ABSTRACT
Aflatoxin B1 has been considered as the most potent liver carcinogen for humans. One factor that stimulates the production of aflatoxins in oilseed products, such as sesame seeds and their products is the presence of high levels of fatty acids. This work presents further evidence that sesame is a favorable substrate for aflatoxin B1 production by Aspergillus parasiticus. Moreover, the use of wild artichoke (Cynara cardunculus L.) extract was examined showing inhibition of aflatoxin B1 production in both sesame and yeast extract sucrose medium, inoculated with A. parasiticus. More specifically, the inhibition of aflatoxin B1 production by A. parasiticus in sesame seeds paste was 99.6% and in yeast extract sucrose medium it was 99.4%.
Introduction
Aflatoxins (AFs) are highly toxic secondary metabolites produced by certain species of Aspergillus, mainly Aspergillus flavus and Aspergillus parasiticus. Among these, aflatoxin B1 (AFB1) represents the most common contaminant and the most potent liver carcinogen, classified by the International Agency for Research on Cancer (IARC) as a Group I carcinogen.[Citation1] AFs have also important economic impact in several crops. The AFB1 exposure associated with the risk through food chain is extensively reported in the literature, and its levels in different foodstuffs like peanuts, cereals, fruits, or their processed products, are constantly examined.[Citation2,Citation3]
Foodstuffs rich in oil, such as nuts and seeds (peanuts, pistachios etc.),[Citation4–Citation7] are sensitive to AF-producing fungal invasion and may, therefore, be contaminated with AFs and particularly AFB1. Sesame (Sesamum indicum L.) is probably the most ancient oilseed cultivated in several countries and is used for oil extraction and edible purposes. Sesame seeds contain high levels of fat and protein. The fat content of sesame seeds is ~50%, whereas the protein content is ~25%.[Citation8] Sesame oil contains fatty acids such as oleic acid (43%), linoleic acid (35%), palmitic acid (11%), and stearic acid (7%) contributing to 96% of total fatty acids.[Citation9]
C. cardunculus L. belongs to sunflower family (Asteraceae). This plant has its origin in edible Cynara cultivars which has been traditionally cultivated in the Mediterranean regions. The edible part of the plant is the fleshy leaf petioles and the immature inflorescence (capitulum or head). Artichoke’s phenolic acid constituents include caffeic acid, mono and di-caffeoyl quinic acids (e.g., cynarin), and chlorogenic acid. These compounds fortify artichoke with important biological properties.[Citation10] Research studies have proved several properties of artichoke, such as antioxidative, anticarcinogenic, antigenotoxic, cholesterol-lowering, hepatoprotective, bile-expelling, diuretic, and anti-inflammatory, as well as antifungal, anti-HIV, and antibacterial effects.[Citation11–Citation14]
In addition, a correlation between the antioxidant capacity of many plants and their antifungal, antimicrobial, and anti-aflatoxigenic activities has been reported in the literature.[Citation15–Citation18] However, data related to the anti-aflatoxigenic properties of artichoke species are scarce. The first aim of this study was to examine if sesame is favorable substrate for the AFB1 production by A. parasiticus comparing the amounts of AFB1 produced in sesame, with those produced in a yeast extract sucrose (YES) medium. Moreover, the Cynara cardunculus L. plant (commonly named cardoon) was investigated for its antiaflatoxigenic efficacy in inoculated with Aspergillus parasiticus, YES medium, and sesame seeds paste (Sesamum indicum L.)
Materials and methods
Sampling and treatment
Sesame seeds (approximately 4 kg) were purchased from a local market in Athens (Greece) during the spring of 2014. Sesame seeds were washed with water, dried with filter paper, and homogenized to form a paste, so that the whole mass to be used as a substrate. Finally, sesame seeds’ paste was autoclaved at 110°C for 2 min, in order to eliminate their natural microbiota before inoculation with A. parasiticus. The above treatment was chosen because the use of sodium hypochlorite solution was an unsuitable treatment for the elimination of the sesame seeds’ natural microbiota, since it was absorbed by the sesame substrate.[Citation19]
After treatment, representative samples were taken from the whole quantity of sesame seeds’ paste and then distributed aseptically into flasks forming a solid mass of one layer. Each flask contained 15 g of sesame seeds paste. Wild artichoke (500 g) or as commonly named cardoon (Cynara cardunculus L./variety: large smooth) was collected from fields of Thebes (Central Greece) during spring of 2014. The artichokes’ heads (inner part of flowers) were washed with water and dried with filter paper. After that, they were chopped and homogenized before analysis.
Apparatus
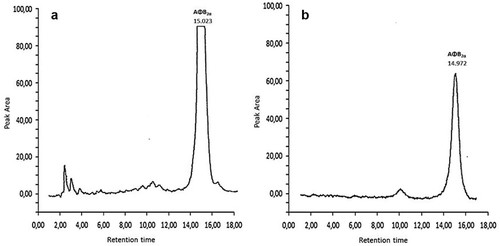
Ultrasound-Assisted Extraction (UAE) procedure was performed using Sonics and Material Inc., Vibra-Cell VCX750 (20 kHz, 750 W) ultrasonics processor equipped with piezoelectric converter and 13 mm diameter probe fabricated from titanium alloy Ti–6Al–4V (Inc. USA). A rotary evaporator (Heidolph, Laborota 4000 efficient, WB eco) was used for the evaporation of extracts. High-performance liquid chromatography (HPLC) analysis conducted using a Hewlett-Packard 1050 (Hewlett-Packard, Waldbron, Germany) liquid chromatograph equipped with a JASCO FP-920 (Jasco Ltd., Tokyo, Japan) fluorescence detector and an HP integrator 3395. The HPLC column used was a C18 Nova-Pak (4.6 × 250 mm, 4 μm,60 Å; Waters-Millipore, Milford, MA, USA). Mobile phase (water: acetonitrile: methanol [20:4:3 v/v/v]) for AFB1 determination was filtered through Millipore HA-VLP (0.45 μm) filters prior to use. The derivative of AFB1 (AFB2a, the hemiacetal of AFB1) was detected at λex = 365 nm/λem = 425 nm. The flow rate was 1 mL min−1 and the retention time was 14.98 ± 0.29 min ().
Figure 1. Chromatographs of AFB2a production: A: sesame seeds’ paste (15 g) inoculated with A. parasiticus on 12th day of observation (dilution 1:40); B: sesame seeds’ paste (15 g) inoculated with A. parasiticus and spiked with C. cardundulus L. head’s extract, on 12th day of observation. All samples were derivatized to AFB2a. The injection volume was 40 mL.
Reagents
AFB1 standard was purchased from Sigma (St. Louis, MO, USA). The Aflatest immunoaffinity columns were obtained from Vicam (Watertown,MA, USA). All reagents used were of analytical grade, while HPLC solvents were of HPLC grade and were purchased from Fisher Chemical (Fisher Scientific, Loughborough, Leicestershire, UK). Hexane and methanol (pro analysis grade) were purchased from Merck (Darmstadt, Germany) andtrifluoroacetic acid was obtained from Fluka (Sigma-Aldrich Chemie GmbH, Steinheim, Germany)
Culture media
Aspergillus Flavus Parasiticus Agar (AFPA) was prepared by dissolving 2 g of yeast extract (Oxoid, Basingstoke, Hampshire, UK), 1 g of bacteriological peptone (Oxoid), 0.05 g of ferric ammonium citrate (Merck, Germany), 0.1 mL of Dichloran 0.2% in ethanol (Fluka Steinheim, The Netherlands), 0.01 g of chloramphenicol (Oxoid), and 1.5 g of agar (Oxoid) per 100 mL of distilled water, final pH 6.0–6.5.
Czapek Dox agar (CZA) was prepared by dissolving 0.2 g of sodium nitrate (Merck), 0.05 g of potassium chloride (Merck), 0.05 g of magnesium sulfate (Merck), 0.001 g of ferric sulfate (Merck), 0.1 g of dipotassium phosphate (Merck), 3 g of sucrose (Merck), 1.5 g of agar (Merck), 0.001 g of zinc sulfate (Merck), and 0.0005 g of copper sulfate (Merck) in 100 mL of distilled water, final pH 6.0–6.5.[Citation20] YES medium was prepared by dissolving 2 g of yeast extract (Oxoid, Basingstoke, Hampshire, UK) and 15 g of sucrose (Merck) in 100 mL of distilled water.
Experimental design
The experimental design of this work is described in . All samples were assayed for AFB1 on days 0, 3, 7, 9, 12, and 15 of incubation. The experiments were repeated in triplicate.
Table 1. Inoculated samples.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) and t-test. The mean differences, which are significantly different, were examined by using the tukey test.[Citation21]
Extraction of Cynara cardunculus L
Ten grams (10 g) of fresh samples were repeatedly extracted (3 times) with 40 mL of MeOH:H2O (80:20 v/v). The homogenized samples and the solvent were inserted in a 250 mL three-neck vessel into an ice bath in order to keep the temperature below 40°C during the extraction time. The extracts were sonicated for 15 min, using 80% amplitude and pulse sonication sequence set to 10 s on and 5 s off. The obtained extracts, were then evaporated to dryness at 40°C and re-dissolved in 40 mL of MeOH:H2O (80:20 v/v).[Citation22]
Preparation of spore inoculum
The aflatoxigenic strain A.parasiticus speare (IMI 283883) that used during this study was purchased from the International Mycological Institute (Engham Surrey, UK). To achieve an inoculum containing 102 conidia, plates (CZA) with 10–100 colony-forming units (cfu) were selected and the quantity (100 spore) used in the present study was estimated, following the method which has been previously described.[Citation20,Citation23]
Inoculation of YES medium and sesame seeds’ paste
For each day of observation, three flasks containing 10 mL of YES medium and three flasks containing 15 g of sesame seeds’ paste were inoculated with 102 conidia flask–Citation1 of A. parasiticus as shown in . Fifty (50) μL of the artichoke extract (in Dimethyl sulfoxide (DMSO)) were added after inoculation with the fungus, into each flask. Furthermore, 50 μL of DMSO alone, were added into three additional flasks of YES inoculated with the fungus and to three additional flasks of inoculated sesame seeds’ paste as well, to be used as control for each day of observation. All flasks were incubated under stationary conditions at 30°C.
AFB1 determination and HPLC analysis
The content of each flask containing sesame seeds’ paste or YES medium spiked with artichoke extract and flasks control, were mixed with 30 mL of aqueous methanol (80:20 v/v) and 30 mL of methanol, respectively, and shaken for 10 min. After filtration, an aliquot of 1 mL from the filtrate was used for AFB1 analysis.
Clean-up
One milliliter from the filtrate was diluted with 10 mL of distilled water and mixed for 1 min. The mixture was transmitted into an Aflatest immunoaffinity column with flow rate 3 mL min–Citation1 and washed twice with 10 mL of distilled water. The column was then allowed to dry by passing air. AFB1 was eluted with 2 mL of acetonitrile (flow rate 0.3 mL min–Citation1). Before derivatization, the eluate was inserted into a glass vial, and the solvent was evaporated to dryness under a gentle stream of nitrogen.
A derivative of AFB1 (AFB2a, hemiacetal of AFB1) was made by adding 200 μL of hexane and 200 μL of trifluoroacetic acid to the evaporated solution of AFB1 eluate, heating at 40°C (in a water bath for 10 min), evaporating to dryness, dissolving in 200 μL with water:acetonitrile (9:1 v/v) and analyzing by HPLC with fluorescence detector (volume injected = 40 μL).[Citation24]
Results and discussion
AFB1 production by A. parasiticus in YES medium and sesame seeds’ paste
Yeast extract is a rich source of B complex vitamins, which have been associated with the stimulation of AFB1 production by A. parasiticus.[Citation25] The presence of magnesium and sucrose in YES medium also enhances AFB1 production by A. flavus and A. parasiticus.[Citation26] Additionally, YES medium is easy to prepare and very suitable for production of higher levels of AF than those reported for other media under the most favorable conditions of incubation at 30°C.[Citation20,Citation27]
AFB1, rather than all four AFs (AFB1, AFG1, AFB2, and AFG2) was studied as it is the most potent carcinogenic mycotoxin and it is usually produced at high levels by the fungus A. parasiticus.[Citation28–Citation30] In addition, the quantity of 102 spores was chosen as it was the minimum concentration found in the literature producing detectable amounts of AFB1 by Aspergilli.[Citation20,Citation26] AFB1 determination in YES medium has been previously characterized in detail by our group.[Citation19] The recovery of the method was found to be 99.2% and the detection limit of the derivatized AFB1 (AFB2a) was 0.02 ng AFB1 mL−1. Furthermore, AFB1 determination in sesame seeds’ paste was characterized and the recovery of the method for AFB1 determination in sesame seeds’ paste was calculated at 111.5% (RSD% = 5.02), while the detection limit was found to be 0.02 ng AFB1 g−Citation1.[Citation31]
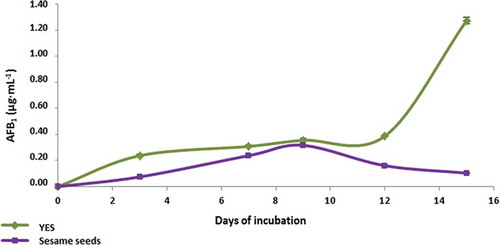
As shown in the AFB1 production in YES medium inoculated with A. parasiticus increased until the 15th day of observation (1.91 μg mL−1). Moreover the AFB1 production in inoculated with the fungus sesame seeds’ paste was increased until the 9th day, where the maximum AFB1 production (0.32 μg mL−1) was observed. The results from the statistical analysis (t-test) showed that there is not statistically significant difference between the amounts of AFB1 production in YES medium and in sesame seeds’ paste for all the days of observation (df = 5; Texp = 1.518; Ttheor = 2.015).
Figure 2. AFB1 production by A. parasiticus in YES medium and sesame seeds’ paste. The AFB1 amounts produced during the whole period of incubation in YES medium and sesame seeds’ paste were not significantly different between them.
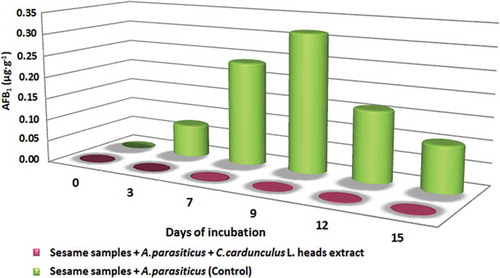
Taking in account the above results, sesame seeds could be assumed as a favorable substrate for the AFB1 production as compared with YES medium, which is an optimum medium for AFB1 production by A.parasiticus. In the treated non-inoculated sesame seeds’ paste, maximum AFB1 levels were observed on 12th day (4.67·10−4 μg g−1). Obviously, the treatment eliminated partially the natural microbiota. As mentioned above, in the treated sesame samples inoculated with the fungus, AFB1 production was ~360 fold higher, compared to the non-inoculated samples at the same day (12th day; ). Comparing the AFB1 occurrence in the treated non-inoculated sesame seeds’ paste, with the AFB1 production in the treated sesame seeds’ paste inoculated with A. parasiticus, significant difference was observed between them (df = 5; Τexp = 3.257; Ttheor = 2.015; ).
Table 2. AFB1 production (μg g−1) by A. parasiticus in inoculated and in non-inoculated sesame seeds’ paste.
Several reports, concerning AF food contamination, indicate an AF/lipid relationship and reveal that the lipid content especially in foodstuffs such as oil seeds, is significant for AF contamination.[Citation28] Earlier, Fabbri[Citation32] showed that the oxidation of unsaturated lipids plays a fundamental role in the induction of AF production by A.flavus and A. parasiticus. As mentioned before, sesame seeds contain high lipid content. This fact confirms that sesame seeds are a favorable substrate for AF production, which agrees with literature data. A survey in Iran exhibited that AFB1 was present in 33 of the 182 samples examined.[Citation33] Moreover, in a study conducted in China, 37 of 100 sesame paste samples analyzed were found to be contaminated with AFB1.[Citation34] AFB1 and AFB2 were also detected in Sudanese sesame oil.[Citation35]
Effect of C. cardunculus L. heads extract on AFB1 production by A. parasiticus in YES medium and sesame seeds’ paste
Plant extracts contain various antioxidant compounds such as polyphenols, phenols, flavonoids, which can be possibly utilized by the food industry for food preservation. It is reported that the previously mentioned plant constituents have an inhibitory effect on growth and production of mycotoxin by Aspergillus spp. and others fungi.[Citation36–Citation39] Recently, in our laboratory, it was shown that saffron, known for its antioxidant properties, inhibited AFB1 production at percentage 99.9%.[Citation40] Redox reactions are essential to cellular metabolism, and antioxidants may delay vital processes. It has been also found that β-oxidation was active during AF biosynthesis on lipid substrates. Inhibition of mitochondrial or peroxisomal β-oxidation by antioxidants may reduce the availability of carbon skeletons for polyketide pathways during growth on lipid-rich seeds. The oxidative pentose phosphate pathway (PPP) is also controlled during AF biosynthesis, and antioxidants could interfere, thus reducing the pool of nicotinamide adenine dinucleotide phosphate (NADP), which is available for the AF biosynthetic reactions.[Citation41]
The purpose of using a natural antioxidant such as C. cardunculus L. heads extract in a favorable for AFB1 production substrate, was to investigate the efficacy of its effect on a substrate which promotes AFB1 biosynthesis. Moreover, little data is in literature concerning the effect of C. cardunculus on AFB1 production. It must be mentioned that Cynara cardunculus L. used during this study, has been previously investigated in our laboratory for the phenolic content and the antioxidant activity of its different parts (heads, bracts, and stems). The spectrophotometric methods, Folin-Ciocalteau, 2,2-diphenyl-1-picryl-hydrazyl radical (DPPH) radical scavenging assay (using the stable DPPH) and Trolox equivalent antioxidant capacity assay (TEAC/using the radical 2,2-azino-bis-[3-ethylbenzothiazoline-6-sulfonic acid] radical [ABTS•+]) were applied. Results showed that the extract obtained from the heads of the plant displayed the maximum total phenolic content and the maximum antioxidant activity compared to the other parts of the plant.[Citation42] Thus, the extract from the heads was chosen to study for its effect on AFB1 production by A. parasiticus.
The extract of C. cardunculus L. heads, which is the edible part of the plant with antioxidant capacity, was used at concentration of 1 g fresh weight (FW) mL−1 DMSO. The above concentration was found to be the most effective against A. parasiticus growth in AFPA when nine different concentrations were examined (from 0.005 to 1 g mL−1; Markaki unpublished data).
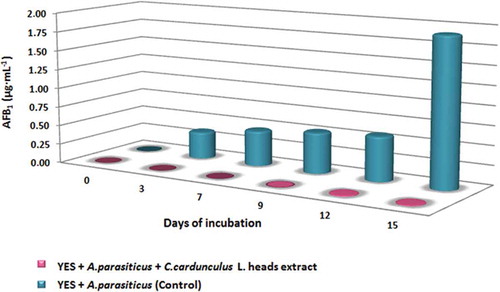
AFB1 production in YES medium inoculated with A. parasiticus with the addition of C. cardunculus L. heads extract (50 μL of 1g FW ml−1 DMSO), was increased until the 12th day (maximum AFB1 production; 128.10·10−4 μg mL−1). After the 12th day, AFB1 decreased. The AFB1 production in control samples (without the extract addition) was continued until the 15th day, which was the last day of incubation and where the maximum production was observed (1.91μg mL−1; ).
Table 3. AFB1 production (μg mL−1) by A. parasiticus in YES medium in comparison with AFB1 production in YES with C. cardunculus L. head extract addition.
The addition of C. cardunculus L. heads extract (50 μL of 1 g FW mL−1 DMSO) in YES medium cultures inoculated with A. parasiticus showed a significant inhibition of AFB1 production compared to control cultures (df = 5; Texp = 2.391; Ttheor = 2.015). In the present study, C. cardunculus L. heads’ extract significantly inhibits the AFB1 production by A. parasiticus in YES medium. The AFB1 reduction amounted from 97.8 to 99.9% after the 3rd day of observation (, ).
Figure 3. C. cardunculus L. heads extract inhibits significantly the AFB1 production by A. parasiticus in YES medium in comparison to AFB1 production in YES medium (control).
The addition of 50 μL (c = 1 g FW mL−1 DMSO) of C. cardunculus L. heads extract, in inoculated sesame seeds’ paste, affected significantly the AFB1 production, compared to control inoculated sesame seeds’ paste (without the C. cardunculus L. heads extract addition). Maximum AFB1 production in inoculated sesame seeds’ paste samples (control) was observed on 9th day of incubation (0.32 μg g−1). Additionally, AFB1 production in inoculated sesame seeds’ paste spiked with C. cardunculus L. heads extract, was increased until the 12th day where the maximum AFB1 production was observed (13.50·10−4 μg g−1). These results revealed that the addition of C. cardunculus L. heads extract in sesame seeds’ paste inoculated with A. parasiticus, inhibited significantly the AFB1 production from 99.2 to 99.9% after the 3rd day of observation (df = 5; Texp = 3.173; Ttheor = 2.015; , ). This inhibition can be attributed to C. cardunculus L. constituents which may be responsible for its antioxidant properties but further investigation is needed. It must be mentioned that differences between the results from literature that concerns AF content in agricultural products could be attributed to the fact that AF production is influenced by the parameters of the system under investigation.
Table 4. AFB1 production (μg g−1) by A. parasiticus in inoculated sesame seeds’ paste with and without C. cardunculus L. head extract addition.
Conclusions
Τhis study presents that sesame seeds’ paste is a favorable substrate for AFB1 production when invasion by an aflatoxigenic fungi such as A. parasiticus occurs. Consequently, it is likely to detect AFB1 in sesame oil, which is also widely used in human diet. The artichoke extract inhibited significantly the AFB1 production both in YES medium (97.8–99.9%) and in sesame seeds’ paste (99.2–99.9%) inoculated with A. parasiticus. The anti-aflatoxigenic efficacy of the wild artichoke heads extract (C. cardunculus L.) can possibly, under the appropriate conditions, to be used in the future as a simple, economic, and safe way for reducing post-harvest AF production or used in predicting AFs control strategies.
Funding
This work was supported in part by the University of Athens, Special Account for Research Grants (11400).
Additional information
Funding
References
- International Agency for Research on Cancer (IARC). Aflatoxins, IARC Monograph on the Evaluation of Carcinogenic Risks to Humans, Vol. 82 IARC. World Health Organization: Lyon, France, 2002; 171–300 pp.
- Costanzo, P.; Santinib, A.; Fattorea, L.; Novellinob, E.; Ritienib, A. Toxicity of Aflatoxin B1 Towards the Vitamin D Receptor (VDR). Food and Chemical Toxicology 2015, 76, 77–79.
- Kollia, E.; Kanapitsas, A.; Markaki, P. Occurrence of Aflatoxin B1 and Ochratoxin A in Dried Vine Fruits from Greek Market. Food Additives and Contaminants Part B: Surveillance 2014, 7, 11–16.
- Abdulkadar, A.H.W.; Al-Ali, A.; Al-Jedah, J. Aflatoxin Contamination in Edible Nuts Imported in Qatar. Food Control 2000, 11(2), 157–160.
- Luttfullah, G.; Hussain, A. Studies on Contamination Level of Aflatoxins in Some Dried Fruits and Nuts of Pakistan. Food Control 2011, 22(3–4), 426–429.
- Milhome, M.A.L.; Lima, C.G.; De Lima, L.K.; Lima, F.A.F.; Sousa, D.O.B.; Nascimento, R.F. Occurrence of Aflatoxins in Cashew Nuts Produced in Northeastern Brazil. Food Control 2014, 42, 34–37.
- Mupunga, I.; Lebelo, S.L.; Mngqawa, P.; Rheeder, J.P.; Katerere, D.R. Natural Occurrence of Aflatoxins in Peanuts and Peanut Butter from Bulawayo, Zimbabwe. Journal of Food Protection 2014, 77(10), 1814–1818.
- Hwang, L.S.; Sesame Oil. In Bailey’s Industrial Oil and Fat Products, Shahidi, F.; Ed.; John Wiley & Sons, Inc.: New York, 2005; 537–545 pp.
- Najeeb, U.; Mirza, M.Y.; Jilani, G.; Mubashir, A.K.; Zhou, W.J. Sesame. In Technological Innovations in Major World Oil Crops; Gupta, S.K.; Ed.; Springer: New York, NY, 2012; 131–145 pp.
- Tuncay, D.; Yagar, H. Comparison of Polyphenol Oxidases Prepared from Different Parts of Artichoke (Cynara Scolymus L.). International Journal of Food Properties 2011, 14, 809–821.
- Prakash, B.; Mishra, P.K.; Kedia, A.; Dubey, N.K. Antifungal, Antiaflatoxin and Antioxidant Potential of Chemically Characterized Boswellia Carterii Birdw Essential Oil and Its in vivo Practical Applicability in Preservation of Piper Nigrum L. Fruits. LWT–Food Science and Technology 2014, 56(2), 240–247.
- Agarwal, R.; Mukhtar, H. Cancer Chemoprevention by Polyphenols in Green Tea and Artichoke. Advances in Experimental Medicine and Biology 1996, 401, 35–50.
- Gebhardt, R. Antioxidative and Protective Properties of Extracts from Leaves of the Artichoke (Cynara Scolymus L.) Against Hydroperoxide-Induced Oxidative Stress in Cultured Rat Hepatocytes. Toxicology and Applied Pharmacology 1997, 144, 279–286.
- Abhishek, R.U.; Mohana, D.C.; Thippeswamy, S.; Manjunath, K. Evaluation of Phyllanthus Polyphyllus L. Extract and Its Active Constituent as a Source of Antifungal, Anti-Aflatoxigenic, and Antioxidant Activities. International Journal of Food Properties 2015, 18(3), 585–596.
- Miadokova, E.; Nadova, S.; Vlckova, V.; Duhova, V.; Kopaskova, M.; Cipak, L.; Rauko, P.; Mucaji, P.; Grancai, D. Antigenotoxic Effect of Extract from Cynara Cardunculus L. Phytotherapy Research 2008, 22, 77–81.
- Makni, M.; Haddar, A.; Kriaa, W.; Zeghal, N. Antioxidant, Free Radical Scavenging, and Antimicrobial Activities of Ajuga Iva Leaf Extracts. International Journal of Food Properties 2013, 16(4), 756–765.
- Barış, D.; Kızıl, M.; Aytekin, Ç.; Kızıl, G.; Yavuz, M.; Çeken B.; Ertekin, A.S. In Vitro Antimicrobial and Antioxidant Activity of Ethanol Extract of Three Hypericum and Three Achillea Species from Turkey. International Journal of Food Properties 2011, 14(2), 339–355.
- Yildiz, H. Chemical Composition, Antimicrobial, and Antioxidant Activities of Essential Oil and Ethanol Extract of Coriandrum sativum L. Leaves from Turkey. International Journal of Food Properties 2016, 19(7), 1593–1603.
- Leontopoulos, D.; Siafaka, A.; Markaki, P. Black Olives as Substrate for Aspergillus Parasiticus Growth and Aflatoxin B1 Production. Food Microbiology 2003, 20, 119–126.
- Vergopoulou, S.; Galanopoulou, D.; Markaki, P. Methyl Jasmonate Stimulates Aflatoxin B1 Biosynthesis by Aspergillus Parasiticus. Journal of Agricultural and Food Chemistry 2001, 7, 3494–3498.
- Fowler, J.; Cohen, L. Practical Statistics for Field Biology. John Wiley and Sons: Chichester, England, 1997.
- Petrović, J.; Papandreou, M.; Glamočlija, J.; Ćirić, A.; Baskakis, C.; Proestos, C.; Lamari, F.; Zoumpoulakis, P.; Soković, M. Different Extraction Methodologies and Their Influence on the Bioactivity of the Wild Edible Mushroom Laetiporus Sulphureus (Bull.) Murrill. Food and Function 2014, 5(11), 2948–2960.
- Kostarelou, P.; Kanapitsas, A.; Pyrri, I.; Kapsanaki-Gotsi, E.; Markaki, P. Aflatoxin B1 Production by Aspergillus Parasiticus and Strains of Aspergillus Section Nigri in Currants of Greek Origin. Food Control 2014, 43, 121–128.
- Daradimos, E.; Markaki, P.; Koupparis, M. Evaluation and Validation of Two Fluorometric HPLC Methods for the Determination of Aflatoxin B1 in Olive Oil. Food Additives and Contaminants 2000, 17(4), 65–73.
- Luchese, R.H.; Harrigan, W.F. Biosynthesis of Aflatoxin—The Role of Nutritional Factors. Journal of Applied Bacteriology 1993, 74, 5–14.
- Abramson, D.; Clear, R.M.A. Convenient Method for Assessing Mycotoxin Production in Cultures of Aspergilli and Penicillia. Journal of Food Protection 1996, 59, 642–644.
- Davis, N.D.; Diener, U.L; Eldridge, D.W. Production of Aflatoxin B1 and G1 by Aspergillus Flavus in a Semisynthetic Medium. Journal of Applied Microbiology 1966, 14, 378–380.
- Meimaroglou, D.M.; Galanopoulou, D.; Markaki, P. Study of the Effect of Methyl Jasmonate Concentration on Aflatoxin Biosynthesis by Aspergillus Parasiticus in Yeast Extract Sucrose Medium. International Journal of Microbiology 2009, 2009, 1–7. DOI:10.1155/2009/842626
- Keller, N.P.; Butchko, R.A.E.; Sarr, B.; Phillips, T.D. A Visual Pattern of Mycotoxin Production in Maize Kernels by Aspergillus spp. Phytopathology 1994, 84, 483–488.
- Burow, G.B.; Nerbitt, T.C.; Dunlap, J.; Keller, N.P. Seed Lipoxygenase Products Modulate Aspergillus Mycotoxin Biosynthesis. Molecular Plant-Microbe Interactions 1997, 10, 380–387.
- Kollia, E.; Tsourouflis, Κ.; Markaki, P.; Aflatoxin B1 in Sesame Seeds and Sesame Products from the Greek Market. Food Additives & Contaminants: Part B: Surveillance 2016. DOI:10.1080/19393210.2016.1179349
- Fabbri, A.A.; Fanelli, C.; Panfili, G.; Passi, S.; Fasella, P. Lipoperoxidation and Aflatoxin Biosynthesis by Aspergillus Parasiticus and A. Flavus. Journal of General Microbiology 1983, 129, 3447–3452.
- Asadi, M.; Beheshti, H.R.; Feizy, J. A Survey of Aflatoxins in Sesame in Iran. Mycotoxin Research 2011, 27(11), 259–263.
- Li, F.Q.; Li, Y.W.; Wang, Y.R.; Luo, X.Y. Natural Occurrence of Aflatoxins in Chinese Peanut Butter and Sesame Paste. Journal of Agricultural and Food Chemistry 2009, 57(9), 3519–3524.
- Idris, Y.M.A.; Hassan, S.A.; Mariod, A.A. Physicochemical Characteristics and Aflatoxin Levels in Two Types of Sudanese Sesame Oil. Journal of the American Oil Chemists’ Society 2013, 90(7), 989–998.
- Shukla, R.; Kumar, A.; Prasad, C.S.; Srivastava, B.; Dubey, N.K. Antimycotic and Antiaflatoxigenic Potency of Adenocalymma Alliaceum Miers. on Fungi Causing Biodeterioration of Food Commodities and Raw Herbal Drugs. International Biodeterioration & Biodegradation 2008, 62, 348–351.
- Molyneux, R.J.; Mahoney, N.; Kim, J.H.; Campbell, B.C. Mycotoxins in Edible Tree Nuts. International Journal of Food Microbiology 2007, 119, 72–78.
- Oliveira, M.S.; Furlong, E.B. Screening of Antifungal and Antimycotoxigenic Activity of Plant Phenolic Extracts. World Mycotoxin Journal 2008, 1, 139–146.
- Mahoney, N.; Molyneux, R.J.; Kim, J.H.; Campbell, B.C.; Waiss, A.C.; Hagerman, A.E. Aflatoxigenesis Induced in Aspergillus Flavus by Oxidative Stress and Reduction by Phenolic Antioxidants from Tree Nuts. World Mycotoxin Journal 2010, 3, 49–57.
- Tzanidi, C.; Proestos, C.; Markaki, P. Saffron (Crocus Sativus L.) Inhibits Aflatoxin B1 Production by Aspergillus Parasiticus. Advances in Microbiology 2012, 2(03), 310–316.
- Maggio-Hall, L.A.; Wilson, R.A.; Keller, N.P. Fundamental Contribution of β-Oxidation to Polyketide Mycotoxin Production in Planta. Molecular Plant-Microbe Interactions 2005, 18(8), 783–793.
- Kollia, E.; Markaki, P.; Zoumpoulakis, P.; Proestos, C. (in press). Antioxidant activity of Cynara scolymus L. and Cynara cardunculus L. extracts obtained by different extraction techniques. Natural Product Research 2016, 1–5.