ABSTRACT
Extract of Withania coagulans fruit was evaluated for milk clotting activity. The highest enzyme activities were detected at pH 4 and 65°C. A 50 mM CaCl2 was considered sufficient to decrease the milk clotting activity to an effective level. Rennet coagulation time was steadily increased with increasing NaCl and decreasing enzyme concentrations. The enzymatic activity of extract was completely inhibited by pepstatin-A, an aspartic protease inhibitor. Proteomic and zymographic analysis discovered an aspartic protease with Mw of about 35 KDa and pI of 5.91. Our data demonstrated that use of this plant in traditional cheese making has the scientific bases due to existence of an effective protease.
Introduction
Milk coagulation that induced by rennin (chymosin) as proteolytic enzyme is a basic step in cheese manufacturing. Over the past decades, much effort has been spent to find appropriate coagulant substitutes due to limited availability of proper stomachs as the main traditional source of rennin and also increase in price of rennin and cheese production. Although, some milk-clotting sources such as fungi[Citation1,Citation2] and bacteria[Citation3–Citation5] have been studied, most of them produced unwanted final products. Attentions have been focused on the production of milk-clotting enzymes (MCEs) from plant sources as rennin substitutes and these researches for new potential plant proteases still continues.
Several plant preparations showed a milk-clotting activity (MCA).[Citation6–Citation12] Withania coagulans Dunal (Family: Solanaceae) is known as an Indian cheese maker and commercially important due to milk coagulation by its fruits.[Citation13–Citation17] This plant is a rigid, gray, under-shrub plant with a height of 60–120 cm that grows in the drier parts of Pakistan, Afghanistan, and India, as well as in Southern Iran. Different parts of W. coagulans showed several biological activities.[Citation18,Citation19] However, the MCA is mostly attributed to the fruit pulp and husk and is due to the presence of an enzyme in its fruits which called plant rennin.[Citation20,Citation21] This work was undertaken to elucidate the properties of W. coagulans fruit extracts in order to use it in cheese making as an alternative to rennet. Also, isolation, partial purification and characterization of a milk-clotting protease from this traditional cheese maker plant were the other aims which addressed in this study for the first time.
Methods
Chemicals and plants
Azocasein, gelatin, sodium dodecyl sulfate (SDS), Tris, and Coomassie Brilliant Blue (CBB) R-250 were purchased from Sigma-Aldrich, Germany. Protease inhibitors were purchased from Roche Applied Science, Germany. Acrylamide/bisacrylamide and immobilized pH gradient (IPG) strips were purchased from Bio-Rad, USA. All other chemicals and salts (analytical grade) were obtained from Merck. Other materials were procured locally. Fruits obtained from 5-year-old W. coagulans in Saravan region of Sistan and Baluchestan Province of Iran (GPS coordinate: 62.334167, 27.370833) and confirmed by a professional botanist.
Extract preparation
Fruits and leaves of W. coagulans were dried at 40°C for 24 h. Then, the dried parts were powdered using grinder and crude powder without filtering by any size of meshes were used for extract preparation. Crude extracts were prepared by homogenizing 10 g of powder in 60 mL of 50 mM sodium citrate buffers, pH 4. Mixtures were stored overnight at 4°C and then centrifuged (2500 × g, 15 min, 4°C). Supernatants were collected and filtered through Whatman No. 1 paper. Leaves extract was prepared only for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis and ammonium sulfate (AS) fractionation, while the fruit extract was used for following analyses.
Rennet coagulation time (RCT) assay
The RCT of extract was determined as described elsewhere with some modifications.[Citation22] Briefly, 2 mL of milk solution containing 10% skim milk and 10 mM CaCl2·2H2O in distilled water was heated to the temperature of 37°C for 5 min. Then, 200 μL of enzyme extract was added and the curd formation was observed manually. The end point was recorded when discrete particles were discernible.
Effects of pH, temperature, enzyme, and salts concentration on RCT
RCT was determined at 37°C, at different pH values using the 50 mM buffer solutions in acidic (sodium citrate, pH 4 and 5), neutral (sodium phosphate, pH 6, 6.5, and 7.0), and basic (Tris/HCl, pH 8.0 and 9.0) pH ranges. To determine the effect of enzyme concentration, the crude extract was added in the range of 0.82, 0.98, 1.23, 1.64, and 2.47 mg/mL of reconstituted (10% w/v) skim milk powder (pH 6.5) and incubated at 37°C until milk coagulation occurred. The optimum temperature for RCT was determined by incubating the reaction mixture containing skim milk 10% in deionized water with 10 mM CaCl2 at different temperatures ranging from 35 to 70°C with 5°C intervals. This assay was performed at the pH that determined as optimum. The effects of CaCl2 and NaCl concentrations on RCT were determined using increasing concentrations of the CaCl2 solution (3, 7, 15, 30, 50, or 80 mM) and NaCl solution (0, 30, 50, or 100 mM).
Effects of milk properties and AS fractionation on RCT
The effects of milk temperature at 35, 40, 45, 50, 55, 60, 65, and 70°C in constant pH 6.5, and the effects of milk pH at 5, 5.5, 6, 6.5, and 7 in constant temperature 37°C on RCT were investigated. AS fractionation was performed by earlier researchers as it was reported previously.[Citation23] Briefly, 50 mg AS was added to 1 mL of fruit extract to give a saturation concentration of 20% and incubated on ice for 30 min. This step was repeated several times to obtain a 90% saturation at every additional 10% of AS. Precipitates were collected by centrifugation (15,000 × g, 10 min, 4°C) and suspended in 600 µL of 50 mM citrate, pH 4. The RCT of each fraction was determined according to the previously mentioned procedure.
Effect of protease inhibitors on proteolytic activity
Total and fractional proteolytic activities were assessed by using the spectrophotometric method based on azocasein.[Citation22] Briefly, 20 μL of the enzymatic extracts were added to 500 µL of 1.5% azocasein in 50 mM Tris-HCl buffer, pH 7.5 at 25°C. The reaction was stopped after 15 min by the addition of 500 µL of 20% trichloroacetic acid (TCA) and centrifugation (6500 × g, 6 min, 4°C). The supernatant was removed and its absorbance at 340 nm was assessed. One unit of total proteolytic activity (U) was ΔA340 nm per min per milliliter of extract. The assay included an appropriate blank, in which TCA was added before the substrate. Different specific protease inhibitors including serine protease inhibitor (phenylmethane sulfonyl fluoride (PMSF), 1 mM), metalloprotease inhibitor (Ethylenediaminetetraacetic acid (EDTA) , 10 mM), aspartic protease inhibitor (pepstatin-A, 0.08 mM), and cysteine protease inhibitor (E-64, 0.01 mM) were added to the 200 µL of enzymatic extract. Samples were incubated at 37°C for 30 min and residual milk-clotting and proteolytic activities were determined and compared to the control, which was incubated without the inhibitors and corresponds to 100% of activity.[Citation24]
TCA-acetone precipitation
Tris-HCl extraction was performed according to the Zukas and Breksa method.[Citation25] Briefly, 500 mg of grounded fruits were suspended in 2.5 mL of 50 mM Tris-HCl buffer, pH 8.8, and homogenized on ice. The homogenate was incubated with gentle shaking at 4°C for 24 h, then was centrifuged (28,000 × g, 30 min, 4°C) and the pellet discarded. Three volumes of cold TCA (10%, w/v, –20°C) in acetone were added to the supernatant, and after overnight incubation at –20°C, the precipitate was collected by centrifugation (16,000 × g, 10 min, 4°C). The pellet was suspended in 1 mL of cold 10% TCA in acetone and sonicated for 1 min on ice. The suspension was centrifuged (16,000 × g, 10 min, 4°C) and the supernatant discarded. After two more rinses with cold 10% TCA in acetone and one with acetone, the resulting pellet was air-dried at room temperature.
Partial purification of milk-clotting protease
AS fractionation was done as described previously for preparing 35, 55, and 80% saturation concentration. For desalting and protein concentration, AS precipitates from 30 mL of each extract were dissolved in 1 mL of the dense SDS buffer consisting of 500 mM Tris-HCl, pH 8.3, 30% sucrose, 1% SDS, 1% 2-mercaptoethanol, plus 0.0001% bromophenol blue. Two parts of protein solution and one part of Tris-buffered phenol, pH 8.8, were mixed well. The mixture was centrifuged (10,000 × g, 5 min, 4°C) and then the upper phenol phase was precipitated with five volumes of 100 mM ammonium acetate in methanol at –20ºC for 20 min. Protein pellets recovered by centrifugation (15,000 × g, 10 min, 4°C) were truly washed with cold methanol and acetone and used for RCT assay.
PAGE
Desalted fractions were analyzed by SDS-PAGE in the presence of 2-mercaptoethanol using 12% polyacrylamide gel.[Citation26] Protein content was determined according to Bradford[Citation27] using the Bio-Rad reagent in the microassay mode and bovine serum albumin as a standard. The running gel was a 12 gr/dl polyacrylamide gel in 1.5 M Tris-HCl buffer, pH 8.8, and 10 g/l SDS. The stacking gel contained 3 g/l acrylamide in 500 mM Tris-HCl (pH 6.8) and 5 g/l SDS. Desalted fractions were added to the loading buffer to give a final concentration of 1 mg/mL protein. The migration buffer comprised 25 mM Tris-HCl, 186 mM glycine, and 0.1% SDS, pH 8.3. Electrophoresis was performed at constant 150 V, and the gel was stained by CBB R-250.
Two-dimensional electrophoresis
After AS fractionation and desalting of the fruit extracts, proteins were well-resolved by 2-DE. In the first dimension, protein samples were dissolved by in-gel rehydration at 20°C in 170 μL of a solution containing 7 M urea, 2 M thiourea, 4% w/v CHAPS, 50 mM Dithiothreitol (DTT), 0.5% v/v carrier ampholytes, and 1% w/v bromophenol blue for 7 cm length IPG strips in the linear pH range 3-10 (Bio-Rad, USA). Strips were rehydrated under passive conditions at 20°C for 14–16 h in the protein isoelectric focusing (IEF) cell (Bio-Rad, USA). The focusing was carried out in three steps 250 V for 30 min, followed by a linear voltage ramping step for 2 h at 4000 V. At the final focusing step, the maximum voltage of the ramp step was maintained at up to 20,000 Vh. Ready strips were then stored at –70°C until second dimension processing. Before, the second dimension separation, ready strips were equilibrated in 15 mL of equilibration buffer contain 0.5 M Tris-HCl pH 8.8, 6 M urea, 30% w/v glycerol, 10% w/v SDS, and 2.5% DTT for 15 min and then for 20 min in the same buffer containing 2% iodoacetamide instead of DTT. SDS-PAGE was performed at 50 V for 15 min followed by approximately 45 min at 170 V using 12% acrylamide gels at 4°C. Resolved proteins were detected by staining with CBB R-250 and modified silver nitrate staining. The proteins of interest were excised from the 2D gels and allowed to dry at 4°C in an air shed. Peptides were analyzed using a Matrix-assisted laser desorption/ionization-time of flight/time offlight-mass spectroscopy (MALDI-TOF/TOF-MS) in reflection mode with delayed extraction.[Citation28–Citation30]
Caseinolytic activity
Hydrolyze of caseins was performed according to the method described previously[Citation9] with some modifications. Briefly, commercial bovine αS-, β-, and κ-casein were dissolved as 2 mg/mL in 100 mM potassium phosphate buffer pH 7.5 and incubated independently with crude extract, AS fractions of fruits and commercial clotting rennin at 30ºC. After 60 min, aliquots were taken and the reaction stopped by adding equal volumes of sample loading buffer (500 mM Tris-HCl pH 6.8, 100% glycerol, 10% SDS, 10% bromophenol blue, and 14.4 M 2-mercaptoethanol) and heated at 100ºC for 5 min. Samples were analyzed by SDS-PAGE and proteins were stained with CBB R-250.
Fruit extract zymography
Electrophoresis was performed and gels (1.5 mm thick) for non-reducing SDS-PAGE in the Laemmli discontinuous gel buffer system were prepared by incorporating appropriate volumes of a 1% (w/v) stock solution of substrate protein, gelatin, into the acrylamide solution (the final gelatin concentration in the gel is 1 mg/mL). The stacking and running gels were 4 and 10%, respectively, and protein samples are not treated with 2-mercaptoethanol or boiling before electrophoresis. PAGE was carried out at room temperature and 150 V. After electrophoresis, the gels were washed for 30 min in 100 mL renaturation buffer (2.5% Triton X-100 in 50 mM Tris-HCl, pH 7.4) with gentle shaking to remove the SDS and then for 30 min with the 100 mL of desired developing buffer (containing 30 Mm Tris-HCl, pH 7.4, 200 mM NaCl, 10 mM CaCl2 and 0.02% Brij-35). The gels were then incubated with gentle shaking in the buffer at 37°C for 4 h, followed by staining with CBB R-250 and were discolored until clear bands indicating proteolytic activity became visible.
Statistical analysis
All assays were done triplicate and the results of the three replicates were pooled and expressed as mean and standard deviation (SD). Kruskal-Wallis test and Mann-Whitney-K tests were used for between groups comparison in all quantitative variables and carried out using SPSS version 20 (Chicago, USA) for Windows. Significance difference was accepted at p-value lower than 0.05.
Results and discussion
Coagulation assessments under different conditions
The effects of the pH and temperature of fruit extracts on RCT were shown in . A minimum RCT was observed at pH of 4 with increasing time in the higher studied pH range 5–9. These data demonstrate that the enzymatic activity of W. coagulans decreases with increasing pH value. Therefore, extraction at pH 4 was used for rennin preparation in this study. In further analysis, we found that the main protease in the fruit extracts of W. coagulans is aspartic protease, and the pH optimum of aspartic protease from W. coagulans was detected at 4 which is similar to that of other aspartic proteases.[Citation31] This indicated that this enzyme is an acid aspartic protease. shows that minimum RCT were at 40 and 45°C. Gradually increasing in RCT was seen until 65°C and then a decrease about 70% in activity occurred in 70°C. Therefore, the optimum temperature for the lowest RCT was found to be 40–45°C. Even at higher temperatures (70°C) the enzyme exhibited about 30% of its original activity indicating the thermal stability of the enzyme. The effect of temperature on the RCT was determined after incubating the enzymatic extracts for a reference time of 30 min. The effects of temperature and pH on the crude enzymatic extract of Ficus racemose[Citation32] and Cynara scolymus[Citation9] were studied. F. racemosa extract enzymes exhibited a broad spectrum of pH optima between pH 4.5–6.5 and showed maximum activity at 60 ± 0.5°C. Chazarra et al.[Citation9] by using C. scolymus extracts showed that between 20–60°C, the RCT decreased as temperature increased. They also reported that maximum activity of extract was observed at pH of around 4, with no significant difference in the studied pH range of 3–7.[Citation9]
Table 1. Mean and SD of effect of pH, temperature (°C), enzyme, NaCl, and CaCl2 concentration (mM) on rennet coagulation time (RCT) of fruits extract of Withania coagulans in 24 h of cultivation.
RCT is dependent on the concentration of enzyme and decreasing with increasing enzyme concentration.[Citation9] We used the model for milk clotting that correlated clotting time with enzyme concentration proposed by Payens et al.[Citation33] showed the negative linear correlation between the enzyme concentration and RCT. Our finding is in agreement with those found by Bringe and Kinsella,[Citation34] which reported that the time taken for clotting milk decreased with increase of enzyme concentration, but the formation and firmness of the gel did not alter.
showed that the RCT of extract was increased hyperbolically with increasing concentrations of NaCl and decreased hyperbolically with increasing concentration of CaCl2. Our results demonstrated that adding of NaCl up to 30 mM couldn’t effect on RCT, but RCT was increased in higher concentration. Other researchers reported that the addition of NaCl to milk leads to an increase in calcium and phosphorus concentrations of the ultrafiltrates[Citation35,Citation36] and increased RCT linearly with NaCl increase. This increasing in RCT is due to a decrease in the enzymatic first-order rate constant, kenz.[Citation35] Similar observation was reported by Chazarra et al.[Citation9] that used NaCl as concentrations of 34, 51, or 102 mM. shows that CaCl2 in concentrations between 30–80 mM could efficiently decreased RCT. The addition of divalent cations such as calcium induces important modifications in the salt distribution between aqueous and micellar phases and about 80% of this ion was associated to casein micelles. Therefore, RCT can affect sufficiently.[Citation35] Our finding is similar to those found by Chazarra et al.[Citation9] that reported a concentration 50 mM of CaCl2 was considered sufficient to increase the rennet strength (RS) to an effective level. Other researchers have reported that the addition of CaCl2 to the milk in the concentration range of 0–10 mM increases the overall enzymatic coagulation rate.[Citation37–Citation39]
The effects of milk pH on RCT are showed in . An increase in the pH of the milk was accompanied by an increasing of the RCT, and at pH 7, approximately 88% of the enzyme activity was lost. Chazarra et al.[Citation9] and Hashem[Citation40] also observed that an increase in the pH of the reaction mixture was associated with a gradual loss of MCA, that equal to increasing of RCT. Decreasing in activity at pH 7 found by Chazarra et al was about 87% but Hashem reported that the MCEs of Penicillium oxalicum still possessed 38% of its original activity at pH 7.[Citation9,Citation40] showed a progressive reduction in RCT as temperature increased from 35 to 45°C; however, at high temperatures, up to 65°C, the coagulation process slowed down. After that, due to protein denaturation and insolubility, RCT was increased. It is well-known that temperature affects both the primary (enzymatic hydrolyze) and secondary (aggregation reaction) phase of milk coagulation.[Citation40] Some researchers reported that increasing the temperature from 26 to 40°C reduces the gelation time[Citation37,Citation39] but another author represented this range between 20–60°C.[Citation27] The optimum temperature and pH for enzyme activity of extracted protease from W. coagulans fruit in recently published study were 70°C and 4, respectively. The extracted protease also exhibited an excellent thermal stability at 60°C for 30 min.[Citation20]
Table 2. Effect of milk temperature and milk pH on rennet coagulation time (RCT) of fruits extract of Withania coagulans in 24 h of cultivation.
Enzyme identification
AS fractionation is often used in protein and enzyme purification and here we used a phenol-based method reported by Wang et al.[Citation23] for quick desalting and concentrating proteins after AS fractionation. This method is quick and does not need much technique. The effect of the different AS fractions on RCT and proteolytic activity of each fraction were shown in . Only the fractions which precipitated with 30–50% AS could decrease the RCT. These results indicated that AS fractions in the range of 30–50% had the highest proteolytic activity and lowest RCT. The W. coagulans fruit extract showed a protein content of 2.47 mg/mL and a proteolytic specific activity of 1.06 U/mg. In the range of 30–50% saturation, MCA of partial purified extract was higher than proteolytic activity and this index can be used to justify the adequacy of enzymatic extract to be used as calf rennet substitute.[Citation40]
Table 3. Mean ± SD of rennet coagulation time (RCT) and proteolytic activity of crude extract and AS fractions of fruits from Withania coagulanse.
As demonstrated in , EDTA, PMSF and E-64 showed low effects on the enzyme activity at high concentrations, while pepstatin A, a specific inhibitor of aspartic proteases, showed to completely inhibit the proteolytic activity. These inhibition indicate that the proteolytic activity is mainly due to the presence of aspartic proteases. However, for metalloproteases it was also found a quite low level of inhibition (7%). The other classes of proteases (such as serine and cysteine proteases) seem to be much less active in the fruits of W. coagulans.
Table 4. Effects of different protease inhibitors on rennet coagulation time (RCT) of enzymatic extract of Withania coagulans fruit as mean ± SD.
Casein hydrolysis comparison between rennin with fruit and leaves extracts is shown in . As demonstrated, casein was hydrolyzed completely by rennin and approximately complete by the fruit extract, but no hydrolysis effects were seen for leaves extract. Also, as demonstrated, the pattern of hydrolysis was different between renin and fruit extract indicating that the respected protease in the fruit extract’s hydrolysis the peptide bonds in different site rather than renin. It had been reported that proteases had different levels in different parts of the plants. Some researchers suggested that fruits or seed had the highest amounts of proteases;[Citation20,Citation41] however, others reported this properties for the other parts of the plants.[Citation42,Citation43] In addition, casein hydrolysis by rennin, leaves extract and AS fractions in the range of 10–50% saturation are shown in and . Although rennin could partially hydrolyze casein, the leaf extracts and all AS fractions could not hydrolyze casein. This means that leaf extract, as raw and partially purified, did not have the appropriate proteolytic activity and the fruit showed higher milk clotting activity than the leaves. Therefore, all other complementary assessments were performed on the fruit extracts.
Figure 1. Comparison of casein hydrolysis activity by rennin, crude fruit’s, and leaves extracts of Withania coagulans (A) and rennin, crude leaves extract of W. coagulans and its ammonium sulfate (AS) fractions (B and C). Different types casein hydrolysis include αs-casein hydrolysis activity by rennin, crude fruit’s extract of W. coagulans and its AS fractions (D and E); β-casein hydrolysis activity by rennin, crude fruit’s extract of W. coagulans and its AS fractions (F) and κ-casein hydrolysis activity by rennin, crude fruit’s extract of W. coagulans and its AS fractions (G, H) are also show. SDS-PAGE (12%) was run and gel was stained with Coomassiee Briliant Blue R-250. C: casein; R: rennin; FE: fruit’s extract; LE: leaves extract.
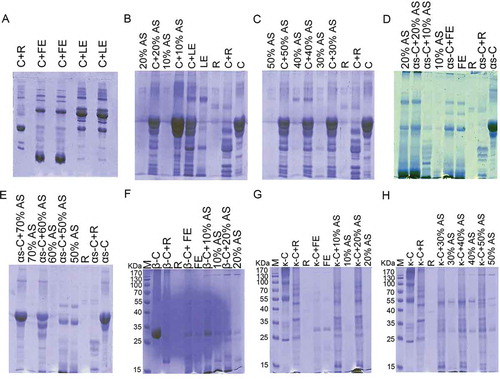
Hydrolysis effects of rennin, fruit extracts and its AS fractions on the each type of commercial bovine caseins included αS-, β-, and κ-caseins were performed. Rennin could cause complete hydrolysis of αS-casien; however, the pattern of hydrolysis was different from fruit extracts and its AS fractions. Fruit extract and 10, 20, and 50% AS fractions could hydrolyzed the αS-casien completely, 60% AS fractions had partially hydrolysis and 70% showed no αs-casein hydrolysis effect. Also, the patterns of αs-casien hydrolysis between AS fractions were different which confirmed of the existence of different proteases ( and ). Renin, fruit extract, and both 10 and 20% AS fractions of fruit extract could hydrolyzed with the β-casein completely with similar pattern of hydrolysis (). Finally, by using κ-casein, it had been demonstrated that fruit extract and all 10–50% AS fractions could hydrolyze this type of casein completely by a different pattern of hydrolysis against renin. The pattern of hydrolysis between fruit extract and its AS fraction also were different ( and ). These results provided evidence of rennin-like activity associated with fruit extract and it’s AS fractions of W. coagulans which may serve as a promising source of vegetable milk coagulant. In another part of this study, two-dimensional electrophoresis plus zymography were performed for evaluating and identification of the respected protease.
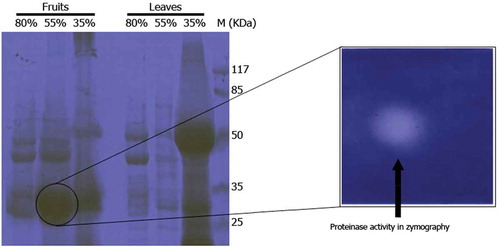
As demonstrated in , and according to the previously mentioned experiments, spots in the Mw of 27-35KDa could be the potential responsible of milk coagulation in A: 30%, B: 40%, and C: 50% AS fractions of fruit extracts. Four spots from all AS fractions of fruit extract with Mw of about 35 KDa (quadrangle area in 50% AS fraction) were extracted from the gel and analyzed by MALDI-TOF/TOF.
Figure 2. Two-dimensional electrophoresis pattern of A: 30%, B: 40%, and C: 50% ammonium sulfate (AS) fractions of fruit extracts of Withania coagulans. Proteins were separated by isoelectric focusing on IPG 3-10 NL as first dimension and then by 12% T SDS-PAGE in second dimension. Coomassiee Briliant BlueR-250 and silver nitrate staining were used for each %AS fraction.
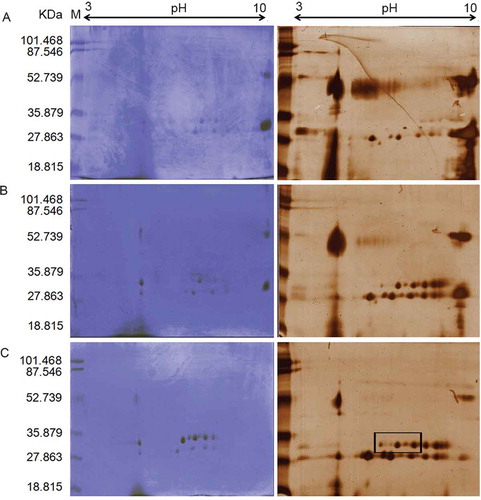
Our results demonstrated that these proteins had similar sequences with aspartate proteinase of Vigna unguiculata (cowpea) with Mw of 55365 Da and pI of 5.91. Also, gelatin zymographic evaluation showed high proteinase activity in only the 55% AS fraction of fruit extract of W. coagulans (). There are neither previous reports about the effects of fruit extract and its AS fractions of W. coagulans on caseins hydrolysis, nor protease identification by 2-DE and zymography. In a recent study conducted by Beigomi et al., a protease from W. coagulans fruits was evaluated for milk-clotting properties. AS fractionation showed that the 40–50% fraction possessed the highest MCA and showed an Mw band of 66 kDa by SDS-PAGE. Although, their study demonstrated that W. coagulans fruits have potential for use as a rennet substitute, but the nature of respected protease(s) was not identified.[Citation20] Other studies also reported that the extract of this plant can be used in cheese making,[Citation15–Citation17] but further investigation about the nature of the related enzymes were not performed. Aspartic proteases with MCA have been reported from C. scolymus L.,[Citation44] Silybum marianum L. Gaertn.,[Citation11] Onopordum turcicum,[Citation45] rice kernels,[Citation46] Centaurea calcitrapa,[Citation47] and Cynara cardunculus.[Citation48] As the first preliminary study, we identified that W. coagulans had an aspartic protease (Mw and pI about 35 KDa and 5.9) which had similar sequences and pI to that extracted from cowpea[Citation49] as seen in National Center for Biotechnology Information (NCBI) database.
Conclusion
In this study it is shown that the extracts obtained from the fruits of W. coagulans contain proteolytic and MCA. On the basis of the results that obtained from inhibition assay, the presence of aspartic proteases and with lower degree metalloproteases were confirmed. The moderate clotting activity with high temperature resistance found in the extracts of the fruits of W. coagulans could be useful in the dairy industry for milk clotting, as an in alternative or in addition to calf rennet. However, understanding the influence of the W. coagulans extract specificity on casein degradation need future studies. However, our study suffered from two limitations; first, lack of any other purification methods, such as chromatography; and second, low detection sensitivity of CBBR-250 staining which used in 1- and 2-DE. Therefore, performing of similar studies which resolving these limitations and also evaluate the kinetic parameters of this enzyme by measured the kinetics of hydrolysis of κ-casein under different conditions are highly recommended.
Acknowledgments
This study was conducted at the Research Center of Medicinal and Ornamental Plants, University of Zahedan, Iran. The authors wish to thank Dr. DM Kordi Tamandani for their kindly help and scientific cooperation.
References
- Kim, S.Y.; Gunasekaran, S.; Olson, N.F. Combined Use of Chymosin and Protease from Cryphonectria Parasitica for Control of Meltability and Firmness of Cheddar Cheese. Journal of Dairy Science 2004, 87, 274–283.
- Zhang, Z.; Wang, C.; Yao, Z.; Zhao, J.; Lu, F.; Yu, G.; Lan, W.; Lu, Z. Isolation and Identification of a Fungal Strain QY229 Producing Milk-Clotting Enzyme. European Food Research and Technology 2011, 232, 861–866.
- Shieh, C.J.; Phan Thi, L.A.; Shih, I.L. Milk-Clotting Enzymes Produced by Culture of Bacillus Subtilis Natto. Biochemical Engineering Journal 2009, 43, 85–91.
- Shih, I.L.; Van, Y.T.; Chang, Y.N. Application of Statistical Experimental Methods to Optimize Production of Poly (γ-Glutamic Acid) by Bacillus Licheniformis CCRC 12826. Enzyme and Microbial Technology 2002, 31, 213–220.
- Shih, I.L.; Yu, Y.T.; Shieh, C.J.; Hsieh, C.Y. Selective Production and Characterization of Levan by Bacillus Subtilis (Natto) Takahashi. Journal of Agricultural and Food Chemistry 2005, 53, 8211–8215.
- Aworh, O.C.; Muller, H.G. Cheese-Making Properties of Vegetable Rennet from Sodom Apple (Calotropis Procera). Food Chemistry 1987, 26, 71–79.
- Aworh, O.C.; Nakai, S. Extraction of Milk Clotting Enzyme from Sodom Apple (Calotropis Procera). Journal of Food Science 1986, 51, 1569–1570.
- Bruno, M.A.; Lazza, C.M.; Errasti, M.E.; López, L.M.I.; Caffini, N.O.; Pardo, M.F. Milk Clotting and Proteolytic Activity of an Enzyme Preparation from Bromelia hieronymi fruits. LWT–Food Science and Technology 2010, 43, 695–701.
- Chazarra, S.; Sidrach, L.; López-Molina, D.; Rodríguez-López, J.N. Characterization of the Milk-Clotting Properties of Extracts from Artichoke (Cynara Scolymus, L.) Flowers. International Dairy Journal 2007, 17, 1393–1400.
- Gupta, C.B.; Eskin, N.A.M. Potential Use of Vegetable Rennet (from Ficus Carica, Ricinus Communis, Benincasa Cerifera) in the Production of Cheese. Food Technology 1977, 5, 62–66.
- Vairo-Cavalli, S.; Claver, S.; Priolo, N.; Natalucci, C.L. Extraction and Partial Characterization of a Coagulant Preparation from Silybum Marianum Flowers. Its Action on Bovine Caseinate. Journal of Dairy Science 2005, 72, 271–275.
- Yamaguchi, T.; Yamashita, Y.; Takeda, I.; Kiso, H. Proteolytic Enzymes in Green Asparagus, Kiwi Fruit and Miut: Occurrence and Partial Characterization. Agricultural and Biological Chemistry 1982, 46, 1983–1986.
- Ali, N.; Ahmad, B.; Bashir, S.; Shah, J.; Azam, S.; Ahmad, M. Calcium Channel Blocking Activities of Withania Coagulans. African Journal of Pharmacy and Pharmacology 2009, 3, 439–442.
- Hemalatha, S.; Kumar, R.; Kumar, M. Withania Coagulans Dunal: A Review. Pharmacognosy Reviews 2008, 2, 351–358.
- Shehla, N.; Masud, T.; Malik A.N. Characterization of Milk Coagulating Properties from The Extract of Withania Coagulans. International Journal of Dairy Technology 2009, 62, 315–320.
- Nawaz, M.A.; Masud, T.; Shehla, S. Quality Evaluation of Mozzarella Cheese Made from Buffalo Milk by Using Paneer Booti (Withania Coagulans) and Calf Rennet. International Journal of Dairy Technology 2011, 64, 218–226.
- Khan, R.S.; Masud, T. Comparison of Buffalo Cottage Cheese Made from Aqueous Extract of Withania Coagulans with Commercial Calf Rennet. International Journal of Dairy Technology 2013, 66, 396–401.
- Budhiraja, R.D.; Sudhir, S. Review of Biological Activity of Withanolides. Journal of Scientific & Industrial Research 1987, 46, 400–408.
- Iqbal Choudhary, M.; Parveen, Z.; Jabbar, A.; Ali, I. Antifungal Steroidal Lactones from Withania Coagulance. Phytochemistry 1995, 40, 1243–1246.
- Beigomi, M.; Mohammadifar, M.A.; Hashemi, M.; Senthil, K.; Valizadeh, M. Biochemical and Rheological Characterization of a Protease from Fruits of Withania Coagulans with a Milk-Clotting Activity. Food Science and Biotechnology 2014, 23, 1805–1813.
- Jain, R.; Kachhwaha, S; Kothari, S.L. Phytochemistry, Pharmacology, and Biotechnology Of Withania Somnifera and Withania Coagulans: A Review. Journal of Medicinal Plants Research 2012, 6, 5388–5399.
- D’ambrosio, A.; Rossano, R.; Ungaro, N.; Riccio, P. Proteolytic and Milk Clotting Activities in Extracts Obtained from the Crustaceans Munida. Journal of Molecular Catalysis B: Enzymatic 2003, 22, 145–150.
- Wang, W.; Liu, Q.-J.; Cui, H. Rapid Desalting and Protein Recovery with Phenol After Ammonium Sulfate Fractionation. Electrophoresis 2007, 28, 2358–2360.
- Merheb-Dini, C.; Gomes, E.; Boscolo, M.; da Silva, R. Production and Characterization of a Milk-Clotting Protease in the Crude Enzymatic Extract from the Newly Isolated Thermomucor Indicae-Seudaticae N31:(Milk-Clotting Protease from the Newly Isolated Thermomucor Indicae-Seudaticae N31). Food Chemistry 2010, 120, 87–93.
- Zukas, A.A.; Breksa, A.P. Extraction Methods for Analysis of Citrus Leaf Proteins by Two-Dimensional Gel Electrophoresis. Journal of Chromatography A 2005, 1078, 201–205.
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685.
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry 1976, 72, 248–254.
- Kazemipour, N.; Qazizadeh, H.; Sepehrimanesh, M.; Salimi, S. Biomarkers Identified from Serum Proteomic Analysis for the Differential Diagnosis of Systemic Lupus Erythematosus. Lupus 2015, 24, 582–587.
- Fattahi, S.; Kazemipour, N.; Hashemi, M.; Sepehrimanesh, M. Alpha-1 Antitrypsin, Retinol Binding Protein and Keratin 10 Alterations in Patients with Psoriasis Vulgaris, a Proteomic Approach. Iranian Journal of Basic Medical Science 2014, 17, 651–655.
- Sepehrimanesh, M.; Kazemipour, N.; Saeb, M.; Nazifi, S. Analysis of Rat Testicular Proteome Following 30-Day Exposure to 900 Mhz Electromagnetic Field Radiation. Electrophoresis 2014, 35, 3331–3338.
- Salvador, S.M.; Novo, C.; Domingos, A. Evaluation of the Presence of Aspartic Proteases from Centaurea Calcitrapa During Seed Germination. Enzyme and Microbial Technology 2006, 38, 893–898.
- Devaraj, K.B.; Gowda, L.R.; Prakash, V. An Unusual Thermostable Aspartic Protease from the Latex of Ficus Racemosa (L.). Phytochemistry 2008, 69, 647–655.
- Payens, T.A.J.; Wiersma, A.K.; Brinkhuis, J. On Enzymatic Clotting Processes I. Kinetics of Enzyme-Triggered Coagulation Reactions. Biophysical Chemistry 1977, 6, 253–261.
- Bringe, N.A.; Kinsella, J.E. Use of Platelet Aggregometer to Monitor the Chymosin-Initiated Coagulation of Casein Micelles. Journal of Dairy Science 1986, 53, 359–370.
- Famelart, M.H.; Le Graet, Y.; Raulot, K. Casein Micelle Dispersions into Water, NaCl and CaCl2: Physicochemical Characteristics of Micelles and Rennet Coagulation. International Dairy Journal 1999, 9, 293–297.
- Gaucheron, F. The Minerals of Milk. Reproduction Nutrition Development 2005, 45, 473–484.
- Bencini, R. Factors Affecting the Clotting Properties of Sheep Milk. Journal of the Science of Food and Agriculture 2002, 82, 705–719.
- Lagaude, A.; Fernandez, L.; Cuq, J.L.; Marchesseau, S. Characterization of Curd Formation During the Rennet Coagulation of Milk by an Optical Microscopic Method. International Dairy Journal 2004, 14, 1033–1039.
- Nájera, A.I.; De Renobales, M.; Barron, L.J.R. Effects of pH, Temperature, CaCl2 and Enzyme Concentrations on the Rennet-Clotting Properties of Milk: A Multifactorial Study. Food Chemistry 2003, 80, 345–352.
- Hashem, A.M. Purification and Properties of a Milk-Clotting Enzyme Produced by Penicillium Oxalicum. Bioresource Technology 2000, 75, 219–222.
- Tajalsir, A.E.; Ebraheem, A.S.A.; Abdallah, A.M.; Khider, F.J.; Elsamani, M.O.; Ahmed, I.A.M. Partial Purification of Milk-Clotting Enzyme from the Seeds of Moringa Oleifera. Journal of Microbiology, Biotechnology and Food Sciences 2014, 4, 58–62.
- Oseni, O.A.; Ekperigin, M.M. Distribution of Proteolytic and Milk Clotting Enzymes in the Plant of Sodom Apple Calotropis Procera (Ait.) R.Br. (Asclepiadaceae). International Journal of Biotechnology and Molecular Biology Research 2013, 1, 24–27.
- Yadav, R.P.; Patel, A.K.; Jagannadham, M.V. Purification and Biochemical Characterization of a Chymotrypsin-Like Serine Protease from Euphorbia Neriifolia Linn. Process Biochemistry 2011, 46, 1654–1662.
- Llorente, B.E.; Bruiti, C.B.; Natalucci, C.L. Partial Characterization of a Milk Clotting Proteinase Isolated from Artichoke (Cynara Scolymus L., Asteraceae). Acta Farmacéutica Bonaerense 1997, 16, 37–42.
- Tamer, I.M. Identification and Partial Purification of a Novel Milk Clotting Enzyme from Onopordum Turcicum. Biotechnology Letters 1993, 15, 427–432.
- Asakura, T.; Watanabe, H.; Abe, K.; Arai, S. Oryzasin as an Aspartic Proteinase Occurring in Rice Seeds: Purification, Characterization, and Application to Milk Clotting. Journal of Agricultural and Food Chemistry 1997, 45, 1070–1075.
- Domingos, A.; Cardoso, P.C.; Xue, Z.; Clemente, A.; Brodelius, P.E.; Pais, M.S. Purification, Cloning and Autoproteolytic Processing of an Aspartic Proteinase from Centaurea Calcitrapa. European Journal of Biochemistry 2000, 267, 6824–6831.
- Shah, M.A.; Mir, S.A.; Paray, M.A. Plant Proteases as Milk-Clotting Enzymes in Cheesemaking: A Review. Dairy Science & Technology 2014, 94, 5–16.
- Cruz de Carvalho, M.H.; d’Arcy-Lameta, A.; Roy-Macauley, H.; Gareil, M.; El Maarouf, H.; Pham-Thi, A.T.; Zuily-Fodil, Y. Aspartic Protease in Leaves of Common Bean (Phaseolus Vulgaris L.) and Cowpea (Vigna Unguiculata L. Walp): Enzymatic Activity, Gene Expression and Relation to Drought Susceptibility. FEBS Letters 2001, 492, 242–246.