ABSTRACT
The aim of this study was to evaluate the antioxidant properties of apple slices stored in edible starch films enriched with white and green tea extracts. All results were compared to that obtained for apple slices stored in polyethylene films. The use of starch films, as carrier of antioxidants, prolonged the storage life and improved quality of fresh-cut apples at the end of storage; however, deteriorated their lightness and increased their browning. Apple slices coated in starch films enriched with tea extracts exhibited higher antioxidant properties than those without these additives, as well as those in polyethylene films.
Introduction
Biodegradable polymers, such as carbohydrates, proteins, lipids, and their mixtures are used in packaging sector for edible film and coating production.[Citation1–Citation3] An edible film is defined as a thin layer which can be consumed, coated on a food, or placed as barrier between the food and the surrounding environment.[Citation2,Citation4] Edible films and coatings do not pretend to replace traditional packaging materials but to provide an additional factor for food preservation. They can also help to reduce the cost and amount of traditional packaging.[Citation3–Citation6] Biodegradable films are also intended to act as a shelf life extenders and quality improvers in the food industry by preventing changes in aroma, taste, texture as well as in appearance of food products.[Citation5,Citation7] Starch polymers due to their functional properties, such as gelling, thickening, bonding, and adhesion are commonly used polysaccharides for film preparations.[Citation4,Citation6] Furthermore, films based on starch polymers can be used as a vehicle for incorporating functional ingredients, such as antioxidants, flavors, colorants, antimicrobial agents, and nutraceuticals.[Citation3,Citation5] Currently, the most frequently used antioxidants in active packaging are butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). However, these synthetic antioxidants are not acceptable by consumers, due to their toxicological aspects. Thus, natural biopolymers enriched with bioactive substances, such as phenolics, can be a great alternative to them.[Citation6,Citation8]
One of the newest research trends in food packaging has focused on the incorporation of bioactive plant extracts rich in antioxidants into dietary polymer-based materials to create novel edible package materials.[Citation3,Citation7,Citation9] For example, tea extracts can be used as an ingredient to improve certain physicochemical properties and bioactivity of films for different applications.[Citation3] White and green tea leaves are one of the richest sources of antioxidants; however, they differ in chemical composition depending on variety, agricultural practises, climate, or methods of production. The major antioxidants in tea extracts are catechins, theaflavins, thearubigins, oxyaromatic acids, flavonols, such as kaempferol, myricetin, quercetin; flavones, such as apigenin; derivatives of gallic acid, such as tannins, etc.[Citation8,Citation10]
Consumers prefer healthy, natural food without synthetic ingredients, but have little time for food preparation. Therefore, the market of fresh-cut, packaged, and ready-to eat products is growing steadily. This trend also indicates a preference for fruits, that are easy to prepare, convenient to eat at work and while traveling, as well as easy to share with family and friends.[Citation9,Citation11] Minimally processed fruits and vegetables have a very short shelf life, due to increased susceptibility to microbial spoilage, increased respiration rate, and ethylene production, as well as cell rupture and releasing intracellular products, such as enzymes. These factors can have a negative effect on the quality of cut apples, for example, on their appearance, color, flavor, texture, or antioxidant properties. To prevent these changes, cut apples can be coated in edible films incorporated in plant extracts rich in antioxidants. They can produce a modified atmosphere, which reduces decay, delays color changes, and improves the appearance of fresh fruit. Furthermore, films enriched with plant extracts can act as a carrier of anti-browning agents, antimicrobials, texture enhancers, nutraceuticals, flavors, and volatile precursors.[Citation11]
Several types of edible films and coatings have been used for preserving apples. They were mostly produced from carrageenan, hydroxypropyl methylcellulose, alginate, zein, or whey protein with different antioxidant additives.[Citation11–Citation16] However, there is little information of potential use of starch edible films (especially obtained from potato starch) on preserving the quality of fruits during storage. Furthermore, due to the fact, that tea extracts can be used as an antioxidant agents in novel biodegradable packaging materials, it appears that such active films will be able to extend the shelf life and to improve the quality of cut fruits. Thus, the aim of this study was to develop an active starch-based edible film enriched with tea extracts and to evaluate their potential use on preserving the quality of model fruits, in this case, apple slices.
Materials and methods
Chemicals
Methanol (99,8%), sodium carbonate, and potassium persulphate, were purchased from POCh (Gliwice, Poland). The other reagents (sodium chloride, potassium dihydrogen phosphate, di-sodium hydrogen phosphate, potassium chloride, acetic acids, iron(III) chloride, hydrochloric acid, iron(II) sulfate, and sodium acetate) were obtained from Chempur (Piekary Śląskie, Poland). The Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2´-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt; ABTS), (2,4,6-tri(2-pirydyl-s-triazine; TPTZ), gallic acid, and Trolox were purchased from Sigma-Aldrich Chemie (Steinheim, Germany).
Materials
Native potato starch, LU-1431-1 superior standard, was obtained from Wielkopolskie Przedsiębiorstwo Przemysłu Ziemniaczanego S.A. (Luboń, Poland), glycerol was purchased from Chempur (Piekary Śląskie, Poland). The white and green tea leaves, as well as polyethylene (PE) film for food wrapping were purchased from a local hypermarket in Krakow, Poland. Apples (Malus domesticus Borkh cv. Champion) were bought from the local fruit and vegetable shop in Krakow.
Film preparation
White and green tea water extracts were prepared according to following recipe: 1 g of tea leaves were weighed and poured with 100 mL of hot water (80 ± 1ºC); brewing lasted 5 min. After that time, extracts were filtered through Whatman no. 1 filter paper and were used for preparing starch films (SFs). The film forming aqueus dispersion contained 2% (m/m) of potato starch and glycerol used as a plasticizer (glycerol to starch ratio 0.5:1 [m/m]). Films were prepared by dissolving starch in distilled water (SFs) or in tea aqueus extract (SFs with green tea extract [SF-GT], SFs with white tea extract [SF-WT]) and heating the slurry in a water bath at 95ºC for 30 min under a stirring speed of 300 rpm. After that time, glycerol was added to the dispersion and was mixing for another 10 min. Then the edible film solutions in the quantity of 55 g were poured into plastic Petri dishes (external diameter 14 cm). The starch suspensions were dried under controlled conditions (40ºC and 30% relative humidity for approx. 24 h) in an environmental chamber Climacell 222 (BMT, Krakow, Poland). Finally, the films were removed from the plates and were used for wrapping apple slices.
Film applications
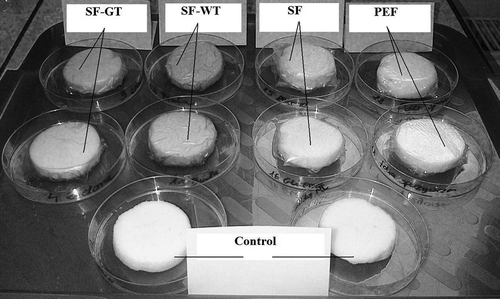
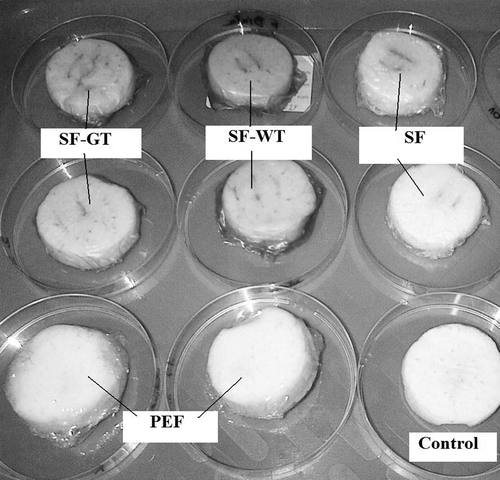
The apples were washed, peeled, and cut into slices with a thickness of 15 mm and diameter of 50 mm. Each slice of fresh cut apple (excepting for the control) was immediately wrapped in edible starch film prepared by dissolving starch in water (SF), green (SF-GT), or white (SF-WT) tea extracts. Due to compare their effect on the storage quality of apple slices, some of them were coated in low density polyethylene (LDPE) films (PEF). Apple slices were then stored on a plastic tray for 6 days in refigerator at 5ºC and 84% RH for further analysis. All of the analyses were done in triplicate on fresh uncoated apple slices (control) at day of packaging (named as day 0) and during 6 days of storage in edible starch and PE films. Picture 1 presents an example of apple slices at day 0, while Picture 2 shows an example of apple samples after 6 days of storage.
Picture 1. An example of apple slices coated in different types of films at day 0 of storage.*Control: apple slices without films. PEF: apple slices stored in polyethylene films. SF: apple slices stored in starch films. SF-GT: apple slices stored in starch films enriched in green tea extract. SF-WT: apple slices stored in starch films enriched in white tea extract.
Picture 2. An example of apple slices coated in different types of films at day 6 of storage at 5ºC.*Control: apple slices without films. PEF: apple slices stored in polyethylene films. SF: apple slices stored in starch films. SF-GT: apple slices stored in starch films enriched in green tea extract. SF-WT: apple slices stored in starch films enriched in white tea extract.
Film thickness
Film thickness was measured with a hand-held micrometer (Falon Tech, Warsaw, Poland) having a precision of 0.01 mm at five random positions on the films and the mean value was used in the calculations.
Weight loss of apple slices
To determine the effectiveness of starch-based edible films as moisture barriers, the weight of apple slices was measured during storage. It was assumed that weight loss corresponded entirely with water loss.[Citation9] The percentage of weight loss relative to the initial weight was calculated by weighing the samples in the day of packaging (0 day), and in 2 and 6 days of storage in films.
Color measurement of apple slices
The effect of SFs on browning of apple slices was determined by investigating their surface in CIELAB system (10º/D65 color spaces, gap 10mm) in reflection by using spectrophotometer Color i5 (X-Rite, USA). The color parameters of the apples were expressed as L* (lightness; from 0 = black, to 100 = white), a* (+a = redness, –a = greenness), and b* (+b = yellowness, –b = blueness) and were used to calculate the browning index (BI). The color parameters of control (uncoated apple) and apples in analyzed films were measured at 0, 2, 4, and 6 days of storage. Films were not removed from apples before measurement, except for the 6th day of storage, in which, color parameters of apple surface were determined both with coatings and after their removal from apples. The BI of apples surface was calculated, as mentioned above, and was used as an indicator of intensity of brown color according to Buera et al.[Citation15,Citation17]
where
Antioxidant activity of apple slices
Extraction process
Extraction process was carried out according to the method of Pająk et al.[Citation18] The blended apples (8 g) after removing the films were extracted for 20 min by shaking with 15 mL of methanol in a screw-capped tube. Extractions were carried out three times. The combined extracts were centrifuged at 10,000 rpm for 15 min and the organic solvent was removed at 35ºC ± 3ºC by using a rotary vacuum evaporator RVO 200A (INGOS Laboratory Instruments Ltd., Prague, Czech Republic). The obtained residue was dissolved in 5 mL of methanol and stored in freezer before the analysis. The extraction process was carried out in triplicate.
Determination of total phenolic content (TPC)
The TPC of methanolic extracts was assayed as described by Meda et al.[Citation19] using Folin–Ciocalteu reagent with final reaction measurements carried out at λ = 760 nm. The 0.1 mL of methanolic extract was diluted with 0.4 mL of deionized water, then the obtained solution was mixed with 2.5 mL of 0.2 M Folin–Ciocalteu reagent and 2 mL of 7.5 % (m/v) sodium carbonate solution. After 2 h of incubation, the absorbance was measured against blank, using an ultraviolet-visible (UV-Vis) spectrophotometer V-530 (Jasco, Tokyo, Japan). The measurements were carried out in triplicate. The TPC was expressed as mg of gallic acids equivalents (GAE) per grams of dry mass (d.m.) of apples.
Determination of ABTS cation radical scavenging activity: Determination of ABTS cation radical scavenging activity was based on the reduction of the ABTS cation radical (dissolved in phosphate buffer saline [PBS]) by methanolic extracts from apples according to metod of Martinez-Villaluenga et al.[Citation20] ABTS cation radical was obtained in the reaction of 2mM phospate-buffered stock solution of ABTS with potassium persulphate. The mixture was left to stand for 24 h, until the reaction was completed and then ABTS solution was dilluted by PBS to obtain the absorbance of 0.800 ± 0.03 at λ = 734 nm. Fifty microliters of appropriately diluted methanolic extract of apples was mixed with 6 mL of the ABTS∙+ solution and the absorbance of the resulting solution was measured after 15 min at λ = 734 nm. The measurements were carried out in triplicate. Antioxidant activity was expressed as mg of Trolox equivalents per gram of d.m. of apple samples.
Determination of DPPH radical scavenging activity
In order to determine DPPH radical scavenging activity the method described by Moure et al.[Citation21] was used with minor modification. The DPPH assay, which has wide spread use in free radical-scavenging assessment, is based on reaction between the free DPPH radical and molecules that can donate hydrogen atoms (such as most antioxidants).[Citation22] An aliquot of 3.9 mL of 0.1 mM DPPH radical in methanol was mixed with 0.1 mL of methanolic extract of the sample. After 60 min of incubation the absorbance of the sample was measured at λ = 515 nm in an UV-Vis spectrophotometer V-530 (Jasco, Tokyo, Japan). The measurements were carried out in triplicate. The DPPH radical scavenging activity in the extracts was expressed as mg of Trolox equivalents per gram of d.m. of apple samples.
Determination of ferric reducing antioxidant power (FRAP)
The ferric ion reducing activity of the methanol extracts was measured according to method of Benzie and Strain[Citation23] with some modifications. At low pH, when a ferric-tripyridyltriazine (FeIII-TPTZ) complex is reduced to the ferrous (FeII) form, an intense blue color with the absorption maximum at 593 nm develops. To 3.3 mL of acetate buffer (pH 3.6) consisting of 18.5 mL 0.2M CH3COOH and 1.5 mL 0.2 M CH3COONa, 0.33 mL 20mM/L FeCl3 and 0.33 ml 10 mM TPTZ (2,4,6-tri(2-pirydyl-s-triazine)) in 40 mM HCl were added. After 5 min of incubation at 37ºC, an aliquot of 0.33 mL of methanol extract was added to the mixture and the absorbance was measured at λ = 593 nm after further 15 min of incubation at 37ºC. The measurements were carried out in triplicate. FRAP was expressed as mmol of Fe2+ per 100 gram of d.m. of apple samples.
Statistical analysis
The results were subjected to a one-way analysis of variance, and the least significant difference (LSD) using a Fisher test at significance level 0.05 was calculated. The Pearson’s linear correlations coefficients between selected parameters were also calculated.
Results and discussion
Film thickness
The thickness of edible films is very important parameter which affects the mechanical and barrier properties of films, as well as the shelf life of the coated food. Film thickness depends on the physical and rheological properties of the polymer solution (such as density, viscosity, and surface tension) but also on the method used in the films formation: dipping, spraying, solvent casting, skimming, etc.[Citation4,Citation6] In our case, the method of films formation and the amounts of all ingredients used for films preparation was the same for all film samples, thus their thickness was similar (of 0.11 ± 0.01 mm). Abdorreza et al.[Citation24] reported that sago SFs exhibited the thickness in the range from 0.12 to 0.16 mm and also observed that the higher the sorbitol and glycerol content, the thicker the film. A similar effect was observed by López et al.[Citation25] in case of films prepared with using native and acetylated corn starch. The last authors reported that plasticizer addition increased film thickness in average 20%, regardless starch type, and films thickness varied from 0.08 to 0.13 mm. The thickness of commercial PE films used in this work for wrapping the apple slices was much lower than that of starch-based films and was of 0.01 mm, which is consistent with the previous study.[Citation26]
Weight loss of apple slices
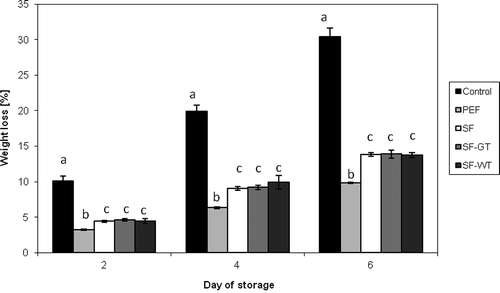
Usually, the weight loss occurs during the fruit and vegetable storage due to their respiratory process, the transference of humidity and some processes of oxidation.[Citation27] Moreover, cutting the apples exposes the skinless tissue to an environment with lower RH, and causes substantial weight loss.[Citation11] As could be expected, the weight loss of control (uncoated) apple slices was the highest during storage compared to other samples (). At the end of storage (day 6), weight loss of control apples was about 30%. A similar weight loss of unpacked apple slices was found by Olivas et al.[Citation11] Regarding the packed samples, the smallest changes in apple weight during storage was observed for apple slices coated in PE films (below 10% at day 6; ). Similar trend was observed by Colla et al.[Citation28] in case of strawberries uncoated and coated in PVC films. These results suggest that synthetic films, such as PE and PVC exhibited better moisture barrier properties than SFs, despite their much lower thickness. As shown in , the SFs (SF, SF-GT, and SF-WT) formed on the surface of apple slices delayed the moisture loss from the fruit tissues into the environment compared to unpacked control. During the storage, weight loss of the last samples was significantly higher (p < 0.05) than that coated in PE films; however, more than twice lower compared to control apple slices. In the contrary to the data reported by Siripatrawan and Harte,[Citation8] there were no differences (p < 0.05) in weight loss (in particular days of storage) between the samples coated in SFs, regardless the type of extract used in film preparation (). The previously mentioned authors reported that incorporation of green tea extract into chitosan films caused the resulting films to become denser and less water vapor permeability (WVP) than non enriched ones. This phenomenon may be because the hydrogen and covalent interactions between chitosan network and polyphenolic compounds limit the availability of hydroxyl groups to form hydrophilic bonding with water, subsequently lead to decrease in the affinity of chitosan film toward water.[Citation8] In our case, it can be assumed that polyphenolic compounds did not affect the WVP and, hence, weight loss of SFs. Krochta and De Mulder-Johnston[Citation29] reported that hydrophilic in nature polysaccharide and protein coatings do not provide a good moisture barrier; however, they are excellent gas, aroma, and lipid barriers.
Figure 1. Weight loss of apple slices stored 6 days at 5ºC. *Control: apple slices stored without films. PEF: apple slices stored in polyethylene films. SF: apple slices stored in starch films. SF-GT: apple slices stored in starch films enriched in green tea extract. SF-WT: apple slices stored in starch films enriched in white tea extract. *Within columns, values subscribed by the same letters did not differ significantly at p < 0.05.
Color measurement of apple surface
The color of food has a significant impact on consumer’s acceptance. One of the main negative attributes detected during fruit and vegetable storage is an enzymatic browning of the cut tissues. shows the variation of the lightness (L*) of apple slices during storage. The significant decrease in lightness of control (uncoated) apple slices was observed during storage (from 84.11 at day 0 to 75.37 at the end of storage). Lightness of samples measured immediately after coating in polyethylene and starch films (PEF and SF at day 0) did not differ significantly (p < 0.05) as compared to the control apples. Moreover, PEF and SF films were the most effective to minimize a decrease in lightness of cut tissues. At the end of storage in these coatings, the values of L* parameter were the highest among the samples (78.98 and 78.70, respectively). However, after removal of the films, the most effective film was SF, because the lightness of apple slices at day 6 did not differ significantly (p < 0.05) as compared to lightness of fresh apple slices (84.40 and 84.11, respectively), as well as fresh packed apple slices (85.60). SFs enriched with green and white tea extracts influenced the lightness of coated apples compared to the control. The fresh-cut apple coated in SF-GT and SF-WT had significantly lower values of L* than control apples and this tendency has continued throughout the storage period. It may be due to the fact that films prepared with using tea extracts were yellowish-brown in color. The lightness of apple slices after removal those films at day 6, was significantly lower (p < 0.05) than control measured at day 0; however, significantly (p < 0.05) higher than lightness of control at day 6 and samples after removal of PE films. The most effective in inhibiting the changes of lightness during apples storage were the SFs without tea extracts (SF). It suggests that SFs prepared with or without tea extracts prevent the loss of apple skin lightness, better than PE films.
Table 1. Lightness (L*) and browning index (BI) of apple slices stored in different films during 6 days at 5ºC.
The BI is a good indicator of color changes during storage.[Citation16] shows the effect of films on the BI values of apple slices during 6 days of storage in refrigerator at 5ºC. The values of BI indicated that films significantly affected the color of cut apples. The enzymatic browning of control samples as well as those packed with PEF increased gradually during 6 days of storage (from 35 to 46.5 BI; ). Application of SFs prepared without tea extracts (SF) significantly reduced BI values compared to control samples. At the end of storage period, values of BI before and after films (SF) removal from apple slices were the lowest compared to remaining uncoated and coated samples (31.2 and 39.5, respectively, at day 6 with films and after their removal). Preventing the effect of edible films on the enzymatic reactions of apple slices was also observed by Olivas et al.[Citation11] in case of alginate coatings enriched with calcium chloride. This tendency was also described by Baysal et al.[Citation30] working on apricot coated in zein films and by Perez-Gago et al.[Citation16] in the case of apples coated in whey protein-lipid based coatings.
Covering the apple slices with SFs enriched with green and white tea extracts significantly decreased some parameters of color of apples. SF-GT and SF-WT films were darker and more yellow than normal SFs, which contributed to the higher values of BI of coated apples. The highest values of BI throughout 6 days of storage exhibited apples coated with SFs enriched with white tea extract (SF-WT); however, after films removal at day 6 the value of BI of apple surface was insignificantly different from the BI of apple wrapped with SF-GT film (61.9 and 64.8, respectively). Darker color and higher BI values of apples stored in SF-WT and SF-GT films (measured after their removal) compared to control and apples stored in PEF and SF (also measured after films removal) may be due to the migration of phenolic compounds (yellowish-brown pigments—i.e., flavonoids) from the packaging (films enriched in tea extracts) into apple surface.
Determination of TPC
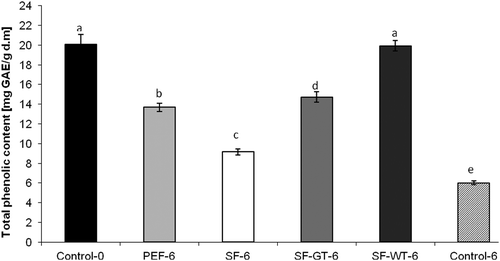
Apples are a source of phenolic compounds with important biological activities. Chlorogenic, p-coumaric and caffeic acid, as well as quercetin, epicatechin, catechin, rutin, phlorizin are the main phenolic compounds present in apples and influenced their antioxidant activity.[Citation31] The TPC of apple slices was measured at day 0 (unpacked control) and at day 6 of storage in analyzed films after their removal from apple surface. Fresh-cut uncoated apples exhibited the value of TPC of 20.07 mg GAE/1 g of plant dry mass. Similar TPCs in fresh S`ampion and Jonagold apples was observed by Leja et al.[Citation32] The authors reported that the values of TPC were 4.18 and 5.20 mg/g of apple fresh mass. Taking into account that the dry mass of apple is about 20%, the content of TPC converting to dry mass of apples will be about 20–26 mg/g. At the end of the storage period, the TPC of almost all of the apple samples was significantly lower as compared to the control (). SFs enriched with white tea extract were the most effective in preventing loss of polyphenolic compounds from apple tissues. There were no significant differences (p < 0.05) in TPC between SF-WT samples and fresh apples (Control-0; ). The highest decrease in the TPC after 6 days of storage was observed for apple slices coated in SFs (SF-6). Hovewer, the loss of polyphenols content was about 30% lower than that for uncoated samples (control-6). Also the apples stored in SFs enriched with green tea extract exhibited significantly higher TPC value than that stored in PE films ().
Figure 2. Changes in total phenolic content of apple slices stored 6 days at 5ºC, expressed as gallic acid equivalent (GAE) in mg per 1 g of plant dry mass. *Control-0: apple slices without films measured at day 0. PEF-6: apple slices stored in polyethylene films measured at day 6. SF-6: apple slices stored in starch films measured at day 6. SF-GT-6: apple slices stored in starch films enriched in green tea extract measured at day 6. SF-WT-6: apple slices stored in starch films enriched in white tea extract measured at day 6. *Within columns, values subscribed by the same letters did not differ significantly at p < 0.05.
Several authors have reported the beneficial effect of edible films on preventing loss of polyphenolic compounds and decrease in antioxidant activity of fruit and vegetable during storage;[Citation33,Citation34] however, no data was found for SFs. As it was demonstrated in this work, incorporation of tea extracts rich in polyphenolic compounds into SFs contributed to the higher values of TPC and AA of apples stored in such types of packaging. It may be due to their better preventing effect, as well as the migration of phenolic compounds from films into the fruit’s surface. Possibly, it may be also explained by the fact, that SFs with the addition of tea extracts accelerate the process of enzymatic browning of apples and presumably these darkening products are responsible for the increase of the values of TPC and AA. Wang et al.,[Citation7] in their review article, gave a few examples of edible films and coatings that provided extra antioxidant activity to the coated food. For example, it has been reported that the addition of antioxidant extracts from borage seeds into fish skin gelatine in process of film forming provided extra antioxidant activity to the horse mackerel patties and protected them from lipid oxidation during the 40 days of frozen storage at –20ºC. Other authors reported that chitosan enriched with oleoresins exerted significant antioxidant activities throughout 5 days storage of packed butternut squash slices.[Citation7] Overall, it has been proposed that polyphenols in these extracts contributed to the antioxidant capacity of these biopolymers. However, it is not clear as to how the phytochemicals interact with each other and whether the interactions lead to some synergistic effects among polyphenols and biopolymers. More work is needed to better understand this phenomenon.
Antioxidant activity
The methanolic extracts of apple slices were analyzed in respect to their antioxidant activity against ABTS and DPPH radicals. The ferric ion reducing activity of the methanolic extracts (FRAP method) was also measured. The results are presented in –. The use of starch and PE films significantly prevented the decrease in antioxidant capacity of apples during storage. The least effective were SFs without tea extract (SF; ). At the beginning of storage (day 0), the ABTS scavenging activity of control was 138 mg Trolox per 1 g of dry mass of apple tissue, and at the end of storage, the radical scavenging activity had decreased to 44 and 66 mg Trolox/1 g of dry mass of apple tissue, respectively, for control samples and apples stored in SF films. The use of SFs enriched with white tea extract for apples coatings exhibited the best prevention effect among all the samples. Antioxidant activity of apples coated in SF-WT films was the highest among the samples, irrespective the method used for AA determination. There was also a significantly higher (p < 0.05) AA against ABTS cation radical in the case of apples stored in SF-GT films compared to that stored in PE films.
Figure 3. Changes in: A: ABTS, B: DPPH scavenging activity of apple slices stored 6 days at 5ºC, expressed as Trolox equivalent in mg per 1 g of plant dry mass, C: FRAP antioxidant reducing power of apple slices stored 6 days at 5ºC, expressed in μmol Fe2+ per 1 g of plant dry mass. *Control-0: apple slices without films measured at day 0. PEF-6: apple slices stored in polyethylene films measured at day 6. SF-6: apple slices stored in starch films measured at day 6. SF-GT-6: apple slices stored in starch films enriched in green tea extract measured at day 6. SF-WT-6: apple slices stored in starch films enriched in white tea extract measured at day 6. Control-6: apple slices without films measured at day 6. *Within columns, values subscribed by the same letters did not differ significantly at p < 0.05.
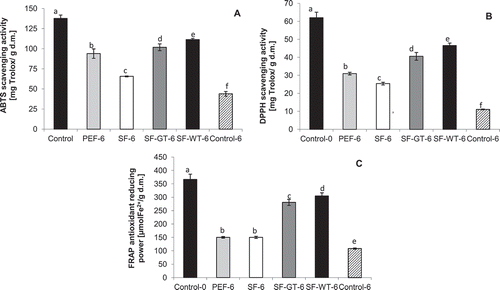
Similar tendency in prevention of decrease of AA in apples was observed when using DPPH assay (). At the end of the storage period, the apple slices coated in SF-WT demonstrated the highest antioxidant activity, followed by FS-GT and PEF. The lowest DPPH scavenging activity among packed samples was observed for apple slices stored in SFs; however, this value of AA was substantially higher than control (25 and 11 mg Trolox per 1 g of plant dry mass, respectively, for SF and the control).
The influence of film types using for apple coatings on the AA expressed as FRAP of the analyzed samples during storage was significant (). The storage of apple slices in SFs enriched with white and green tea extracts resulted in almost two-fold higher values of AA as compared to apples stored in SF and PEF films. The highest decrease in reducing ability at the end of storage was observed for uncoated control samples. The AA of the unpacked sample decreased from 367 to 108 µmol Fe2+ per 1 g of plant dry mass from the beginning to the end of storage, respectively. There were no differences (p < 0.05) between the AA of apple slices stored in PE and SFs (without tea extract; (). It suggests that SFs without or with tea extracts exhibited similar or better effect on AA of analyzed apples as PE films, and thus, may be a good alternative to that synthetic material in some applications.
There were high and significant linear correlations (at significance level 0.05) between total polyphenols content and antioxidant activity of apples evaluated using ABTS, DPPH, and FRAP assays (r = 0.965, 0.950, and 0.914, respectively), and these results suggest that phenolic compounds are good predictors of in vitro antioxidant activity. A decrease in the TPC as well as antioxidant activity in pear wedges stored in different polysaccharide-based edible coatings was also observed by Oms-Oliu et al.[Citation34] The authors reported that the use of gellan, pectin, and alginate coatings does not seem to substantially contribute to enhancement of antioxidant capacity of fresh-cut pears. The incorporation of antioxidant agent such as N-acetylcysteine and glutatione into the previously mentioned coatings helped to prevent fresh-cut fruits from a considerable decrease in AA. Similar results were obtained in this work. White and green tea extracts incorporated in SF matrix act as antioxidant agent, which led to prevent from decrease in AA and TPC as compared to other examined samples.
Conclusions
The use of starch-based films were very effective as a packaging material of fresh-cut apples. Incorporation of green and white tea extracts helped to maintain high quality of apple slices during refrigerated storage including TPC and antioxidant activity. Starch-based films, especially those enriched with an antioxidant agents, may successfully compete with PE films for the ability to inhibit weight loss, darkening, and decrease in antioxidant activity and content of phenolic compounds in apple slices during storage. Furthermore, plant extracts may provide unique colors and flavors of films which may positively influence apples’ appearance and consumer acceptance.
References
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. Journal of Texture Studies 2013, 44(3), 176–186.
- Özyurt, G.; Özkütük, A.S.; Şimşek, A.; Yeşilsu, A.F.; Ergüven, M. Quality and Shelf Life of Cold and Frozen Rainbow Trout (Oncorhynchus Mykiss) Fillets: Effects of Fish Protein-Based Biodegradable Coatings. International Journal of Food Properties 2015, 18(9), 1876–1887.
- Wang, Y.; Liu, A.; Ye, R.; Li, X.; Han, Y.; Liu, Ch. The Production of Gelatin-Calcium Carbonate Composite Films with Different Antioxidants. International of Food Properties 2015, 8(11), 2442–2456.
- Skurtys, O.; Acevedo, C.; Pedreschi, F.; Enrione, J.; Osorio, F.; Aguilera, J.M. Food Hydrocolloid Edible Films and Coatings. In Food Hydrocolloids: Characteristics, Properties and Structure, Ch. 2; Hollingworth, C.S.; Ed.; Nova Science Publishers: New York, NY, 2010; 41–80;
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food and Bioprocess Technology 2011, 4, 849–875.
- Pająk, P.; Fortuna, T.; Przetaczek-Rożnowska, I. Protein- and Polysaccharide-Based Edible Packagings: Profile and Applications. Food Science Technology Quality 2013a, 2(87), 5–18.
- Romero-Bastida, C.A.; Bello-Pérez, L.A.; García, M.A.; Martino, M.N.; Solorza-Feria, J.; Zaritzky, N.E. Physicochemical and Microstructural Characterization of Films Prepared by Thermal and Cold Gelatinization from Non-Conventional Sources of Starches. Carbohydrate Polymers 2005, 60, 235–244.
- Siripatrawan, U.; Harte, B.R. Physical and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocolloids 2010, 24, 770–775.
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L-T. Fortification of Dietary Biopolymers-Based Packaging Material with Bioactive Plant Extracts. Food Research International 2012, 49, 80–91.
- Yashin, A.; Yashin, Y.; Nemzer, B. Determination of Antioxidant Activity in Tea Extracts, and Their Total Antioxidant Content. American Journal of Biomedical Sciences 2011, 3(4), 322–335.
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Cánovas, G.V. Alginate Coatings for Preservation of Minimally Processed “Gala” Apples. Postharvest Biology and Technology 2007, 45, 89–96.
- Baldwin, E.A.; Nisperos, M.O.; Chen, X.; Hagenmaier, R.D. Improving Storage Life of Cut Apple and Potato with Edible Coating. Postharvest Biology and Technology 1996, 9, 151–163.
- Le Thien, C.; Vachon, C.; Mateescu, M.A.; Lacroix, M. Milk Protein Coatings Prevent Oxidative Browning of Apples and Potatoes. Journal of Food Science 2001, 66, 512–516.
- Lee, J.Y.; Park, H.J.; Lee, C.Y.; Choi, W.Y. Extending Shelf-Life of Minimally Processed Apples with Edible Coatings and Antibrowning Agents. LWT–Food Science and Technology 2003, 36, 323–329.
- Perez-Gago, M.B.; Serra, M.; Alonso, M.; Mateos, M.; del Río, M.A. Effect of Solid Content and Lipid Content of Whey Protein Isolate-Beeswax Edible Coatings on Color Change of Fresh-Cut Apples. Journal of Food Science 2003, 68, 2186–2191.
- Perez-Gago, M.B.; Serra, M.; Alonso, M.; Mateos, M.; del Río, M.A. Effect of Whey Protein- and Hydroxypropyl Methylcellulose-Based Edible Composite Coatings on Color Change of Fresh-Cut Apples. Postharvest Biology and Technology 2005, 36, 77–85.
- Buera, M.P.; Lozano, R.D.; Petriella, C. Definition of Colour in the Nonenzymatic Browning Process. Die Farbe 1985, 32–33, 318–322.
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic Profile and Antioxidant Activity in Selected Seeds and Sprouts. Food Chemistry 2014, 143, 300–306.
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chemistry 2005, 91, 571–577.
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskuła, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time Dependence of Bioactive Compounds and Antioxidant Capacity During Germination of Different Cultivars of Broccoli and Radish Seeds. Food Chemistry 2010, 120, 710–716.
- Moure, A.; Franco, D.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Lema, J.M. Antioxidant Activity of Extracts from Gevuina Avellana and Rosa Rubiginosa Defatted Seeds. Food Research International 2001, 34, 103–109.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts From Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16, 45–60.
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power:” The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76.
- Abdorreza, M.N.; Cheng, L.H.; Karim, A.A. Effects of Plasticizers on Thermal Properties and Heat Sealability of Sago Starch Films. Food Hydrocolloids 2011, 25, 56–60.
- López, O.V.; Lecot, C.J.; Zaritzky, N.E.; García, M.A. Biodegradable Packages Development from Starch Based Heat Sealable Films. Journal of Food Engineering 2011, 105, 254–263.
- Pająk, P.; Madej, M.; Krystyjan, M. Edible Coatings as an Alternative to Synthetic Films. Potravinarstvo 2013b, 7, 200–203.
- Ayranci, E.; Tunc, S. A Method for the Measurement of the Oxygen Permeability and the Development of Edible Films to Reduce the Rate of Oxidative Reactions in Fresh Foods. Food Chemistry 2003, 80, 423–431.
- Colla, E.; Sobral, P.J.A.; Menegalli, F.C. Effect of Composite Edible Coating from Amaranthus Cruentus Flour and Stearic Acid Refrigerated Strawberry (Fragaria Ananassa) Quality. Latin American Applied Research 2006, 36, 249–254.
- Krochta, J.M.; De Mulder-Johnston, C. Edible and Biodegradable Polymer Films: Challenges and Opportunites. Journal of Food Technology 1997, 51(2), 61–74.
- Baysal, T.; Bilek, S.E.; Apaydin, E. The Effect of Corn Zein Edible Film Coating on Intermediate Moisture Apricot (Prunus Armenica L.) Quality. GIDA 2010, 35(4), 245–249.
- Heras-Ramírez, M.E.; QuinterRamos, A.; Camacho-Dávila, A.A.; Barnard, J.; Talamás-Abbud, R.; Torres-Muňoz, J.V.; Salas-Muňoz, E. Effect of Blanching and Drying Temperature on Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace. Food and Bioprocess Technology 2012, 5, 2201–2210.
- Leja, M.; Mareczek, A.; Ben, J. Antioxidant Properties of Two Apple Cultivars During Long-Term Storage. Food Chemistry 2003, 80, 303–307.
- Viña, S.Z.; Mugridge, A.; García, M.A.; Ferreyra, R.M.; Martino, M.N.; Chaves, A.R.; Zaritzky, N.E. Effects of Polyvinylchloride Films and Edible Starch Coatings on Quality Aspects of Refrigerated Brussels Sprouts. Food Chemistry 2007, 103, 701–709.
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible Coating with Antibrowning Agents to Maintain Sensory Quality and Antioxidant Properties of Fresh-Cut Pears. Postharvest Biology and Technology 2008, 50, 87–94.
