree of Crystallinity
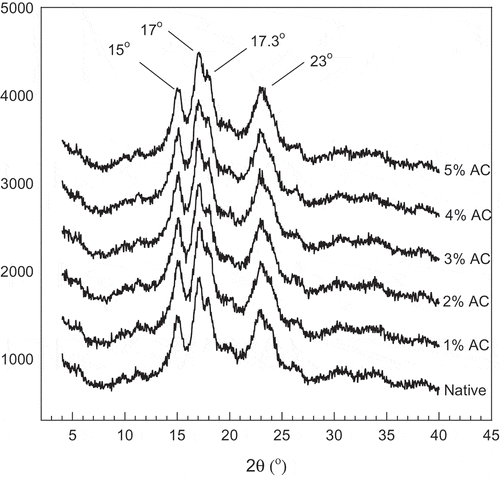
Because starch generally presents a semi crystalline structure, X-ray diffraction (XRD) has been employed extensively to characterize the physicochemical properties of starch. The XRD pattern of the starch samples are shown in . The native jackfruit seed starch showed the typical A-type XRD pattern with appearance of peaks of 2θ at 15, 17, 17.3, and 23°. An A-type XRD diffraction pattern had been previously reported for the native jackfruit seed starch.[Citation7] The degree of crystallinity of the native jackfruit seed starch was 29.6%. The results showed that no marked differences in XRD pattern were observed between the native starch and the oxidized starches. The relative crystallinity is normally calculated based on the total area and amorphous area of the X-ray diffractograms, and a significant decrease in the amorphous area results in an increase in relative crystallinity.[Citation13] When the hypochlorite level increased, there was a decrease in relative crystallinity suggesting that either the amorphous region or the crystalline region was already affected after being treated with 1–5% active chlorine. In addition, the jackfruit seed starch oxidized with higher concentrations of active chlorine caused a greater depolymerization of the amylopectin chains. This explanation of the current result is correlated well with the finding in the oxidized corn starch as reported by Kuakpetoon and Wang[Citation14] and Chavez-Murillo et al.[Citation14] Kuakpetoon and Wang[Citation14] reported an increase in relative crystallinity of corn starch after oxidative treatment with 0.8% sodium hypochlorite and they also found a decrease in relative crystallinity when the concentration of hypochlorite was increased to 2 and 5%. According to these authors, they suggested that the increase of relative crystallinity with 0.8% sodium hypochlorite may be due to the occurrence of oxidation is mainly taken place in the amorphous region of the granules with degradation of amylose molecules. For the crystalline region, it may be slightly degraded. However, they suggested that the reduced relative crystallinity with hypochlorite concentrations of 2 and 5% was due to the occurrence of high degradation of the crystalline region. In addition, Vanier et al.[Citation10] also found that high levels of oxidation caused a decrease in the relative crystallinity of bean starch. On the other hand, an increase in relative crystallinity was detected in oxidized corn, barley,[Citation13] and banana starch.[Citation9] This result might be explained because the partial hydrolysis as affected by oxidation mainly occurred in the amorphous region of the starch granules.
Swelling Power
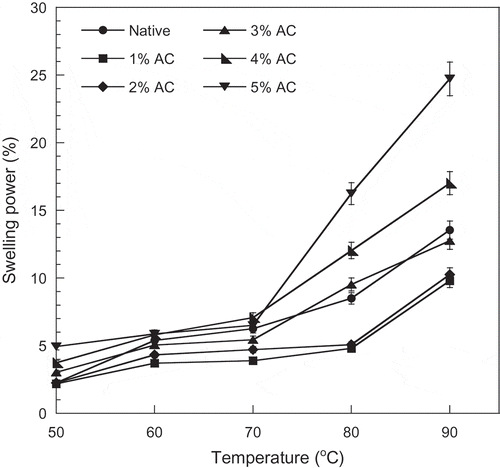
The heating of starch in excess water results in the extensive swelling of the starch granules. The swelling ability of starch contributes to important characteristics of most starchy food products, such as the pasting and rheological behaviors. This swelling power can be used to assess the extent of the interaction between the starch chains, within the amorphous and crystalline regions of the starch granules. The swelling power of starch has been reported to depend upon the water holding capacity of the starch molecules through hydrogen bonding. The hydrogen bonds stabilizing the structure of the double helices in crystallites are broken during gelatinization and are replaced by hydrogen bonds with water, and the swelling is regulated by the crystallinity of the starch.[Citation34,Citation35] In this study, the swelling power of the jackfruit seed starch was evaluated over temperatures of 50–90°C at 10°C intervals. The swelling power of all starch samples increased as the incubation temperature increased (). Normally, starch cannot be dissolved in cool water and this is attributed to the starch crystal structure. The starch molecules start to integrate with water as the temperature increases, then the amylose and amylopectin were dissociated in suspension. The insoluble starch granules started to swell because of hydration. Increases in temperature weakened the intragranular binding forces of the native and the modified starches, thus facilitating less restricted swelling as the temperature increased.[Citation36] Regarding to the effect of oxidation, lower swelling power was found in the starch after being treated with 1–3% active chlorine compared to the native starch. In addition, the swelling power of the oxidized starch tended to decrease with a lower active chlorine level, particularly at a low level of active chlorine (1–3%). Wang and Wang[Citation12] revealed that swelling power of the oxidized corn starches (0.5–3.0% active chloride) was lower than the native corn starch. Similar observations were also found in the oxidized bean starches (0.5–1.5% active chlorine) and the oxidized banana starches (0.75 and 1% active chlorine) and the oxidized sword bean starch (1% active chlorine) as reported by Vanier et al.,[Citation10] Sanchez-Rivera et al.,[Citation9] and Adebowale et al.,[Citation18] respectively. This is probably why the oxidized starches has lower swelling power to its native starch, as these highly associated starch granules with an extensive and strongly bonded micellar structure display relatively great resistance toward swelling.[Citation18] However, the explanation for this phenomenon is still unclear and further study is required. On the other hand, the swelling power of the samples oxidized by higher concentrations of active chlorine, especially at 4–5%, increased compared to the swelling power of native starch. The higher swelling capability of the starch oxidized with high concentrations of active chlorine can also be attributed to there being a higher content of carboxyl groups. It is believed that the repulsion between carboxyl groups in adjacent chains helps increase hydration, leading to increments in swelling power.[Citation21,Citation37] Starch with high swelling power could be utilized in starch applications such as food thickening and the preparation of hydrogels.
Solubility
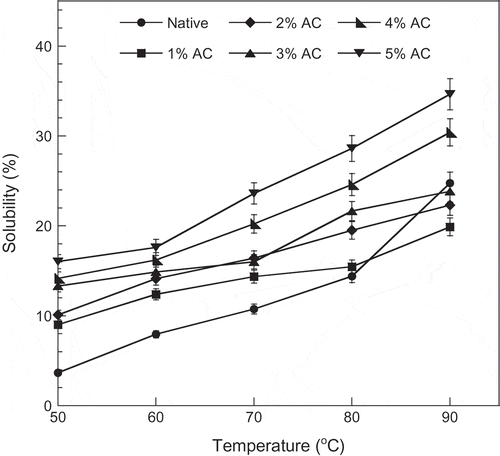
Temperature also affected the solubility of the native and the oxidized starches (). The solubility increased when both the temperature and the concentration of active chlorine increased. The increase in solubility after oxidation was due to the disintegration and structural weakening of the starch granule.[Citation15] Lawal and Adebowale[Citation19] also found that the increase in solubility was probably influenced by the leaching of the amorphous regions of starch granules. The depolymerization of starch chains, particularly at higher concentrations of active chlorine, caused an increase in solubility. This explanation was correlated well with the work of Wang and Wang,[Citation19] who analyzed the average chain length of the oxidized common and waxy corn starches and reported that the oxidation could promote the breaking glucosidic linkages of both amylose and amylopectin, leading to the enhancement of solubility of the oxidized starch.
Thermal Properties
Starch gelatinization was assessed by the differential scanning calorimetry (DSC) because of its accuracy. As previously mentioned, the starch granule has a semi-crystalline structure. It is swollen when treated in the endothermic process. At the same time, the ordering of the crystallinity became disordered.[Citation38] The gelatinization properties of the starches are summarized in . No significant difference in peak temperature (Tp) and end temperature (Te) was found between the native and the oxidized starches. Furthermore, the oxidized starches had lower onset temperatures (To) than the native starch, indicating that the oxidized starches had greater capacity to hydrate and gelatinize. The decrease in To of starch upon oxidation might be due to the weakening of the starch granules, which leads to the early rupture of the amylopectin double helices.[Citation19] In addition to starch degradation, the hypochlorite oxidation introduced high amounts of carboxyl groups into the starch molecules. The negatively charged carboxyl groups could readily adsorb water and facilitate hydration. A lower To was also found in the oxidized normal corn and waxy corn starches compared to their native counterpart starches as shown by Wang and Wang.[Citation12] The enthalpy of gelatinization (∆H) represents the amount of energy required for the gelatinization process. In addition, the enthalpy of gelatinization is regarded as an indicator of the loss of molecular order due to hydrogen bonds breaking within the granule. The enthalpy of gelatinization of the starch oxidized with 1–3% active chlorine was slightly higher than that of the native starch. This result was in agreement with the work of Sanchez-Rivera et al.[Citation9] who studied the oxidized banana starch (0.25–0.50% active chlorine). These noted that carboxyl groups introduced in the starch molecules stabilize the structure and provides the energy necessary to carry out and increase the starch gelatinization process. However, a decrease in the enthalpy of gelatinization was observed after oxidation with high concentrations of active chlorine (4–5%). This suggested that high starch degradation in the crystalline region occurs at high levels of AC as evidenced by the reduction in crystallinity (). High oxidation levels led to a weakening of the starch granules, consequently less energy was needed to gelatinize the starch granules. In addition, high content of carboxyl group might promote the repulsive force between carboxyl groups in adjacent chains, resulting in the enhancement of hydration and gelatinization. Furthermore, a broadening of the gelatinization temperature range (Te-To, ΔT) took place after oxidation. This indicated an increase in inhomogeneity within both the amorphous and crystalline regions of the granules. A broadening of the temperature range of starch treated with oxidation was also found in bean starch.[Citation10]
Table 2. Thermal and pasting properties of the native and the oxidized jackfruit seed starches.
Pasting Properties
The heating of a starch-water dispersion under shear strain above its gelatinization temperature yields starch pastes. The pasting profiles of a starch provide an effective method for relating starch functionality with its structural features. This gives access to the potential industrial application in products dependent on the viscosity and thickening behavior of starch. To compare the pasting properties of the native and the oxidized starches, the pasting temperature (PT), peak viscosity (PV), breakdown (BK), final viscosity (FV), and setback (SB) were calculated from the viscosity-temperature versus time curves obtained from RVA (). The pasting temperature provides an indication of the minimum temperature required to cook the starch. A higher pasting temperature implies a higher cooking time for the starch. It was found that the pasting temperature decreased after oxidation. Further increases in the active chlorine concentration reduced the pasting temperature progressively. The reduction in pasting temperature following oxidation is a consequence of structural weakening and disintegration by the depolymerization of starch molecules during oxidation.[Citation13] In addition, Chavez-Murillo et al.[Citation13] and Kuakpetoon and Wang [Citation14] analyzed the amylopectin chain length of the corn and barley starches and found that the modification by oxidation caused a decrease in amylopectin chain length, resulting in the reduction in pasting temperature. A decrease in the pasting temperature of corn and barley starch oxidized with active chlorine was also observed.[Citation12,Citation13] According to these authors, the oxidized starch granules can swell more easily and can swell to a greater extent. This is because the association forces between the molecules in the native starch are weakened by the electrical repulsion of the carboxyl groups. Thus, more water is allowed to penetrate into the granules, leading to the promotion of gelatinization. When a sufficient number of granules become swollen, a rapid increase in viscosity occurs, known as peak viscosity. Peak viscosity is indicative of water binding capacity. There was a decrease in the peak viscosity of the oxidized starches as compared to the native starch. In addition, increments in the concentration of active chlorine caused a further decrease in the peak viscosity. The decrease in the peak viscosity might be attributed to the partial cleavage of glycosidic linkages due to extensive oxidation, leading to the reduction in the molecular weights of the starch molecules.[Citation10,Citation13] This partially degraded network is not resistant to shearing and the integrity of the starch granules cannot be maintained, thereby producing a low viscosity. Similar decreases in the peak viscosity of the oxidized starch were observed in pine nut starch,[Citation22] banana starch,[Citation9] and corn starch.[Citation16] On the other hand, Vanier et al.[Citation10] reported that the bean starches oxidized with 0.5% active chlorine showed higher peak viscosity than its native counterpart. They suggested that the starch oxidized with low active chlorine levels showed the characteristics of slightly crosslinked starches because chemical crosslinking brought about improved starch integrity. The same pattern was previously reported by Wang and Wang[Citation12] who studied the physicochemical properties of the common and waxy corn starches oxidized with sodium hypochlorite at low levels (0.25–0.50% active chlorine). However, using high concentrations of active chlorine (more than 1%) also caused a decrease in the peak viscosity of the common and waxy corn starches.[Citation12] The breakdown viscosity of all oxidized starches was lower than its native counterpart. An increase in the level of oxidation caused an increase in the breakdown viscosity. The breakdown is caused by the disintegration of the gelatinized starch granule structure during continued stirring and heating. The difference in breakdown viscosity is related to differences in the rigidity of the swollen granules. The lower breakdown implies a higher hot paste stability (the resistance to shear thinning during cooking) of the oxidized starch. Vanier et al.[Citation10] also revealed that the breakdown viscosity of the oxidized bean starch decreased with a higher concentration of active chlorine. Lawal and Adebowale[Citation19] suggested that the reduction in the breakdown of the oxidized starches resulted from the introduction of new substituent groups into the oxidized starches. When the system is cooled, the amylose leached in the aqueous phase starts to re-associate and gradually forms a gel. The gel formation leads to the final equilibrium viscosity. This increase in viscosity from the breakdown value to the final equilibrium viscosity is known as the setback in the pasting curve. Setback viscosity is a measure of the degree of re-association during cooling among starch molecules involving the leaching of amylose from swollen starch granules. It is generally used as a measure of the gelling ability or retrogradation tendency of starch. In addition, high setback is usually related to the amylose content of the starch. The amylose component of the starch retrogrades more readily than amylopectin due to its essentially linear structure. The straight chain structure of amylose allows it to readily form hydrogen bonds between molecules, resulting in a rigid gel. Generally, the oxidized jackfruit seed starches had lower values in terms of setback viscosity and final viscosity as compared to the native starch. This result indicated that the oxidized starches were less prone to reassociation. The substitution of hydroxyl groups with larger size carbonyl and carboxyl groups during oxidation can hinder the interaction between starch chains. This could have caused the oxidized starch molecules to show a lower setback value.[Citation9,Citation10,Citation13,Citation20]
Gelation Capacity
describes the LGC of the native and the oxidized starches. The LGC was used as the index of gelation. The LGC of the jackfruit seed starch increased after oxidation. A reduction in the LGC value is an indication of better gelating properties. Starch gelation is a complex process that involves gelatinization, swelling, and absorption of water to build a three-dimensional network that offers structural rigidity in various food applications. The building of the structural network also involves the bridging of the intergranular binding forces among the starch molecules, which largely involves hydrogen bonding.[Citation19] The results show that the native starch did not form a gel below a 5% concentration while the LGC of starch oxidized with 1% and 2–3% active chlorine was 6 and 7%, respectively. The appropriate concentration for gelation was 9% for the starches oxidized with 4–5% active chlorine. The introduction of carbonyl and carboxyl groups following oxidation probably limited this interaction and caused electrostatic repulsion among the starch molecules, thus increasing the LGC.
Table 3. Gelation capacity of the native and the oxidized jackfruit seed starches.
Turbidity and Paste Clarity
The tendency of retrogradation of the native and the oxidized starches was also determined by following the changes in the light transmittance of starch pastes during storage at 4°C. The light transmittance of all the starches declined as the days of storage increased from the first day to the seventh day (). A high-transmittance value shows the starch paste has more clarity than at a low-transmittance value. The results show that the transmittance values of all the starch pastes decreased continuously with increasing storage times. The increase in turbidity during storage is probably due to the interactions between leached amylose and amylopectin chains that lead to the development of junction zones. These reflect or scatter a significant amount of light. Amylose aggregation and crystallization have been reported to be complete within the first few hours of storage, while amylopectin aggregation and crystallization occurs during later stages.[Citation37] In addition, retrogradation is a time-dependent process, thus longer days of storage enhances structural reordering after gelatinization. Regarding the effect of oxidation, the pastes prepared from the oxidized starches showed much higher light transmittance than the native starch, suggesting that the oxidized starches had a lower tendency to molecular re-association. Moreover, the starch paste turbidity was reduced as the level of oxidation increased. The introduction of negatively charged carboxyl groups into the starch molecules limits the formation of binding forces by electrostatic repulsion and this accounts for the reduction in the turbidity of the oxidized starch. Paste clarity is valued in starch applications such as food additives because it enhances consumer acceptability of the product, particularly those products that could stay on the shelf for a long time. The observation here is consistent with the literature where light transmittance of cassava starch increased after oxidation,[Citation21] suggesting reduction in paste turbidity.
Table 4. Transmittance value and syneresis of the native and the oxidized jackfruit seed starches.
Syneresis: Stability Relating to Refrigeration
Water mobility has an important role in the quality of systems containing starch. A common phenomenon observed after the refrigeration food containing starch was the release of water (syneresis); this was mainly related to amylose retrogradation. High syneresis is not seen as beneficial in the food industry, since starches with this property readily absorb and eliminate water, like a sponge.[Citation39] The result of syneresis in all samples is shown in . An increase in syneresis was observed with respect to the storage time. The native jackfruit seed starch had higher syneresis and, therefore, lower stability in refrigeration than did the oxidized starches. This indicates that during storage the oxidized starches showed the capacity to retain more water. These results suggest that the oxidized starches are more stable during refrigerated storage than are native starches. This suggests that this type of modification to jackfruit seed starch greatly improves its functionality for products requiring refrigerated storage. The presence of hydrophilic functional groups, especially carboxyl groups, in the oxidized starches might be responsible for the retardation of syneresis.
Conclusion
This investigation dealt with the evaluation of the changes in the functional properties of starch isolated from jackfruit seed following oxidation. The degree of starch oxidation and the resultant starch physicochemical properties varied greatly with the amount of active chlorine. The modification of jackfruit seed starch by oxidation could greatly improve the whiteness, paste clarity, and stability of starch gel during low-temperature storage. This study has shown that the oxidized jackfruit seed starch can be used as a thickener or stabilizer in products where paste clarity is required, as well as for products that stored at low temperatures.
Acknowledgment
The authors thank Dr. Anthony Gethin Hopkin for kindly revising the English of the manuscript.
Funding
This research was funded by the Faculty of Agricultural Product Innovation and Technology, Srinakharinwirot University.
Additional information
Funding
References
- Tongdang, T. Some properties of starch extracted from three Thai aromatic fruit seeds. Starch/Starke 2008, 60, 199–207.
- Madrigal-Aldana, D.L.; Tovar-Gomez, B.; Oca, M.M.; Sayago-Ayerdi, S.G.; Gutierrez-Meraz, F.; Bello-Perez, L.A. Isolation and characterization of Mexican jackfruit (Artocarpus heterophyllus L) seeds starch in two mature stages. Starch/Starke 2011, 63, 364–372.
- Kittipongpatana, O.S.; Kittipongpatana, N. Preparation and physicochemical properties of modified jackfruit starches. LWT-Food Science and Technology 2011, 44, 1766–1773.
- Dutta, H.; Sa Paul, S.K.; Kalita, D.; Mahanta, C.L. Effect of acid concentration and treatment time on acid-alcohol modified jackfruit seed starch properties. Food Chemistry 2011, 128, 284–291.
- Oates, C.G.; Powell, A.D. Bioavailability of carbohydrate material stored in tropical fruit seeds. Food Chemistry 1996, 56, 405–414.
- Bobbio, F.O.; El-Dash, A.A.; Bobbio, F.O.; Rodrigues, L.R. Isolation and characterization of the physicochemical properties of the starch of jackfruit seeds (Artocarpus Heterophyllus). Cereal Chemistry 1978, 55, 505–511.
- Rengsutthi, K.; Charoenrein, S. Physico-chemical properties of jackfruit seed starch (Artocarpus heterophyllus) and its application as a thickener and stabilizer in chilli sauce. LWT-Food Science and Technology 2011, 44, 1309–1313.
- Mukprasirt, A.; Sajjaanantakul, K. Physico-chemical properties of flour and starch from jackfruit seeds (Artocarpus heterophyllus Lam.) compared with modified starches. International Journal of Food Science and Technology 2004, 39, 271–276.
- Sanchez-Rivera, M.M.; Garcia-Suarez, F.J.L.; Velazquez del Valley, M.; Gutierrez-Meraz, F.; Bello-Perez, L.A. Partial characterization of banana starches oxidized by different levels of sodium hypochlorite. Carbohydrate Polymer 2005, 62, 50–56.
- Vanier, N.L.; Zavareze, E.R.; Pinto, V.Z.; Klein, B.; Botelho, F.T.; Dias, A.R.G.; Elias, M.C. Physicochemical, crystallinity, pasting, and morphological properties of bean starch oxidised by different concentrations of sodium hypochlorite. Food Chemistry 2012, 131, 1255–1262.
- Kuakpetoon, D.; Wang, Y.J. Locations of hypochlorite oxidation in corn starches varying in amylose content. Carbohydrate Research 2008, 343, 90–100.
- Wang, Y.J.; Wang L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydrate Polymer 2003, 52, 207–217.
- Chavez-Murillo, C.E.; Wang, Y.J.; Bello-Perez, L.A. Morphological, physicochemical, and structural characteristics of oxidized barley and corn starches. Starch/Strake 2008, 61, 206–213.
- Kuakpetoon, D.; Wang, Y.J. Structural characteristics and physicochemical properties of oxidized corn starches varying in amylose content. Carbohydrate Research 2006, 341, 1896–1915.
- Chong, W.T.; Uthumporn, U.; Karim, A.A.; Cheng, L.H. The influence of ultrasound on the degree of oxidation of hypochlorite-oxidized corn starch. LWT-Food Science and Technology 2013, 50, 439–443.
- Sandhu, S.K.; Kaur, M.; Singh, N.; Lim, T.S. A comparison of native and oxidized normal and waxy corn starches: Physicochemical, thermal, morphological, and pasting properties. LWT-Food Science and Technology 2008, 41, 1000–1010.
- Sangseethong, K.; Termvejssyanon, N.; Sriroth, K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized cassava starches. Carbohydrate Polymer 2010, 82, 446–453.
- Adebowale, K.O.; Afolabi, T.A.; Olu-Owolabi, B.I. Functional, physicochemical, and retrogradation properties of sword bean (Canavalia gladiata) acetylated and oxidized starches. Carbohydrate Polymer 2006, 65, 93–101.
- Lawal, O.S.; Adebowale, K.O. Physicochemical characteristics and thermal properties of chemically modified jack bean (Canavalia ensiformis) starch. Carbohydrate Polymer 2005, 60, 331–341.
- Simsek, S.; Ovando-Martinez, M.; Whitney, K.; Bello-Perez, L.A. Effect of acetylation, oxidation, and annealing on physicochemical properties of bean starch. Food Chemistry 2012, 134, 1796–1803.
- Lawal, O.S. Composition, physicochemical properties, and retrogradation characteristics of native, oxidized, acetylated, and acid-thinned new cocoyam (Xanthosoma sagittifolium) starch. Food Chemistry 2004, 87, 205–218.
- Conto, L.C.; Plata-Oviedo, M.S.V.; Steel, C.J.; Chang Y.K. Physico-chemical, morphological, and pasting properties of Pine nut (Araucaria angustifolia) starch oxidized with different levels of sodium hypochlorite. Starch/Strake 2011, 63, 198–208.
- Eggleston, G.; Swennen, R.; Akoni, S. Physicochemical studies on starch isolated from plantain cultivars, plantain hybrids, and cooking bananas. Starch/Starke 1992, 44, 121–128.
- AOAC. Official Methods of Analysis of the Association of Official Chemists International, 16th Ed; The Association of Official Analytical Chemists International, Gaithersburg, 2000.
- Gunaratne, A.; Hoover, R. Effect of heat moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydrate Polymer 2002, 49, 425–437.
- Nollet, L.M.L. Handbook of Water Analysis; Marcel Dekker, Inc: New York, 2007, 191–197.
- Chattpadhyay, S.; Singhal, R.S.; Kulkarni, P.R. Optimization of synethesis of oxidized starch from corn and amaranth for use in film-forming application. Carbohydrate Polymer 1997, 34, 203–213.
- Smith, R.J.; Characterization and Analysis of Starches. In Starch Chemistry and Technology Vol II; Whistler, R.L.; Paschall, E.F.; Eds.; Academic Press: New York, 1976; 620–625.
- Zhu, K.; Kanu, P.J.; Claver, I.P.; Zhu, K.; Qian, H.; Zhou, H. A method for evaluating Hunter whiteness of mixed powders. Advanced Powder Technology 2009, 20, 123–126.
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana starch: Production, physicochemical properties, and digestibility—A review. Carbohydrate Polymers 2005, 59, 443–458.
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Relationship between granule size and in vitro digestibility of maize and potato starches. Carbohydrate Polymers 2010, 82, 480–488.
- Wang, Y.J.; White, P.; Pollak, L.; Jane, J. Characterization of starch structure of 17 maize endosperm mutant genotypes with Oh43 inbred line background. Cereal Chemistry 1993, 70, 171–179.
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical, and physicochemical aspects of starch granule size, with emphasis on small granule starches—A review. Starch/Strake 2004, 56, 9–99.
- Debet, M.R.; Gidley, M.J. Three classes of starch granule swelling: Influence of surface proteins and lipids. Carbohydrate Polymers 2006, 64, 452–465.
- Leach, H.W.; McCowen, L.D.; Schoch, T.J. Structure of starch granule. Swelling power and solubility patterns of various patterns of various starches. Cereal Chemistry 1959, 36, 534–544.
- Li, J.Y.; Yeh, A.I. Relationships between thermal, rheological characteristics, and swelling powder of various starches. Journal of Food Engineering 2001, 50, 141–148.
- Lawal, O.S.; Adebowale, K.O.; Ogunsanwo, B.M.; Barba, L.L.; Llo, N.S. Oxidized and acid thinned derivatives of hybrid maize: Functional characteristics, wide-angle X-ray diffractometry, and thermal properties. International Journal of Biological and Macromolecules 2005, 35, 71–79.
- Gujral, H.S.; Sharma, P.; Kaur, H.; Singh, J. Physicochemical, pasting, and thermal properties of starch isolated from different barley cultivars. International Journal of Food Properties 2013, 16, 1494–1506.
- Singh, H.; Sodhi, N.S.; Singh, N. Structure and functional properties of acetylated sorghum starch. International Journal of Food Properties 2012, 15, 312–325.