ABSTRACT
Walnuts contain significant amounts of bioactive compounds such as polyphenols. The aim of the present study was to determine the differences in phenolic composition and polyphenolic in vitro availability of 10 genotypes of walnuts. All analyzed genotypes showed high flavonoid and non-flavonoid phenolic compounds, especially Eureka and Tehama genotypes. Ellagic acid and syringic acid were the mayor non-flavonoids. Regarding flavonoids, catechin and procyanidin B2 were the most abundant, covering about 98% of total flavonoids. The in vitro digestion showed flavonoids as the most available polyphenols, especially catechin, gallocatechin, and epigallocatechin gallate.
Introduction
Walnuts (Juglans regia, L), originated in central Asia and the Mediterranean region, are an essential food in Mediterranean Diet, consumed as snacks, desserts, or part of a meal. Today, walnuts are cultivated for commercial purposes throughout southern Europe, northern Africa, eastern Asia, the United States, and western South America.[Citation1] Several epidemiological studies suggest that walnut consumption is inversely associated with the incidence of different diseases such as cardiovascular disease, obesity, diabetes, brain illness, and some kinds of cancer.[Citation2–Citation4] Walnuts have shown healthy properties, both in terms of prevention and promotion of health, mainly due to its polyphenolic composition. Due to this evidence, walnuts have been incorporated into recommended dietary guidelines in some countries, for example, the United States, Canada, and Spain.[Citation5] Walnuts are comprised of significant amount of phytochemicals, particularly phenolic compounds, including hydrolyzable and condensed tannins, flavonoids, or phenolic acids.[Citation6,Citation7]
The highest content of polyphenols in walnuts has been found in the hull, reporting favorable effects on human health due to their apparent antiatherogenic and antioxidant properties.[Citation8,Citation9] The slight astringency of the nuts has been associated with the presence of phenolic compounds.[Citation10] A handful of walnuts provides more polyphenols than other foods from the Mediterranean diet.[Citation8,Citation11,Citation12] Finally, ellagitannins have been reported as the main phenolic compounds from the seeds of Juglans regia L.[Citation9,Citation13]
Establishing the bioavailability of phenolic compounds is essential for their effect on the organism. A major challenge in the evaluation of the role of health-promoting components in walnuts is the lack of information about its bioavailability regarding the gastrointestinal track. Previous reports have determined the in vivo bioavailability of walnuts in humans. However, previous scientific reports evaluating the in vitro availability of total polyphenol content are limited. The objective of the present work was to determine the differences in phenolic composition and polyphenolic in vitro availability from 10 genotypes of walnuts, grown in Region of Murcia under the same agricultural practices.
Materials and methods
Source of the walnuts
Ten different varieties of walnut samples were obtained from an experimental cultivar of the Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario (IMIDA; Torre Pacheco, Spain). These commercial walnuts varieties were: Algaida, Amigo, Chico, Eureka, Franquette, Payne, Pedro, Serr, Sunland, and Tehama. All varieties were grown under the same agricultural conditions. Cultivation references were 6 × 8 m2. The soil has a clay loam texture according to the classification criteria of the U.S. Department of Agriculture (USDA). After the analysis of soil saturation, the extract field had the following grading: 31.77% sand, 32.53% silt, and 35.7% clay. For each variety, 2 kg of walnuts were harvested. Mesocrap-striped walnuts were obtained at optimum ripeness, discarding those with defects like cracks or microbiological contamination symptoms. From each batch, 50 randomly selected fruits were shelled to remove kernels. Finally, walnut kernels were vacuum-packed in plastic bags and stored at –80°C until analysis. Prior to analysis, 12 g of walnuts were homogenized with an Ultraturrax IKA® T18 Basic at 24,000 rpm after the addition of 60 mL of distilled water.
For in vitro availability, a pepsin solution was prepared by adding 4 g of pepsin (P7000 Sigma-Aldrich, Germany) in 25 mL of distilled water. A pancreatin mixture solution was prepared with 0.42 g of NaHCO3 (Sigma-Aldrich, Germany), with 1.25 g of bile salts (Sigma-Aldrich, Germany) and 0.2 g of pancreatin (P7545 Sigma-Aldrich, Germany), the mixture was dissolved in 50 mL of distilled water.
In vitro availability method
The procedure was adapted from the previous work of Gil-Izquierdo et al.[Citation14] This method allows knowing the grade of release from the food matrix as well as the stability of phenolic compounds. Forty milliliters of the previously prepared mixture of water and walnuts were used. The pepsin solution was prepared with 4 g of pepsin (Sigma-Aldrich) and was added to 25 mL of distilled water and stirred. The pancreatin solution was prepared with 0.42 g of NaHCO3, with 1.25 g of bile salts (Sigma-Aldrich) and 0.2 g of pancreatin (Sigma-Aldrich); the mixture was dissolved in 50 mL of distilled water.
First, the pH was measured and the sample was titrated with 0.6 N HCL to pH 2. Then, 6 mL of the solution of pepsin and acid digestion was completed for 2 h at 37°C in a bath with constant mild agitation, mimicking the peristalsis and human body temperature. During this time, the pH was monitored in order to maintain a of 2.
Second, an aliquot of 20 mL (aliquot 1) of the sample was added 5 mL of solution of bile salts and pancreatin, and titrated with NaOH to pH 7. Another aliquot (aliquot 2) of ±20 mL, remained in an ice bath so that the acid digestion was stopped. Third, the aliquot 2 was subjected to a second digestion and dialysis at 37°C for 2 h in a water bath with moderately and constant stirring, simulating human conditions. Membranes were filled with 25 mL of water and known amounts of NaHCO3 equivalent to the previous valuate acidity (NaHCO3 equivalents necessary for dialyzed mixture of pepsin and biliary-pancreatic extracts at pH 7). Five milliliters of the mixture of biliary-pancreatic extracts was added, and enzyme was allowed to act for 2 h at physiological temperature, obtaining a balance between the dialyzed fraction (bioavailable) and the non-dialyzed fraction (not bioavailable). Finally, dialysate was collected, filtered through a membrane filter 0.45 µm Millex-HV13 (Millipore, USA), and stored at –80°C until analysis. All the analyses were replicated (n = 3) and expressed as mean values ± standard deviation.
Identification and quantification of phenolic compounds
Sample preparation
For preparation of the samples, 3 g of walnuts were homogenized in an Ultraturrax T-18 Basic at 24,000 rpm for 1 min with 20 mL of a dissolution methanol:water (80:20; v:v). The extracts were centrifuged at 5000 × g for 10 min in a centrifuge Heraeus Biofuge stratos. The methanol was evaporated and the aqueous phase was extracted with hexane (1:1; v:v) and filtered through solid phase extraction “Sep-Pak” (Millipore, USA). The cartridges were previously activated with 10 mL methanol, 10 mL of deionized water, and finally with 10 mL of air. After elution of the sample volume, the cartridge was washed with 10 mL of water. The remaining volume in the cartridge was eluted with 2 mL of methanol. The methanol fraction was filtered through a Millex-HV13 0.45 µm (Millipore, USA) filter and analyzed by high-performance liquid chromatography-diode array detector (HPLC-DAD).[Citation15,Citation16]
For determination of ellagitannins, a hydrolysis was carried out according to the procedure of Häkkinen, et al.,[Citation17] and modified by Cerdá et al.[Citation15] One gram of the nuts were homogenized with 5 mL of 2 M ClH. The mixture was placed in an oven at 85°C for 20 h. Then, the solution was extracted with 8 mL of ether. This process was repeated three times. The organic phase was dried and the residue was redissolved in 1 mL of methanol, and filtered through a membrane filter 0.45 μm Millex-HV13 (Millipore, USA). The filtrate was collected in amber vials and analyzed by HPLC-DAD.
Determination of total phenolic compounds
The chromatograph used was Shimadzu model LC-10AD HPLC with DAD SPD-M10A (Agilent Technologies Spain). The chromatographic separation was performed on a reserve phase C18 column Lichrospher RP-18, 25 cm long, 0.4 cm in diameter, and 5 μm particle size, coupled to a pre-column of the same material and 1 cm length.
Two solvents are used as mobile phases: a solvent consisting of water with 1% formic acid (99:1, v:v) and solvent B, 100% methanol. Elution was performed with a flow rate of 1 mL min−1 and gradient with initial conditions 5% B, which increased to 15% B at 3 min, 20% B at 5 min, 25% B at 12 min, 30% B at 15 min, 40% B at 20 min, 45% B at 30 min, 50% B in 40 min and reached 70% of B at 45 min, returning to initial conditions which were maintained for 10 min to recondition the column between analyses. The injection volume was 20 µL.
The ultraviolet spectra of the different compounds were recorded between 240 and 400 nm. Detection was carried out at 280 and 320 nm. The identification of peaks was confirmed by comparing their retention times with pure standards and quantified by comparing the peak areas in the chromatograms of the samples with those of the standards.
Gallic acid, gallocatechin, catechin, procyanidin B2, epigallocatechin gallate, epicatechin gallate, and epicatechin were quantified at 280 nm, and chlorogenic acid, p-coumaric acid, and syringic acid at 320 nm.[Citation18] Ellagitannins were quantified as free ellagic acid at 360 nm after the hydrolysis previously described. All measurements were performed in triplicate at 25 °C, expressing the result as mean values ± standard deviation of milligrams or micrograms of phenolic compound/100 g of walnut or mean values ± standard deviation of milligrams of walnut EA/100 g.
Statistical analysis
For statistical analysis the IBM® SPSS® Statistics (version 19.0.) statistical package was used. Analysis of variance (ANOVA) was used once the assumption of normality was tested. In variables where significant differences (p < 0.05) were obtained, the Tukey honest significant difference (HSD) test was applied to determine the existence of differences between means, establishing a confidence level of 95%. To study the relationship between qualitative variables, Pearson and Kendall correlation were accomplished contrast to both linear and non-linear association.
Results and discussion
The total polyphenolic profile of walnuts has been extensively studied.[Citation11,Citation19–Citation21] The majority of the previous studies focused on the content of phenolic compounds from the leaves of walnut[Citation22,Citation23] and in the pericarp.[Citation24] In the present research, 10 varieties analyzed showed 11 different phenolic compounds (six flavonoid compounds and five non-flavonoid compounds). , , and show the different phenolic compounds chromatograms obtained at 280, 320, and 360 nm after the analysis of Tehama genotype.
Figure 1. Tehama variety chromatogram which identifies:(1) gallic acid, (2) gallocatechin, (3) catechin, (4) procyanidin B2, (5) gallate epigallocatechin, (6) epicatechin and (7) gallate epicatechin at 280 nm. (8) syringic acid, (9) chlorogenic acid and (10) p-coumaric acid at 320 nm and (11) ellagic acid identified at 360 nm.
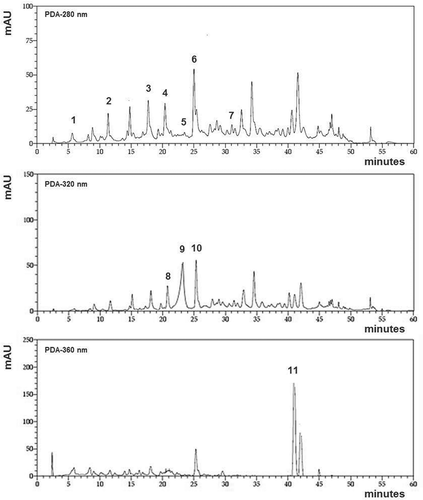
Availability of flavonoid phenolic compounds by HPLC
Other researches have reported the presence of flavonoids in the seeds of walnuts; however, the individual characterization of the different polyphenolic compounds is still limited; it has been described the presence of monomeric flavan-3-ol (catechin and epicatechin) and oligomeric proanthocyanidins (procyanidin B2).[Citation25–Citation27]
Tannins has been proposed to be responsible for the astringency of the walnuts.[Citation28] We identified six flavan-3-ols (gallocatechin, catechin, procyanidin B2, epigallocatechin gallate, epicatechin, and epicatechin gallate) in 10 different genotypes of walnut studied. The content of the different flavan-3-ols identified is shown in . As shown, Algaida (38.47 ± 9.65 mg/100 g walnut), Amigo (37.36 ± 9.59 mg/100 g walnut), and Payne (36.12 ± 9.25 mg/100 g walnut) showed the highest flavonoid content of all the varieties studied, showing statistically significant differences (p < 0.05). On the contrary, Eureka (25.51 ± 6.50 mg/100 g walnut), Franquette (25.03 ± 6.44 mg/100 g walnut), and Serr (25.70 ± 6.60 mg/100 g walnut) varieties showed the minor flavonoid content (p < 0.05).
Table 1. Flavonoid phenolic compounds quantified in the different genotypes of walnuts.
In every genotype, catechin and procyanidin B2 were the major flavonoids identified (assuming 47.50 and 50.07% of the flavonoid content, respectively). Finally, the remaining 2.5% were trace amounts of epicatechin, epicatechin gallate, gallocatechin, and epigallocatechin gallate. Catequin was mainly found in Amigo (20.02 ± 0.06 mg/100 g walnut) and Sunland (19.92 ± 0.37 mg/100 g walnut) genotypes (p < 0.05). Meanwhile, the highest quantity of procyanidin B2 was found after the analysis of Pedro genotype (20.78 ± 0.80 mg/100 g of walnut), followed by Algaida (19.83 ± 2.37 mg/100 g walnut) and Payne (19.19 ± 1.40 mg/100 g walnut) varieties (p < 0.05). Worthy of mention, Eureka was the genotype which showed the mayor (p < 0.05) content of epicatechin (390.55 ± 1.16 µg/100 g walnut); however, it did not showed remarkable amounts of any other flavan-3-ol. In turn, epicatechin gallate was mainly found in Tehama (190.72 ± 0.56 µg/100 g walnut), Eureka (183.75 ± 1.10 µg/100 g walnut), and Sunland (181.88 ± 1.36 µg/100 g walnut) genotypes. Finally, Franquette was the variety which showed the highest amount (p < 0.05) of gallocatechin (183.58 ± 4.26 µg/100 g walnut), while epigallocatechin gallate was mainly found (p < 0.05) in the Payne genotype (87.48 ± 3.66 µg/100 g walnut). Jakopic and Veberic,[Citation29] in a study evaluating different solvents for extraction of phenolic compounds from walnut, obtained similar results to the current study in terms of catechin content (±0.2 mg/g walnut) when using methanol for the extraction.
The content of the different flavonoid compounds identified showed a significant decrease after the in vitro digestion (). As mean values, the content on flavonoids after the simulated digestion dropped more than 50%. The most available compound were gallocatechin, catechin, and epigallocatechin gallate (p < 0.05). The best availability rate was found for gallocatechin, showing 41% of the initial content. Moreover, similar availability rates were found for catechin and epigallocatechin gallate, showing 39 and 38% of initial values, respectively. Contrarious, Procyanidin B2, epicatechin, and epicatechin gallate were the minor available compounds. In fact, the availability of Procyanidin B2 was 29%, while the availability of epicatechin and epicatechin gallate were 24%, respectively.
Figure 2. Availability of flavonoid compounds identified after “in vitro” digestion. *Indicates statistical differences (p < 0.05).
As before in vitro digestion, the two mayor flavonoids identified after the simulated digestion were catechin and procyanidin B2. However, the poor availability rate showed by procyanidin B2 compared to catechin, led to a lower concentration of the first to the second. Furthermore, despite the large availability of gallocatechin, it remains as the minor flavonoid due to its low initial concentration.
The variation on the availability of flavonoids after in vitro digestion was patent for the different genotypes (). The main available genotype was Amigo, showing the highest (p < 0.05) post-digestion content of catechin (8.20 ± 0.02 mg/100 g walnut), epicatechin gallate (61.06 ± 1.01 µg/100 g walnut) and gallocatechin (98.04 ± 8.30 µg/100 g walnut). Similar than Amigo, Algaida genotype showed high content of every flavonoid, being the most abundant variety (p < 0.05) on epigallocatechin gallate (42.50 ± 2.92 µg/100 g walnut) and procyanidin B2 (8.13 ± 0.97 mg/100 g walnut) after in vitro digestion. Finally, epicatechin was mainly found (p < 0.05) in Pedro (148.62 ± 1.91 µg/100 g walnut) and Sunland (144.19 ± 6.14 µg/100 g walnut) genotypes. The poorest availability of flavonoids have been found for Eureka genotype (1.48 ± 0.01 mg catechin/100 g walnut), Tehama genotype (33.69 ± 0.38 µg/100 g epicatechin and 10.70 ± 0.52 µg /100 g epigallocatechin gallate), Pedro genotype (144.19 ± 6.14 µg/100 g gallate epicatechin, and 2.91 ± 0.11 mg/100 g procyanidin B2) and Serr genotype (144.19 ± 6.14 µg/100 g gallocatechin).
Table 2. Phenolic acids quantified in the different genotypes of walnuts.
Table 3. Flavonoid phenolic compounds quantified in the dialyzed fraction of the different genotypes of walnuts.
Non-Flavonoid Compounds by HPLC
Previous reports showed identified a large a number of non-flavonoid phenolic compounds in seeds of walnut. The main components correspond to phenolic acids (gallic, ellagic, vanillic, and syringic) and glycosides derivatives, besides hydroxycinnamic acids (p-coumaric, sinapic, caffeic, and ferulic) and soluble derivatives such as chlorogenic acid.[Citation9,Citation10,Citation20,Citation27,Citation30–Citation32]
In the present study, we identified three phenolic acids (gallic acid, ellagic acid, and syringic acid) and two hydroxycinnamic acids (chlorogenic acid and p-coumaric acid) from the genotypes studied. The differences between the non-flavonoid phenolic compounds identified were more relevant than those observed after the identification of flavonoid compounds. The different non-flavonoid phenolic compounds identified in walnuts seed are shown in . From all genotypes, Eureka (136.57 ± 29.18 mg/100 g walnut) showed the highest content on non-flavonoid compounds (p ≤ 0.05), followed by Sunland (112.19 ± 20.80 mg/100 g walnut), Algaida (115.01 ± 24.03 mg/100 g walnut), and Tehama (109.06 ± 27.45 mg/100 g walnut). Contrary to the above varieties, Amigo and Serr were the varieties that showed the minor amounts on total non-flavonoid compounds (61.13 ± 9.86 mg/100 g walnut and 63.64 ± 8.73 mg/100 g walnut, respectively). It should be noted that Amigo was the second genotype showing the higher flavonoid compounds, despite its poor non-flavonoid content, allowing for the understanding of the poor correlation (r = 0.08; p > 0.05) between flavonoid and non-flavonoid content in the genotypes analyzed. On the contrary, a strong correlation (r = 0.95; p < 0.05) was found between flavonoid phenolic compounds and total phenols. In this sense, many studies in different food matrices have established a direct relationship between both parameters.[Citation33]
Availability of benzoic acid content
Ellagic acid (resulting from the hydroxylation of ellagitannins) was the mayor phenolic compound found in walnuts in the present study, covering 35.50% of total polyphenols and 47.07% of non-flavonoid phenolic compounds. Previous studies pointed walnuts as a main source of ellagitannins.[Citation11,Citation15,Citation34]
Moreover, Fukuda et al.[Citation35] found a 15.8% ratio of ellagic acid in a phenolic extraction from nuts. Among the different genotypes analyzed, Eureka (70.47 ± 1.90 mg/100 g walnut) and Tehama (69.18 ± 10.49 mg/100 g walnut) showed significantly higher content of ellagic acid (p ≤ 0.05) than the other genotypes, excepting Sunland variety. These values are similar to those obtained by Daniel et al.,[Citation36] who found about 59 mg of ellagic acid per 100 g walnut. Likewise, Christopoulos and Tsantili[Citation34] showed 68.1 mg of ellagic acid per 100 g of walnut, close values to those found in the present survey. However, they are slightly lower than those obtained by Li et al.[Citation37] On the contrary, Serr and Amigo showed the most minor content of ellagic acid out of all the varieties.
Syringic acid was the second most abundant non-flavonoid compound, contributing almost to 32% of the total content on non-flavonoid compounds in walnuts. The average content of syringic acid was 28.88 ± 10.09 mg/100 g walnut, similar value that described by Colaric et al.[Citation10] (about 33.82 mg syringic acid per 100 g walnut) and Christopoulos and Tsantili[Citation34] (about 31.02 mg syringic acid per 100 g walnut). In our study, syringic acid was found in high amounts in Eureka (44.59 ± 5.72 mg/100 g walnut) and Sunland (41.16 ± 1.05 mg/100 g walnut) genotypes, showing significant differences (p ≤ 0.05) with respect to the rest of varieties.
Finally the 15.77% of the quantified non-flavonoid compounds correspond to gallic acid, showing a mean value of 15.40 ± 3.07 mg/100 g of walnut. From all varieties, Tehama (21.92 ± 1.67 mg/100 g walnut) is the genotype with higher values of gallic acid. On the contrary, Payne (11.58 ± 0.80 mg/100 g walnut) and Amigo (11.73 ± 0.80 mg/100 g walnut) were the two varieties showing minor values of gallic acid.
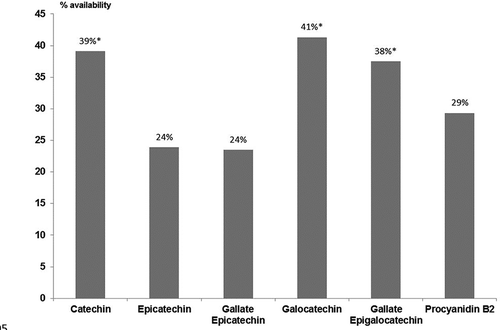
The different benzoic acids identified showed a marked significant decrease after the in vitro digestion (). In fact, the most available (p < 0.05) benzoic acid was gallic acid, reaching only 27% of initial mean values before simulated digestion. In addition, availability of ellagic acid and syringic acid was even lower, showing 19 and 22% of initial mean values before simulated digestion, respectively.
Figure 3. Availability of non-flavonoid compounds identified after “in vitro” digestion. *Indicates statistical differences (p < 0.05) with ellagic acid and syringic acid.
The variation on the availability of benzoic acids after in vitro digestion was patent for the different genotypes (). As observed for flavonoids, the Algaida genotype showed high availability compared with the other varieties. That fact was especially manifest (p < 0.05) for gallic acid (6.89 ± 0.58 mg/100 g walnut) and ellagic acid (16.25 ± 2.90 mg/100 g walnut). Algaida genotype showed high content on syringic acid; however, the Sunland genotype was the variety showing the highest content (p < 0.05) on this benzoic acid (12.76 ± 0.33 mg/100 g walnut). On the contrary, minor content on gallic acid, ellagic acid, and syringic acid were found in Sunland genotype (1.93 ± 0.19 mg/100 g walnut), Serr genotype (2.82 ± 0.28 mg/100 g walnut), and Amigo genotype (3.41 ± 0.20 mg/100 g walnut), respectively.
Table 4. Phenolic acids quantified in the dialyzed fraction of the different genotypes of walnuts.
Availability of hydroxycinnamic acid content
Although at lower amounts, hydroxycinnamic acids were also identified in all the genotypes analyzed. Chlorogenic acid comprised about 5% of total non-flavonoid compounds present in walnuts, while the content of p-coumaric acid was only a 0.20% of total non-flavonoid compounds identified. Some authors have reported the presence of hydroxycinnamic acids in walnuts. Gómez-Caravaca et al.[Citation27] and Colaric et al.[Citation10] found minor content of chlorogenic acid and p-coumaric acid in walnuts than the obtained in our study (4.87 ± 1.70 mg/100 g walnut and 194.35 ± 27.97 µg/100 g of walnut, respectively). Among the different genotypes analyzed, Payne (7.82 ± 1.62 mg/100 g walnut) and Algaida (7.01 ± 0.75 mg/100 g of walnut) showed the highest content on chlorogenic acid. In contrast, Tehama, one of the varieties with higher content of hydroxybenzoic acids, was the variety with the lowest content of chlorogenic acid. Finally, the higher content (p < 0.05) of p-coumaric acid was found in the genotypes: Tehama (273.21 ± 27.92 µg/100 g walnut), and Sunland (260.66 ± 18.04 µg/100 g walnut).
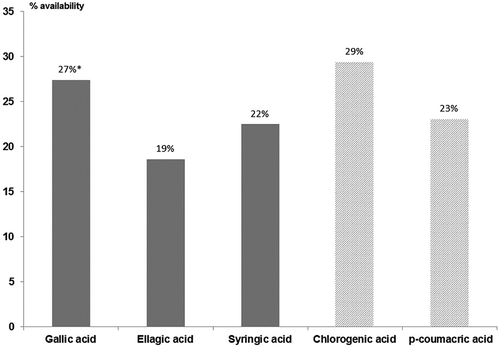
Values of hydroxycinnamic acids obtained after the in vitro digestion are showed in . The highest availability was found for chlorogenic acid, reaching 29% of the initial content previous to simulated digestion. Meanwhile, the analysis of p-coumaric acid reported only 23% of initial values obtained before in vitro digestion.
Regarding the different genotypes, Payne (2.43 ± 0.50 mg chlorogenic acid/100 g walnut) and Eureka (103.27 ± 7.96 µg p-coumaric acid/100 g walnut) were the varieties that showed the highest availability for both hydroxycinnamic acids. Meanwhile, Chico and Pedro were the genotypes with minor content on chlorogenic acid (0.46 ± 0.14 mg/100 g walnut) and p-coumaric acid (23.44 ± 2.12 µg/100 g walnut) after in vitro digestion, respectively.
Conclusion
All analyzed genotypes show high content on flavonoids and non-flavonoids phenolic compounds, highlighting Eureka and Tehama genotypes. From all the phenolic compounds identified, non-flavonoid compounds are the largest group in walnuts. Especially, ellagic acid and syringic acid were the major non-flavonoids, mainly found in Eureka and Tehama genotypes. Regarding flavonoids, catechin, and procyanidin B2 are the most abundant in the analyzed genotypes, covering about 98% of total flavonoids. From all varieties, Algaida, Amigo, and Payne genotypes are those with the highest content on flavonoid phenolics. The in vitro digestion of the different phenolic compounds showed flavonoids as the most available compounds, especially catechin (39% availability), gallocatechin (41% availability), and epigallocatechin gallate (38% availability). From non-flavonoids, chlorogenic acid (29% availability) and gallic acid (27% availability) showed the highest availability rate; however, it was minor than flavonoids. From all the different genotypes, Amigo showed the highest availability of flavonoids due to its post-digestion content of catechin (8.20 ± 0.02 mg/100 g walnut), epicatechin gallate (61.06 ± 1.01 µg/100 g walnut), and gallocatechin (98.04 ± 8.30 µg/100 g walnut). In turn, Payne (2.43 ± 0.50 mg chlorogenic acid/100 g walnut) and Eureka (103.27 ± 7.96 µg p-coumaric acid/100 g walnut) showed to exert the highest availability of non-flavonoids, respectively. Meanwhile, Chico and Pedro were the genotypes with minor content on chlorogenic acid (0.46 ± 0.14 mg/100 g walnut) and p-coumaric acid (23.44 ± 2.12 µg/100 g walnut) after in vitro digestion, respectively.
Funding
The authors acknowledge the financial support of Consejería de Ciencia, Tecnología, Industria y Comercio de la Región de Murcia and Catholic University of San Antonio de Murcia.
Additional information
Funding
References
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans Regia L.): Genetic Resources, Chemistry, by‐Products. Journal of the Science of Food and Agriculture 2010, 90, 1959–1967.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly) Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxidants & Redox Signaling 2013, 18, 1818–1892.
- Feldman, E.B. The Scientific Evidence for a Beneficial Health Relationship Between Walnuts and Coronary Heart Disease. The Journal of Nutrition 2002, 132, 1062S–1101S.
- Sabaté, J.; Oda, K.; Ros, E. Nut Consumption and Blood Lipid Levels: A Pooled Analysis of 25 Intervention Trials. Archives of Internal Medicine 2010, 170, 821–827.
- Chen, C.; Blumberg, J.B. Phytochemical Composition of Nuts.Asia Pacific Journal of Clinical Nutrition 2008, 17, 32–332.
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682.
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive Identification of Walnut Polyphenols by Liquid Chromatography Coupled to Linear Ion Trap–Orbitrap Mass Spectrometry. Food Chemistry 2014, 152, 340–348.
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut Polyphenolics Inhibit in Vitro Human Plasma and LDL Oxidation. The Journal of Nutrition 2001, 131, 2837–2842.
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative Polyphenols from Walnuts (Juglans Regia L.). Phytochemistry 2003, 63, 795–801.
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic Acids, Syringaldehyde, and Juglone in Fruits of Different Cultivars of Juglans Regia L. Journal of Agricultural and Food Chemistry 2005, 53, 6390–6396.
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of Phenol Content and Antioxidant Capacity of Nuts. Food Science and Technology (Campinas), 2010, 30, 254–259.
- Vinson, J.A.; Cai, Y. Nuts, Especially Walnuts, Have Both Antioxidant Quantity and Efficacy and Exhibit Significant Potential Health Benefits. Food & Function 2012, 3, 134–140.
- Ito, H.; Okuda, T.; Fukuda, T.; Hatano, T.; Yoshida, T. Two Novel Dicarboxylic Acid Derivatives and a New Dimeric Hydrolyzable Tannin from Walnuts. Journal of Agricultural and Food Chemistry 2007, 55, 672–679.
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in Vitro Method to Simulate Phenolic Compound Release from the Food Matrix in the Gastrointestinal Tract. European Food Research and Technology 2002, 214, 155–159.
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of Antioxidant and Chemopreventive Ellagitannins from Strawberries, Raspberries, Walnuts, and Oak-Aged Wine in Humans: Identification of Biomarkers and Individual Variability. Journal of Agricultural and Food Chemistry2005, 53, 227–235.
- Salcedo, C.L.; De Mishima, B.a.L.; Nazareno, M.A. Walnuts and Almonds as Model Systems of Foods Constituted by Oxidisable, Pro-Oxidant and Antioxidant Factors. Food Research International 2010, 43, 1187–1197.
- Häkkinen, S.H.; Kärenlampi, S.O.; Mykkänen, H.M.; Heinonen, I.M.; Törrönen, A.R. Ellagic Acid Content in Berries: Influence of Domestic Processing and Storage. European Food Research and Technology 2000, 212, 75–80.
- García‐Alonso, F.; Periago, M.; Vidal‐Guevara, M.; Cantos, E.; Ros, G.; Ferreres, R.; Abellán, P. Assessment of the Antioxidant Properties During Storage of a Dessert Made from Grape, Cherry, and Berries. Journal of Food Science 2003, 68, 1525–1530.
- Almeida, I.F.; Fernandes, E.; Lima, J.L.; Costa, P.C.; Bahia, M.F. Walnut (Juglans Regia) Leaf Extracts Are Strong Scavengers of Pro-Oxidant Reactive Species. Food Chemistry 2008, 106, 1014–1020.
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L. Bioactive Properties and Chemical Composition of Six Walnut (Juglans Regia L.) Cultivars. Food and Chemical Toxicology 2008, 46, 2103–2111.
- Samaranayaka, A.G.; John, J.A.; Shahidi, F. Antioxidant Activity of English Walnut (Juglans Regia L.). Journal of Food Lipids 2008, 15, 384–397.
- Amaral, J.S.; Seabra, R.M.; Andrade, P.B.; Valentao, P.; Pereira, J.A.; Ferreres, F. Phenolic Profile in the Quality Control of Walnut (Juglans Regia L.) Leaves. Food Chemistry 2004, 88, 373–379.
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human Cancer Cell Antiproliferative and Antioxidant Activities of Juglans Regia L. Food and Chemical Toxicology 2010, 48, 441–447.
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L.; Pereira, J.A. Total Phenols, Antioxidant Potential and Antimicrobial Activity of Walnut (Juglans Regia L.) Green Husks. Food and Chemical Toxicology 2008, 46, 2326–2331.
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. Journal of Nutrition 2004, 134, 613–617.
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.; Moutsatsou, P. Walnut Extract (Juglans Regia L.) and Its Component Ellagic Acid Exhibit Anti-Inflammatory Activity in Human Aorta Endothelial Cells and Osteoblastic Activity in the Cell Line KS483. British Journal of Nutrition 2008, 99, 715–722.
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Caboni, M.F.; Fernández-Gutiérrez, A. Development of a Rapid Method to Determine Phenolic and Other Polar Compounds in Walnut by Capillary Electrophoresis–Electrospray Ionization Time-of-Flight Mass Spectrometry. Journal of Chromatography A 2008, 1209, 238–245.
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors Influencing Their Sensory Properties and Their Effects on Food and Beverage Preferences. The American Journal of Clinical Nutrition 2005, 81, 330S–335S.
- Jakopic, J.; Veberic, R. Extraction of Phenolic Compounds from Green Walnut Fruits in Different Solvents. Acta Agriculturae Slovenica 2009, 93, 11.
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant Phenolic Compounds from Walnut Kernels (Juglans Regia L.). Food Chemistry 2009, 113, 160–165.
- Bolling, B.W.; Chen, C.-Y.O.; Mckay, D.L.; Blumberg, J.B. Tree Nut Phytochemicals: Composition, Antioxidant Capacity, Bioactivity, Impact Factors. A Systematic Review of Almonds, Brazils, Cashews, Hazelnuts, Macadamias, Pecans, Pine Nuts, Pistachios and Walnuts. Nutrition Research Reviews 2011, 24, 244–275.
- Bakar, N.B.A.; Makahleh, A.; Saad, B. In-vial Liquid–Liquid Microextraction-Capillary Electrophoresis Method for the Determination of Phenolic Acids in Vegetable Oils. Analytica Chimica Acta 2012, 742, 59–66.
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic Acid Content and Radical Scavenging Activity of Extracts from Medlar (Mespilus Germanica L.) Fruit at Different Stages of Ripening. Food Chemisrty 2011, 124, 271–277.
- Christopoulos, M.V.; Tsantili, E. Storage of Fresh Walnuts (Juglans Regia L.)–Low Temperature and Phenolic Compounds. Postharvest Biology and Technology 2012, 73, 80–88.
- Fukuda, T.; Ito, H.; Yoshida, T. Effect of the Walnut Polyphenol Fraction on Oxidative Stress in Type 2 Diabetes Mice. Biofactors 2004, 21, 251–254.
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.-H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, Stability, and Quantitation of Ellagic Acid in Various Fruits and Nuts. Journal of Food Composition and Analysis 1989, 2, 338–349.
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Zhu, H.; Young, J.C. Polyphenolic Profiles and Antioxidant Activities of Heartnut (Juglans Ailanthifolia var. Cordiformis) and Persian Walnut (Juglans Regia L.). Journal of Agricultural and Food Chemistry 2006, 54, 8033–8040.
