ABSTRACT
Assay of the antioxidant content and reducing power of the plant extracts is a crucial point in phytochemistry, the food industry, and nutrition. On the other hand, green synthesis of noble metal nanoparticles, such as gold by the aid of plant extract is dependent on the reducing power and antioxidant content of the extract. In this work, cyclic voltammetry was utilized as a facile technique for screening antioxidant properties and estimating the reducing power of the medicinal plant extracts. The effect of different extraction conditions on the reducing power of the aqueous extract was also determined through the cyclic voltammetry technique. The extract of Eucalyptus oleosa leaves obtained at optimum conditions of this study (with maximum reducing power based on cyclic voltammetry assay) was utilized for the green synthesis of gold nanoparticles. Furthermore, the kinetic of the synthesis reaction was investigated by the aid of ultraviolet-visible spectrophotometry based on surface Plasmon resonance for gold nanoparticles at 552 nm. Meanwhile, the synthesized gold nanoparticles were characterized by the transmission electron microscopy technique.
Introduction
The electron donating capacity of the bioactive compounds is defined as the reducing power and/or the antioxidant activity. In other words the antioxidant property of a compound or extract could be described as the ability of reducing and inactivation of the oxidants or as a redox reaction in which a reaction species (antioxidant) is reduced at the exposure of the oxidant. Commonly, the in vitro antioxidant property of the plant extracts is determined by different traditional techniques, i.e., β-carotene/linoleic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and reducing power assays.[Citation1] On the other hand, cyclic voltammetry (CV) by providing information about the electrochemical behavior of the extract solution could be utilized as a facile and promising assay technique rather than traditional routes.
The biosynthesis of nanoparticles as a cost-effective and environment friendly method is beneficial over conventional chemical and physical routes, especially it is not require to utilize high temperature, pressure, energy, and toxic chemicals.[Citation2] However, synthesis of nanoparticles by the aid of plant extracts is preferred over other biological routes due to the elimination of the maintaining cell cultures as an elaborate process and ability for facile scaling up in order to large-scale synthesis of nanoparticle.[Citation3,Citation4] This route operates based on the electrochemical reduction of metallic ions via antioxidant components presented in the plant extract. Thus, the reducing power of the extract has a considerable role in the green synthesis reaction kinetic and yield of process. In other words, measuring the reducing power of the extract could be helpful for controlling the synthesis kinetic reaction. During recent years, metallic nanoparticles, for example, silver and gold, have attracted much attention due to their various and widespread applications. They attracted considerable interests in different fields such as medicine, electronics, catalysis, optics, information storage, magnetics, and also surface-enhanced Raman scattering. Meanwhile, they exhibited considerable potential for utilizing in modern materials, especially in microelectronics and biocatalysts.[Citation4,Citation5] The intrinsic properties of the nanoparticles are strongly depending on their size, structure, shape, stability, composition, and crystalline form.[Citation6]
During recent years, numerous reports have published about the preparation and characterization of gold nanoparticles (GNPs), due to its extraordinary physicochemical properties and wide range applications.[Citation7–Citation9] Preparation of metallic nano-gold via physical routes (i.e., laser ablation) provides GNPs with narrow particle size distribution; however, they require expensive equipment and also have low yield.[Citation7] Hazardous side-effects of organic solvents and toxically effects of reducing reagents utilized for the chemical synthesis of GNPs caused attention to be drawn toward the development of the alternative green routes.[Citation8,Citation9]
E. oleasa is belonging to the Myrtaceae families, while it has Australian originality. Phytochemical studies on this plant revealed that it possesses wide variety of the chemical constituents, i.e., α-pinene, 1,8-cineole and α-terpineol which are well-known as the antifungal and antioxidant agents.[Citation10] The commercial distillation of Eucalyptus oil has a vast array applications mainly for fragrance, flavor, and also pharmaceutical to treat diarrhea, bronchitis, asthma, and respiratory tract infections.[Citation11,Citation12]
This article reports two important criteria about E. oleosa leaf including measuring the reducing power of the plant extracts obtained under different extraction temperatures and various amounts of plant materials in a constant volume of water. Furthermore, investigation on the possible synthesis of GNPs by reduction of Au(III) ions using the aqueous extract of this plant. To achieve this aims, the influence of the temperature of extraction process on the reducing power of the resulted plant extract was measured by electrochemical method. Then, the formation and growth of the GNPs in the extract solution was monitored by ultraviolet-visible (UV-Vis) spectrophotometry, while the shape and size of the resulted GNPs were determined by transmission electron microscopy (TEM). In our previous study, the E. oleosa leaf was utilized for the preparation of silver nanoparticles.[Citation13] The reducing power for the methanol extract of this plant has been investigated by traditional technique.[Citation10] However, to the best of our knowledge, there is no information on the electrochemical determination of reducing power of E. oleosa aqueous extract and its usage for the synthesis of GNPs in the literature.
Materials and methods
Plant material, chemicals, and apparatus
The leaves of E. oleosa cultivated in Kashan (Isfahan Province, Iran) were obtained in September 2013. The leaves were dried in the shade (at room temperature). The voucher specimen of the plant was deposited at the herbarium of the Kashan botanical garden (KBG 1493). Tetrachloroauric (III) acid trihydrate (purity 99.5%) was obtained from Merck chemicals. 50 mL of aqueous solution of the chloroauric acid with the concentration of 1 × 10−3 M was prepared in a flask. UV-Vis spectrophotometric analysis was carried out on a spectrophotometer model cintra 6 made by GBC (Australia) operated at a resolution of 1 nm. TEM was carried out on a Ziess-EM900 system. The sample preparation was performed by drop-coating the GNPs solution onto carbon-coated copper grids.
Extraction
The aqueous solution of the plant leaves extract was prepared by weighting the required amount of cutted plant leaves in 50 mL of distilled water and then keeping the resulted mixture at the desired temperature during a definite time. Then, the mixture was filtered through a filter paper of Whatman No.1, and then the filtrate was stored in the airtight amber colored bottle at 10°C and used within 24 h.
Determination of antioxidant activity by CV
CV of the plant extracts solution were carried out on a potentiostat-galvanostat system (SAMS500, electroanalyzer system, Iran). In order to measurement, the plant extract sample is introduced into a well-equipped with three electrodes including: the working electrode (glassy carbon), the reference electrode (Ag/AgCl), and the auxiliary electrode (platinum wire). The working electrode was polished with the alumina on a polishing cloth before performing the assay. The utilized supporting electrolyte was 0.2 M phosphate buffer with pH 7.0. The scan was carried out at a potential range of –0.2 to 0.9 V. The cyclic voltammograms for the plant extract solutions were recorded at the scan rates of 20, 50, 100, 200, 300, and 400 mV.s−1. During the cyclic voltammmetry experiment, a potential current curve was recorded (cyclic voltammogram).
GPNs synthesis procedure
In order to prepare GNPs, 2 mL of the leaf extract was added to the chloroauric acid solution at room temperature, while observing a pinkish-red solution indicates the formation of GNPs.[Citation14,Citation15] The reduction of gold ions in the extract solution was monitored by sampling the reaction solution at regular intervals and the maximum absorption was determined by UV-Vis spectrophotometric analysis, at the wavelength range of 250–700 nm.
Results and discussion
Assay of reducing power of extract
Extraction conditions, i.e., temperature and mass of plant material used for extraction in a constant volume of solvent, could effect on the composition and characteristics of the plant extracts and hence playing an important role in determining the reducing power of the extract solution.[Citation16,Citation17] On the other hand, different methods might be utilized to evaluate the antioxidant properties (reducing power) of plant extract ingredients, i.e., enzymatic or non-enzymatic determining the inhibiting effects of lipid peroxidation, measurement of the active oxygen species scavenging capability and radical scavenging activity prediction, i.e., 1,1-diphenyl-2-picrylhydrasyl (DPPH) radical.[Citation18,Citation19] It is obvious that the resulted data by these assays only reflect the specific reducing power in the corresponding system. But the reduction capacity is not depending only on a specific reaction and various mechanisms might be involved in the reduction power. Thus, evaluation the overall reduction capability of the plant extracts could be carried out simply by electrochemical techniques without application of a certain reactive species.
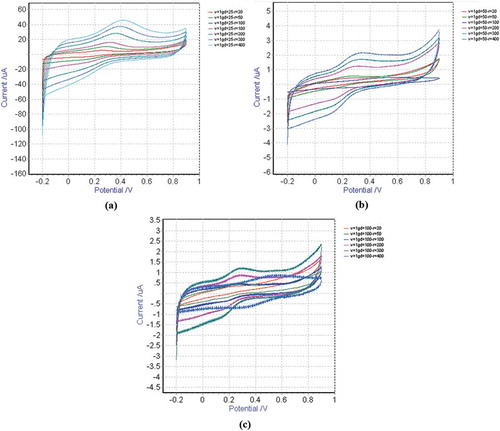
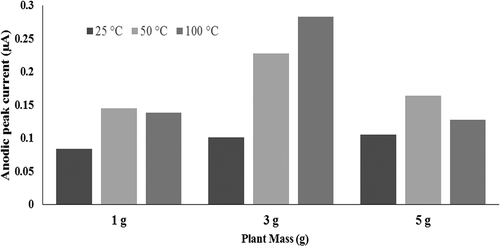
In this study, the effect of extraction conditions (i.e., temperature and mass of plant) on the reducing power of extracts was investigated. On the other hand, CV as a routine technique was utilized to characterize the redox behavior of the plant extracts.[Citation20] Thus, CV tests were carried out on the plant extracts obtained at different temperatures (i.e., 25, 50, and 100°C. The cyclic voltammograms for the plant extracts obtained at different temperature are shown in . As seen in these figures, a single anodic peak was appeared in the cyclic voltammograms of the different extracts. For all the extracts this anodic current peak was observed around +0.3 V at the scan rate of 200 mV.s−1. On the other hand, presents the plot of anodic peak current (Ipa) values for the extract solutions obtained at different extraction temperatures. As seen, the value of anodic peak currents (Ipa) for the plant extracts obtained at the temperature of 50°C are highest value. This figure also shows that the reducing power for the extracts obtained at lower temperature (25°C) is considerably lower. Meanwhile, this item for the extracts obtained at higher temperature of 100°C is lower than those resulted at 50°C which may be attributed to the degradation of thermal reliable components at higher temperature. This curve shows that increasing the extraction temperature from 25 to 50°C increases and from 50 to 100°C reduces the antioxidant property of the plant extract solution. In other words, the plant extract obtained at the temperature of 50°C possesses the highest antioxidant content and, hence, the maximum reduction power. The other investigated extraction condition is the mass of plant material utilized for the extraction by a constant volume of water as solvent. plots the anodic peak current (Ipa) values for the extract solutions obtained by different mass of plant materials at the various investigated temperatures. As seen in this curve, the antioxidant property of the plant extract solution increases by enhancing the mass of plant material. In other words, anodic peak current (Ipa) for the extract obtained by 5 g of plant material has maximum value, while it was minimum for the plant extract obtained from 1 g of plant material. Considering the resulted values of the peak anodic current (Ipa) from cyclic voltammograms of the plant extract solutions prepared at different conditions of extraction process (i.e., temperature and mass of plant material) exhibited that the maximum reducing power could be achieved by extraction of 5 g plant material at the temperature of 50°C.
GNPs synthesis
In the next step of this study, the plant extract obtained at optimum conditions of the extraction (with maximum reducing power determined by CV studies) was utilized for the preparation of GNPs in aqueous medium. UV-Vis spectrophotometry is an indirect technique to detect the formation of nanoparticle and up to now it is valid as an excellent indicator toward the formation of nano-scaled metal particles. Thus, the bio-reduction process of GNPs was monitored via sampling the reaction mixture at the regular intervals and scanning by UV-Vis spectrophotometry, at the wavelength range of 250–700 nm.
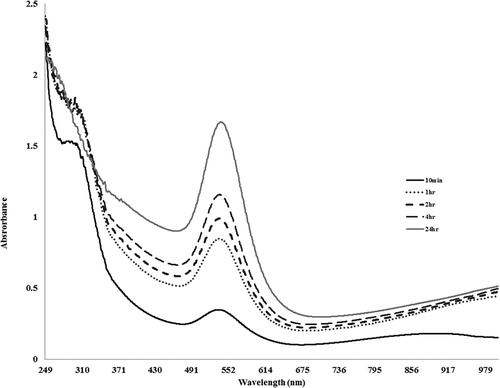
The results of absorption spectrophotometry for the Au(III) ions solution are displayed in . As seen, at t = 10, i.e., 10 min after introducing of Au(III) ions, the solution turns pink and the absorption spectrum of the reaction solution shows a small peak at the 552 nm. This peak is assigned to the surface Plasmon resonance (SPR) band of the formed GNPs due to the reduction of Au(III) ions. However, by increasing the reaction time to 1, 2, 4, and 24 h, the intensity of the peak increased due to the enhancing the concentration of the formed GNPs in the reaction solution.
Characterization of the formed nanoparticles by TEM
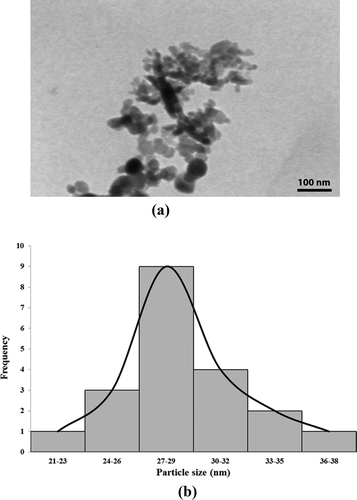
The shape and size of the biosynthesized gold particles were elucidated by the aid of TEM. The resulted image of transmission electron microscopic for the prepared GNPs sample is shown in . As seen, the majority of resulted gold particles were more or less spherical, while the average size of produced GNPs was about 28 nm. Meanwhile, the frequency curve corresponds to the size distribution of GNPs is shown in .
Conclusion
The composition of the medicinal plant extracts is complicated and hence synergistic interactions between the antioxidants are possible. This study revealed that CV as a rapid and simple electrochemical technique is efficient for characterization the antioxidant properties of the plant extracts. The effect of extraction temperature and mass of plant on the reducing power of the extract was investigated by CV technique. Furthermore, a facile, eco-friendly and novel synthesis route to prepare GNPs was utilized by E. oleosa aqueous extract as the green reducing and capping agent. The resulted GNPs were almost in spherical shape. UV-Vis spectra confirmed the formation of GNPs by exhibiting the peak at the wavelength of 552 nm, while the saturation of the process was observed after approximately 24 h. GNPs synthesized by the proposed procedure have a good compatibility for medical applications and also pharmaceutical drugs delivering. Furthermore, the procedure possesses appropriate potential for production in large commercial scales.
References
- Rahimi-Nasrabadi, M.S.; Pourmortazavi, M.; Sadat Shandiz, S.A.; Ahmadi, F.; Batooli, H. Green Synthesis of Silver Nanoparticles Using Eucalyptus Leucoxylon Leaves Extract and Evaluating the Antioxidant Activities of Extract. Natural Product Research 2014(28), 1964–1969.
- Feldheim, D.L.; Foss, C.A. Metal Nanoparticles. Marcel Dekker: New York, NY, 2002.
- Templeton, A.C.; Wuelfing, W.P.; Murray, R.W. Monolayer-Protected Cluster Molecules, Accounts of Chemical Research 2000, 33, 27.
- Weare, W.W.; Reed, S.M.; Warner, M.G.; Hutchison, J.E. Improved Synthesis of Small (dCORE ≈1.5 nm) Phosphine-Stabilized Gold Nanoparticles. Journal of the American Chemical Society 2000, 122, 12890.
- Hata, K.; Fujihara, H. Preparation and Electrochemical Polymerization of New Multifunctional Pyrrolethiolate-Stabilized Gold and Palladium Nanoparticles. Chemical Communications 2002(22), 2714–2715.
- Sun, Y.G.; Xia, Y.N. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176.
- Safavi, A.; Absalan, G.; Bamdad, F. Effect of Gold Nanoparticle as a Novel Nanocatalyst on Luminol–Hydrazine Chemiluminescence System and Its Analytical Application. Analytica Chimica Acta 2008, 610, 243.
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold Nanoparticles in Delivery Applications. Advanced Drug Delivery Reviews 2008, 60, 1307.
- Daniel, M. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology D. Chemical Reviews 2004, 104, 293.
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Nazarian, S.; Ahmadi, F.; Batooli, H.; Chemical Composition, Antioxidant, and Antibacterial Activities of the Essential Oil and Methanol Extracts of Eucalyptus Oleosa Leaves. International Journal of Food Properties 2013, 16, 1080–1091.
- Rahimi-Nasrabadi, M.; Ahmadi, F.; Batooli, H. Essential Oil Composition of Eucalyptus Procera Dehnh., Leaves from the Central of Iran. Natural Product Research 2012, 26, 637–642.
- Ali, S.; Chatha, S.A.S.; Ali, Q.; Hussain, A.I.; Hussain, S.M.; Perveen, R. Oxidative Stability of Cooking Oil Blend Stabilized with Leaf Extract of Eucalyptus Citriodora, International Journal of Food Properties 2016, 19, 1556–1565.
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Rahimi-Nasrabadi, M. Procedure Optimization for Green Synthesis of Silver Nanoparticles by Aqueous Extract of Eucalyptus Oleosa. Spectrochimica Acta Part A 2015, 136, 1249–1254.
- Parashar, U.K.; Saxenaa, P.S.; Srivastava, A. Bioinspired Synthesis of Silver Nanoparticles. Digest Journal of Nanomaterials and Biostructures 2009, 4, 159–166.
- Basu, S.; Ghosh, S.K.; Kundu, S.; Panigrahi, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, T. Biomolecule induced nanoparticle aggregation: Effect of particle size on interparticle coupling. Journal of Colloid and Interface Science 2007, 313, 724–734.
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical Fluid Extraction in Plant Essential and Volatile Oil Analysis. Journal of Chromatography A 2007, 1163, 2–24.
- Aghel, N.; Yamini, Y.; Hadjiakhoondi, A.; Pourmortazavi, S.M.; Talanta. Supercritical carbon dioxide extraction of Mentha pulegium L. essential oil 2004, 62, 407–411.
- Lee, Y.H.; Choo, C.; Waisundara, V.Y. Determination of the Total Antioxidant Capacity and Quantification of Phenolic Compounds of Different Solvent Extracts of Black Mustard Seeds (Brassica Nigra). International Journal of Food Properties 2015, 18, 2500–2507.
- Gholivand, M.B.; Rahimi-Nasrabadi, M.; Batooli, H.; Ebrahimabadi, A.H. Chemical Composition and Antioxidant Activities of the Essential Oil and Methanol Extracts of Psammogeton Canescens. Food and Chemical Toxicology 2010, 48, 24–28.
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological Aspects About in-Vitro Evaluation of Antioxidant Properties. Analytica Chimica Acta 2008, 613, 1–19.