ABSTRACT
Electrical bio-impedance measurements were conducted on local strawberry fruits. A non-destructive device was designed to obtain the impedance spectrum of the whole fruit. Four electrical variables were tested: low frequency resistor R0 (related to extracellular resistances), the high frequency resistor R∞ (related to intracellular resistances), and constant phase element (magnitude and phase, related to the membrane capacitances and heterogeneity, respectively). In parallel with the electrical bio-impedance measurement, color and firmness were correlated to the ripeness stage. The results indicated that the strawberries at the highest stage of ripeness had significantly lower constant phase element and R0 values.
Introduction
The strawberry is a non-climacteric fruit that must be harvested at its full ripeness stage to achieve the maximum quality of flavor and color. Strawberry quality components include appearance (color, size, shape, and the absence of defect and decay), firmness, flavor, and nutritional value.[Citation1] The firmness affects the susceptibility of the strawberries to physical damage and, consequently, their suitability for shipping.[Citation2] The color of fresh strawberries is a highly variable trait of seedling populations and is one of the most important traits for selecting commercially acceptable cultivars.[Citation3] Electrical bio-impedance is a simple technique using the passive electrical properties of materials and, particularly for biological tissues, the ability to oppose the flow of an electric current.[Citation4] This technique has been used with destructive measurement methods to characterize the electrical properties of a wide variety of biological materials. However, the same technique has been used in plant tissues, such as fruits, in a non-destructive way.[Citation5] One of the most widely used models used to analyze electrical bio-impedance data is the Cole model. Investigations of this field have been related to the determination of the extent of damaged apple tissue caused by bruising,[Citation6] the evaluation of ripening and chilling injury development in persimmon fruits,[Citation7] the assessment of the ripening of kiwi fruits,[Citation8] and the characterization of changes in intracellular and extracellular resistance, as well as changes in the condition of membranes, during the ripening of nectarines.[Citation9] The same technique was used on apples stored at room temperature to assess the degree of conservation of the fruit.[Citation10] Additionally, the electrical bio-impedance method has been used to assess the soluble solids content and acidity of apples,[Citation11] to assess the conditions of unripe and ripe mango fruits harvested using a robotic arm,[Citation5] and to distinguish the internal quality of healthy and rotten apples.[Citation12] The non-destructive determination of the impedance spectrum has also been used to assess fresh (intact) and artificially bruised (pressed) apples with or without skin.[Citation13] A recent study of citrus fruit was published by Jaunsah et al.;[Citation14] in this work, the authors dealt directly with frequency-dependent parameters (resistance and reactance) and found the highest correlations with ripeness at 1 MHz after analyzing different parts of the fruit. Finally, non-contact methods, such as magnetic induction spectroscopy, used for the impedance measurements of biological samples have been addressed.[Citation15] When using magnetic induction, rather than electrodes, to produce eddy currents across a sample, a secondary magnetic field is generated. The impedance of the sample affects the magnitude and phase of the magnetic field due to this secondary field. This technique is more challenging and requires higher sensitivity than direct electrical impedance. In whole strawberry fruits, electrical impedance spectroscopy was used to assess the changes in the electrical resistance of the apoplast and symplast.[Citation16] Carbon dioxide treatments reduced the resistance of the apoplast (resistance at 50 Hz) below that of the control fruit. In this article, the variables obtained using the electrical bio-impedance spectrum of strawberries were used to classify parameters by ripeness stage.
Materials and Methods
Materials
“Camino real” strawberries were obtained from a commercial orchard in the town of Irapuato, in the state of Guanajuato, Mexico. The fruit remained at 4°C inside a portable refrigerator throughout the experiment. A graded color chart (subjective classification) and a range equatorial diameter table (objective classification), referenced in the Mexican Norm (NMX-FF-062-SCFI-2002), were used to classify three ripeness degrees, 2, 4, or 6 (of a scale range from 0 to 6, where 0 is not ripe and 6 is fully ripe), and two sizes, B or C (of three sizes available: A, B, and C, where A is the smallest and C the largest). Twenty-five strawberries were measured for each ripeness degree and size; a total of 150 fruits were evaluated in this study.
Experimental Set-up
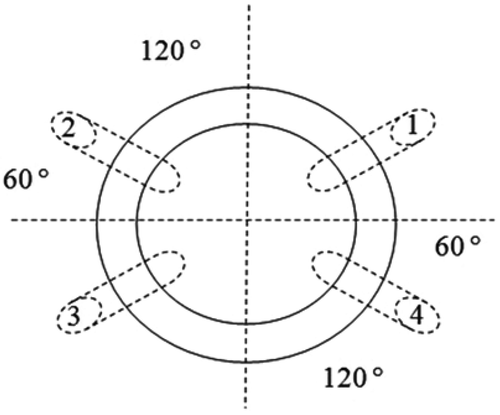
Electrical bio-impedance measurements were performed using an impedance analyzer (Solartron SI 1260) and a frequency range between 1 Hz and 1 MHz, allowing for the characterization of the α and β dispersions, and a 1 Vrms generator voltage. A circular device with a four electrode configuration was used; the two current stimulus electrodes were separated from each other by 120°, and the other two electrodes for the voltage measurement were in a symmetrical array, as shown in . The electrodes gently touched the intact fruit through a conductive gel between the four copper electrodes and the skin of the fruit to ensure good electrical contact. The strawberries were placed on the container with the stem end-calyx axis vertical so that the electrodes were placed around the fruit’s equator.
Figure 1. Schematic diagram of the device used for measuring strawberry ripeness by electrical bio-impedance.
Two impedance spectra were obtained per fruit. Using both the Nyquist plot and the Cole model, four variables were obtained, as shown in . The four electrical variables were: the low frequency resistor R0 (related to extracellular resistance), the high frequency resistor R∞ (which includes information about intracellular resistance), the constant phase element (CPE; and with magnitude [CPE-T], sometimes called the pseudocapacitance and phase [CPE-P]); R0 and R∞ are derived from the series and parallel resistances of the equivalent circuit (Rs and Rp, respectively). The magnitude CPE-T is related to the membrane capacitances and the phase CPE-P is related to the heterogeneity of the sizes and shapes of the cell. The equivalent “real” capacitance is, C = Rp(1 – α) /α(CPE-T)1/α, where CPE-P = α. Thus, the Tau (τ) parameter (τ = RpC) or the relaxation time would be, τ = (Rp × CPE-T)1 /α, without addressing the physical meaning and dimensions.[Citation17] We decided to work with the CPE-T and CPE-P parameters and with R∞ (Rs) and R0 (Rp – Rs) for classification purposes. The mean value of both measurements for each fruit was used for analysis.
Figure 2. Nyquist plot of the impedance spectrum (Re [Z] versus Im [Z]), left; single-dispersion Cole model, right.
![Figure 2. Nyquist plot of the impedance spectrum (Re [Z] versus Im [Z]), left; single-dispersion Cole model, right.](/cms/asset/086bca2b-8815-4129-9d51-3e92cefc2577/ljfp_a_1199033_f0002_b.gif)
The external skin color was evaluated on three sides of the fruit using a Konica Minolta spectrophotometer CM-508d, and the results were expressed in CIE L*, a*, and b* space. The D65 light source was calibrated using a white standard tile. The average measurements of the three sides were used for analysis. Data acquisition of the strawberry firmness (Fx) assessment was performed using a TA-XT2 texture analyzer. A 50 kg load cell was used for the determination of the firmness of the fruit. A probe (4 mm diameter) with a flat tip punctured twice at a speed of 1 mm/s into the intact skin of the fruit (5 mm depth), rotating 90°. This procedure provided a force-distance curve where the breakpoint obtained was related to the firmness (N).
Statistical analysis was performed using analysis of variance (ANOVA), test discriminant analysis or multiple linear regression (MLR) with the Statgraphics plus 5.1 program. Parametric statistics were used because the data exhibited a normal distribution.
Results
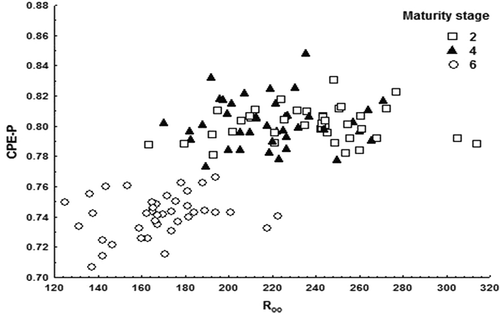
ANOVA was used to search for statistically significant differences among the ripeness stage parameters. No significant differences were found between the ripeness stages and weight or diameter (p > 0.05), as expected. Moreover, no significant differences were found between the size and color or firmness, in agreement with the fact that the size is independent of ripeness. Statistically significant differences were found between the ripeness stages and color (L*), firmness (Fx), impedance resistance (R∞), and the CPE-P of the constant phase element at the last ripeness stage (); no statistically significant differences were found for the other impedance parameters R0 and CPE-T. The stages of ripeness were visually and manually established according to color by experts. One expert was a strawberry farmer with many years of experience. The farmer may not be a formally trained agronomist, but his years of experience in the orchards make him an expert.
Table 1. Lightness (L*), firmness (Fx), resistance (R∞), and homogeneity degree (CPE-P) of the strawberries1.
To quantify the capacity of the electrical bio-impedance parameters used to rank the ripeness stages of strawberries, multi-linear regression analysis and forward stepwise discriminant analysis were performed for all parameters. With the ripeness set as the only dependent variable and the rest set as independent variables, the software was able to identify redundant information and select the most useful variables or combination of variables to be considered in the results.
Using the color variables and the destructive firmness to segregate the ripeness stages, 99.3% of fruits were appropriately classified. This classification result was expected because the manual classification was made based on a visual inspection of the color by an expert during the harvest and was confirmed using the classification conducted in the lab.
In the case of the electrical bio-impedance parameters, the first approach was a multi-linear regression analysis that was used to predict the color variables and firmness related to the impedance variables. The color variables, L*, hue angle (tg−1[b* /a*]), and firmness were significantly related to the impedance variables. Approximately 76.7% of the variance of the L* variable and 70.1% of the variance of the hue angle could be explained by the impedance variables. Because color is the main parameter for classification, the color change DE (a, b), based on L, with “a” and “b” color coordinates was calculated in reference to the non-ripe strawberries (L0, a0, b0) using Eq. (1) and was compared to the ripeness stages.
As expected, because the gold standard classification was performed using color, the color change increased significantly during ripening (p < 0.001). The color variable “a” (directly proportional to red color) was significantly related to the impedance variables. Approximately 77.1% of the variance of the “a” color coordinate could be explained by the impedance variables Rs, CPE-P, and Rp, according to Eq. (2).
In this work, color and firmness were considered for comparison purposes only; the final aim was to discover how well the impedance measurement can replace color and firmness for ripeness stage classification. Therefore, a forward stepwise discriminant analysis was performed to classify the ripeness stages using the electrical bio-impedance variables. There was 77.3% of appropriately classified strawberries obtained, as shown in . The bio-impedance variables CPE-P (related to the tissue homogeneity), R∞ (related to the intracellular resistance), and R0 (related to extracellular resistance) were selected for this analysis.
Table 2. Classification matrix of the discriminant analysis of the previously established and predicted ripeness stages (2, 4, and 6; from least to most ripe) according to the bio-impedance variables (CPE-P, R0, and R∞).
The preliminary results showed that there were no significant differences between adjacent stages; therefore, we decided to report the differences found for non-adjacent stages (2, 4, and 6). Strawberries from the highest ripeness stage (6) were perfectly classified (100% of fruit correctly classified). However, ripeness stages 2 and 4 (low and intermediate ripeness stages) were misclassified in approximately one-third of the cases. Strawberries with the highest ripeness stage (6) had lower CPE-P and R∞ values than the other strawberries (p < 0.05), as shown in . More mature fruits had lower tissue homogeneity and lower intracellular resistance due to the textural degradation. In all cases, strawberries from the highest ripeness stage (6) had a CPE-P value lower than 0.77 and an R∞ value less than 223 ohms. The lack of the tissue homogeneity, detected using only the CPE-P bio-impedance variable, could be used to detect fruits in the last ripeness stage.
Discussion
Color and firmness parameters have been, until now, the best options to classify strawberry fruits according to their ripeness stage. The use of these parameters is expected for ripeness classification because these parameters are used by the experts to classify fruit during harvest. Nevertheless, firmness is generally a destructive test, leaving color as one of the only options to assess ripeness in a non-destructive way.
The electrical bio-impedance technique has been used in both destructive and non-destructive ways to evaluate fruit quality and is proposed as a non-destructive alternative for performing ripeness classification according to color. However, the comparison may not be equal because the bio-impedance data gives information about the whole fruit (mainly internal quality); however, color addresses the external quality of the fruit, and the process of ripening is non-homogeneous. One-third of the strawberries that were classified by bio-impedance as stage 2 already had enough red color on the surface to be classified by color as stage 4; one-third of the fruits classified by bio-impedance as stage 4 did not have enough red surface color or had a non-homogeneous surface color, so they were classified by color as stage 2. Further studies should be conducted to correlate all of the bio-impedance parameters with the average quality of the fruit (surface color, firmness, and internal tissue condition).
Conclusions
Because expert harvesters use visual color assessment to classify strawberries, the fruit evaluation using those parameters almost completely discriminated the samples considered in this work (99.3% of fruits were appropriately classified). Strawberries from the low and intermediate ripeness stages were misclassified using the bio-impedance parameters. However, through the use of the electrical bio-impedance parameters CPE-P and R∞, a complete classification of the last ripeness stage (completely ripe fruit) was obtained. Strawberries at the highest ripeness stage had significantly lower CPE-P (lower than 0.77) and R∞ (less than 223 ohms) values than the other strawberries. More mature fruits had lower tissue homogeneity and lower intracellular resistance due to texture degradation. The lack of tissue homogeneity detected using only the CPE-P bio-impedance variable could be used to detect fruits in the last ripeness stage.
Acknowledgments
The authors would like to express their gratitude to Mr. and Mrs. Abraham from Irapuato, Mexico for providing the fruits, and to Mr. Juan Manuel Noriega from the University of Guanajuato for his technical support.
Funding
The authors would like to thank the University of Guanajuato for the financial support.
Additional information
Funding
References
- Cordenunsi, B.R.; Do Nascimento, O.J.R.; Lajolo, F.M. Physico-Chemical Changes Related to Quality of Five Strawberry Fruit Cultivars During Cool-Storage. Food Chemistry 2003, 83, 167–173.
- Kader, A. Quality and Its Maintenance in Relation to the Postharvest Physiology of Strawberry. In The Strawberry into the 21st Century; Dale, A., Luby, J.J.; Eds.; Timber Press: Portland, OR, 1991; 145–152.
- Sacks, E.J.; Shaw, D.V. Optimum Allocation of Objective Color Measurements for Evaluating Fresh Strawberries. Journal of American Horticultural Science 1994, 119(2), 330–334.
- Grimnes, S.; Martinsen, O.G. Bioimpedance and Bioelectricity Basics. Academic Press: London, 2008.
- Rehman, M.; Abu Izneid Basem, A.J.A.; Abdullah, M.Z.; Arshad, M.R. Assessment of Quality of Fruits Using Impedance Spectroscopy. International Journal of Food Science and Technology 2011, 46, 1303–1309.
- Jackson, P.J.; Harker, F.R. Apple Bruise Detection by Electrical Impedance Measurement. Postharvest Biology and Technology HortScience 2000, 35(1), 104–107.
- Harker, F.R.; Forbes, S.K. Ripening and Development on Chilling Injury in Persimmon Fruit: An Electrical Impedance Study. New Zealand Journal of Crop and Horticultural Science 1997, 25, 149–157.
- Bauchot, A.D.; Harker, F.R.; Arnold, W.M. The Use of Electrical Impedance Spectroscopy to Assess the Physiological Condition of Kiwifruit. Postharvest Biology and Technology 2000, 18(1), 9–18.
- Harker, F.R.; Maindonald, J.H. Ripening of Nectarine Fruit: Changes in the Cell Wall, Vacuole, and Membranes Detected Using Electrical Impedance Measurements. Plant Physiology 1994, 106, 165–171.
- Alzate, A.V.; Noreña, P.T.; Segura, B.; Aristizábal, W.; Rorales-Rivera, A. Estudio de la Manzana Mediante la Técnica de Espectroscopia de Impedancia Eléctrica. Revista Colombiana de Física [Electrical Impedance Spectroscopic study of apples. Colombian Journal of Physics] 2009, 41(1), 175–177.
- Fang, Q.; Liu, X.; Cosic, I. Bioimpedance Study on Four Apple Varieties. IFMBE Proceedings 2007, 17, 114–117.
- Li, Q.; Wang, J.; Ye, Z.; Ying, Y.; Li, Y. Detection of Fruit Quality Based on Bioimpedance Using Probe Electrodes. In Optics East 2005. International Society for Optics and Photonics, 2005. 59940W-59940W-11.
- Vozáry, E.; Benko, P. Non-Destructive Determination of Impedance Spectrum of Fruit Flesh Under the Skin. International Conference on Electrical Bioimpedance. Journal of Physics: Conference Series 2010, 2010, 012142.
- Harker, F.R.; Elgar, H.J.; Watkins, C.B.; Jackson, P.J.; Hallet, I.C. Physical and Mechanical Changes in Strawberry Fruit After High Carbon Dioxide Treatments. Postharvest Biology and Technology 2000, 19, 139–146.
- Juansah, J.; Budiastra, I.W.; Dahlan, K.; Seminar, K.B. Electrical Properties of Garut Citrus Fruits at Low Alternating Current Signal and Its Correlation with Physicochemical Properties During Maturation. International Journal of Food Properties 2014, 17, 1498–1517.
- O’Toole, M.D.; Marsh, L.A.; Davidson, J.L.; Tan, Y.M.; DArmitage. D.W.; Peyton, A.J. Non-Contact Multi-Frequency Magnetic Induction Spectroscopy System for Industrial-Scale Bio-Impedance Measurement. Measurment Science and Technology 2015, 26(3), 035102.
- Gore, C.M.; White, J.O.; Wachsman, E.D.; Thangadurai, V. Effect of Composition and Microstructure on Electrical Properties and CO2 Stability of Donor—Doped, Proton Conducting BaCe1−(x+y)ZrxNbyO3. Journal of Materials Chemistry A 2014, 2, 2363–2373.