ABSTRACT
The phenolic profiles of six varieties of avocado (Persea americana) grown in Sicily were investigated. The ultra-high-performance liquid chromatography-heated electrospray-mass spectrometry method was developed to determine qualitative and quantitative changes in fruits at two different ripening stage. Nineteen individual phenolic compounds were detected in avocado pulp extracts. Gallic acid, sinapinic acid, vanillin, p-coumaric acid, and gentisic acid were present only in ripe fruits. On the contrary, epicatechin decreased with fruit ripening, whereas protocatechuic acid, 4-hydroxybenzoic acid, chlorogenic acid, and benzoic acid were relatively stable or exhibited erratic changes with fruit ripening. The different avocado cultivars exhibited different phenolic profiles and total quantities and it was found that, among the tested cultivars, “Orotawa” avocados may provide the highest nutritional and health contribution to human diet. The qualitative and quantitative differences among cultivars and maturation stages by multivariate analysis allowed for the individuation of a set of phenolic compounds that have a great potential in the discrimination and identification of different genetic groups.
Introduction
The diet of the Western world has become more and more rich and varied, for the introduction of different nature and origin foods. Nonetheless, the process of food preservation, the intensive cultivation, the indiscriminate use of growth factors lead to the decrease in the overall food quality and to the need of controls on food to protect consumer health. In the last few years, many studies have been performed to characterize or quantify each class of substances in various edible matrices such as fish, meat, vegetables, and their derivatives.[Citation1–Citation6] Several different approaches have been developed to fight the frauds and they include both panel tests and analytical techniques.[Citation7] These analytical methods can be used to analyze either the minor components or the principal components of foods.[Citation8]
It is worth mentioning that foods intrinsically rich in nutraceuticals, vitamins, and tocopherols integrated in the diet may lead to an improvement of several biochemical parameters correlated to the well-being of the individuals.[Citation9] On the other hand, the commercial success of some foods is frequently linked to the ability to highlight nutritional characteristics that enable their preferential marketing. Recently, several articles have tried to obtain a fine characterization of many foods such as oils, wines, cheeses, meats, and fruit.[Citation1] Furthermore, the medical literature frequently recommend consumption of fruits with high bioactivity, because only such fruits are effective in the prevention and treatment of various diseases. Avocado (Persea americana Mill.) is a tropical and subtropical tree. Although avocados are native to southern Mexico, currently, they are grown in places as far from America as Australia, South Africa, or Spain.[Citation10]
The fruit has long been used as a healthy food and recent researches have shown that avocado can improve hypercholesterolemia and be useful in the treatment of hypertension, in type 2 diabetes mellitus, hypertension, and dyslipidemia and can play an important role in cardiovascular health.[Citation11,Citation12] Avocado tissues are interesting natural sources of rich-phenolic extracts with high antioxidant and antimicrobial potential.[Citation13] The lipophilic extract of avocado inhibits prostate cancer cell growth,[Citation14] induces apoptosis in human breast cancer cells,[Citation15] and suppresses liver injury.[16] The avocado fruit is also a very important natural source of monounsaturated food lipids and essential fatty acids (linoleic and linolenic acid).[Citation17] The fruits have a long harvesting period depending on cultivar and the estimation/identification of physiological and commercial maturity is difficult due to externally undetectable changes. A unique feature of all varieties of avocado is that the fruits mature on the tree and ripen after harvest. The ripening process takes 5–7 days at room temperature. Fruits are ripe when they yield to gentle pressure.[Citation18]
Considering that phenolic composition of avocado fruits is assumed to differ due to the difference in cultivation environment and varieties, the aim of this study was to investigate the changes of phenolic bioactive compounds profile before and after ripening of six avocado varieties (“Hass”, “Bacon,” “Fuerte,” “Pinkerton,” “Rincon,” and “Orotawa”) grown in a collection of the Department of Agricultural and Forest Sciences, University of Palermo, in Sicily.
In the present work, liquid chromatography quadrupole Orbitrap mass spectrometer (Q Exactive) was used to identify and quantify phenolic compounds. The quantitative data were further submitted to linear discriminant analysis (LDA), a multivariate exploratory technique that have been successfully used in previous works,[Citation19,Citation20] in order to best evaluate differences or affinity among varieties and between ripening stages of the sampled fruits.
Materials and methods
Chemicals and standards
Phenolic standards: protocatechuic acid, gallic acid, gentisic acid, 4-hydroxybenzoic acid, vanillic acid, chlorogenic acid, caffeic acid, homovanillic acid, syringic acid, epicatechin, vanillin, p-coumaric acid, ferulic acid, sinapinic acid, 3-hydroxycinnamic acid, rutin, taxifolin, benzoic acid, narirutin, naringin, myricetin, isorhamnetin, neohesperidin, quercetin, luteolin, trans-cinnamic acid, poncirin, naringenin, apigenin, kaempferol, galangin, chrysin were purchased from Sigma-Aldrich (Steinheim, Germany). All solvents and other chemicals were of analytical grade purity and were supplied from Merck (Darmstadt, Germany). Methanol, acetonitrile and acetone (liquid chromatography-mass spectrometry [LC-MS] grade) were purchased from Biosolve B.V. (Valkenswaard, The Netherlands). Acetic acid (100%), formic acid (98–100%) were purchased from VWR International B.V. (Roden, The Netherlands);
Plant material and extraction of phenolic compounds
Fruits of six varieties (“Hass,” “Bacon,” “Fuerte,” “Pinkerton,” “Rincon,” and “Orotawa”) were collected at two different ripening stages (unripe: just harvested; ripe: ready for consumption) from adult trees. All the varieties were grown under identical environmental conditions (soil, rain, light, etc.) and cultural management. The samples used in this study were part of a collection of the Department of Agricultural and Forest Sciences, Palermo, Sicily. Extracts were prepared according to Hurtado-Fernández et al.[Citation21] Briefly, for each varieties and for each ripening stage, the pulp of three fruits was homogenized, and samples of 10 g were mixed with 40 mL of pure methanol. The mixtures were stirred for 30 min at ambient temperature, transferred in centrifuge tubes and finally centrifuged at 4500 rpm for 10 min; subsequently, supernatants were filtered, evaporated to dryness and kept at –18°C for further analysis. This procedure gave 36 independent extracts that were submitted to further analysis.
Identification and quantification of phenolic compounds
Ultra-high-performance liquid chromatography (UHPLC) was conducted using a Dionex Ultimate 3000 System equipped with an autosampler controlled by Chromeleon 7.2 Software (Thermo Fisher Scientific, Bremen, DE and Dionex Softron GmbH, Germering, DE). A UHPLC column (Phenomenex Luna C18[2] 50 × 1 mm, 2.5 μ) was used. A flow rate of 50 µL min−Citation1 was set for separation of the selected compounds. The best separation was achieved using eluent A, water containing 0.1% acetic acid (v/v) pH 3.2, and eluent B, acetonitrile. The gradient elution program was: 0–2 min 5% B; 2–4.5 min linear increase to 10% B; 4.5–16 min linear increase to 25% B; 16–29 min linear increase to 95% B, 29–31 min hold 95% B; 31–31.5 min back to 5% B; and 31.5–40 min hold 5% B coming back to the initial conditions and being equilibrated. The column temperature was set at 35°C and the injection volume at 1 μL. Heated electrospray ion source (HESI)was used for the ionization. The HESI parameters were optimized as follows: sheath gas flow rate 30 arbitrary units; auxiliary gas unit flow rate 10 arbitrary units; capillary temperature 250°C; auxiliary gas heater temperature 150°C; spray voltage 2.8 kV; and S lens RF level 50.
MS parameters and quantification approach
Negative mode for the MS method was selected since previous works indicate that this mode is the best for analysis of low-molecular phenolic compounds.[Citation1] Detection of the compounds was performed using a quadrupole Orbitrap mass spectrometer (Q Exactive; Thermo Scientific, Germany). Full scan data were acquired in negative ion mode at a resolving power of 17,500 Full Width Half Maximum (FWHM) (at m/z 200). For the compounds of interest, a scan range of 100–900 m/z was chosen. The automatic gain control (AGC) target for a maximum capacity in C-trap was set at 1e6 ions for a maximum injection time of 200 ms. The scan-rate was set at 2 scans s−Citation1.
In addition to the full scan acquisition method, to enhance sensitivity, a targeted Singol Ion Monitoring (SIM) analysis was performed using the mass inclusion list and expected retention times of the target analytes, with a 15 s time window. The Orbitrap spectrometer was operated in negative mode at 17,500 FWHM (at m/z 200). The precursor ions are filtered by the quadrupole with an isolation window of 0.4 m/z. A maximum injection time of 200 ms was set. Collision energy was optimized by injecting working mix standard solution at a concentration of 40 μg mL−Citation1. For the quantitation of phenol compounds an external calibration procedure was performed. The SIM traces (chromatograms) of several compounds being analyzed, are reported in , for reader convenience.
Figure 1. SIM traces of several compounds found in avocado samples, gallic acid and 4-idrossibenzoic acids have lower retention times (7.48 and 4.72 min, respectively), are not shown.
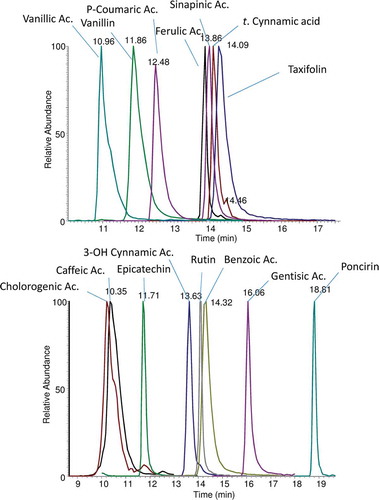
Detection was based on calculated exact mass and on retention time of target compounds, presented in . Data were evaluated by the Quan/Qual Browser Xcalibur 3.0 (Thermo Fisher Scientific San Jose, CA, USA). The mass tolerance window was set to 5 ppm for the two analysis modes. Linearity of the MS response was verified with solutions containing all standards at six different concentration levels over the range from 0.250 or 5 ppm. Each point of the calibration graph corresponded to the average of five independent injections.
Table 1. Formula, retention time and exact mass of studied compounds in avocado fruits analyzed by UHPLC-HESI-MS.
Limits of detection (LODs) and limits of quantification (LOQs) for each individual compound of the standard solution were estimated through the regression curve and the blank signal collected (averaging five blanks injected between standards) at the same time window of the individual substance elution. The LOD concentration was calculated as the concentration giving a signal corresponding to the sum of the blank signal and three time its standard deviation, LOQ concentration was calculated as the concentration giving a signal corresponding to the sum of the blank signal and eight time its standard deviation ().
Table 2. Concentration (mg analyte/kg fresh sample) of phenolic compounds in the pulp of unripe (green) and ripe fruit from six avocado cultivars.
Standard solutions
Standard stock solutions were prepared individually at a concentration of approximately 0.1 mg/mL by dissolving approximately 10 mg of each standard in 20 mL of 70:30 MeOH/H2O (v/v). A STD mix solution at 5 ppm was prepared mixing 1 mL of each individual standard solution in a 100 mL volumetric flask and diluting with methanol up to the mark. The other diluted solutions (at 2.5, 1.0, 0.5, 0.25 ppm) were prepared by dilution of the STD mix. All solutions were corrected for purity and no internal standard was used in this study. Calibration curves were constructed by injecting each standard mix solution at each concentration level in quadruplicate. The peak areas were calculated and plotted against the corresponding concentrations of the standard compounds using linear regression (least squares) to generate standard curves ().
Data analysis
Data of total phenolic concentrations were analyzed by two-factor analysis of variance using cultivar and maturation stage as main factors and cultivar × maturation stage as the sole interaction. Tukey’s honestly significant difference (HSD) at P = 0.05 was used to separate means. Multivariate LDA was performed using concentrations of individual phenolic compounds to attempt classification of treatment groups and individuate the set of compounds that would allow for discrimination of cultivars and/or maturation stages. Complete group discrimination (no miss-classified cases) was obtained with forward stepwise procedures at a P-to-enter threshold of 0.05. The multivariate analysis was validated through an external validation process using the “leave one out” criterion. All tests were conducted using procedures of the Statistica 7 software package (StatSoft Inc., Tulsa, OK, USA).
Results and discussion
It is well-known that genetic and environmental factors could play important roles in the composition and content of phenolic compounds and significant varietal difference in phenolic contents were found. Of the 33 phenolic compounds investigated () only 19 were quantified in the methanolic extracts of avocado pulp (). The amount of flavonoid compounds detected was less than the LOD. Substantial variation in metabolic composition of avocado extracts was observed. Some compounds were detected in all the cultivars under study, whereas other compounds were specific of a particular cultivar or ripening stage. For example, among the compounds studied, gentisic acid, 4-hydroxybenzoic acid, chlorogenic acid, and epicatechin were present in nearly all cultivars and ripening stages, whereas p-coumaric and ferulic acids (hydroxycinnamic acids) were only present in ripe fruits of all cultivars but not in “Bacon” (). Gallic acid, sinapinic acid and vanilin were also present only in ripe fruits of three out of six cultivars. A similar increasing trend with fruit ripening was shown also for gentisic acid suggesting that all these compounds are synthesized only after harvest. On the contrary, epicatechin exhibited a decreasing trend with fruit ripening, whereas protocatechuic acid, 4-hydroxybenzoic acid, chlorogenic acid, and benzoic acid were relatively stable or exhibited erratic changes with fruit ripening.
Among the investigated compounds, flavonoids were not observed in concentrations worthy of mention. Many of them like myricetin, luteolin, apigenin, kaempferol chrysin, and galangin were not found at all (). Other flavonoids like naringin, neohesperidin, and quercetin were present only in traces and they could not be quantified. Only narirutin and poncirin were significantly detected in unripe fruits of “Pinkerton.”
Among cultivars and considering ripe fruits, “Hass” and “Fuerte” had the most wide phenolic profile (11 compounds) followed by “Orotawa” (nine compounds), whereas in “Bacon” and “Rincon” only five compounds were detected. In terms of concentration, epicatechin and p-coumaric acid were the most abundant compounds followed by cholorogenic and benzoic acids.
Indeed, total phenols showed a generalized increase with fruit maturation, but with different intensity depending on the cultivars (). Specifically, “Rincon” exhibited the sharpest increase (4.3-fold) from unripe to ripe fruit followed by “Fuerte” (3-fold), “Orotawa” (1.9-fold), and “Bacon” (1.6-fold). No increase was detected in Hass, and a 60% decrease was found in “Pinkerton.”
Table 3. Total phenolic compounds (mg/kg of analyte in fresh sample) in green and ripe fruit of the six cultivars in the trial.
In unripe fruit, “Pinkerton” exhibited the greatest concentration of total phenols followed by “Orotawa” and “Hass,” whereas “Rincon” and “Bacon” exhibited the smallest concentration of total phenols. In ripe fruit, “Orotawa” exhibited the greatest concentration of total phenols followed by “Fuerte” and “Pinkerton,” while “Bacon” exhibited the smallest concentration of total phenols (). From a general health standpoint, the fruit of “Orotawa” seems to represent the best edible option.
In previous studies, flavonoids like naringenin, kaemferol, rutin, taxifolin, benzoic acid, narirutin, naringin, myricetin, isorhamnetin, neohesperidin, luteolin, poncirin, naringenin, apigenin, kaempferol, galangin, and chrysin were found in very few samples.[Citation21–Citation23] In our case, the presence of some of these flavonoids was detected in ripe “Hass” and unripe “Pinkerton” fruits. The absolute concentrations found in our samples are somewhat different from the data previously reported, and this could be due to several reasons. First, the analytical approach used to determine biophenols. Indeed, the most widely used approach is the determination of total polyphenol content with the Folin-Ciocalteu method.[Citation13] There are also few studies reporting individual polyphenol amounts using methods different from ours, and in particular gas chromatography (GC) with two “detectors” (Flame Ionization Detector (FID) and Atmospheric Pressure Chemical Ionization / Mass Spectrometry (APCI/MS)),[Citation22] and capillary electrophoresis/MS.[Citation23] Discrepancies between our results and previously published data could also be due to genetic (different avocado cultivars), environmental (different geographic locations), and cultural (different tree/soil management) conditions.
Looking at the common varieties analyzed (Hass and Pinkerton) some comparison of compunds concentration can be made with the results obtained by Hurtado-Fernandenz[Citation21] taking into consideration an average water content of the avocado pulp about 80%. Taking in consideration the Hass variety a good agreement between the found concentration of caffeic acid, chlorogenic acid, ferulic acid, gallic acid, epicathechin, p-coumaric acid, and vanillin. The concentration of benzoic acid is higher in our determination (about 5-fold) while for epicathechin an inverse trend between ripe and uniripe fruit content has been found.
As concern the “Pinkerton” variety again some correlation has been found for: p-coumaric acid, caffeic acid, ferulic acid, gallic acid, epicathechin, and vanillin. The concentration of benzoic acid is lower (n.d.) in our determination while for epicathechin a lower value (5-fold) in our ripe fruit has been found. Discrepancies between our results and previously published data could also be due to genetic (different avocado cultivars), environmental (different geographic locations), and cultural (different tree/soil management) conditions.
Multivariate analysis
The multivariate models used in this study proved to be useful for the discrimination of cultivars and maturation stages based on phenol profiles and respective amounts. LDA on the 19 fruit phenolic compounds included in the analysis was able to completely separate the six cultivars (). Specifically, both Mahalanobis distances () and the canonical score plot show that “Rincon” and “Pinkerton” tend to form the most distant groups with the rest of the cultivars in between them. The high F-values and p-values below 0.01 (representing a 99% confidence level) indicate a high significance of the prediction model (). The canonical discriminant functions after a stepwise analysis included 4-hydroxybenzoic acid, benzoic acid, chlorogenic acid, ferulic acid, narirutin, p-coumaric acid, and vanillin. In other words, a linear combination of those seven compounds allows for full discrimination of the six cultivars.
Table 4a. Mahalanobis squared distance between cultivar pairs from linear discriminant analysis.
Table 4b. Tests of significance of squared Mahalanobis f-tests with 7 and 18 degrees of freedom of the variety separation model, independently from the ripening stage.
Figure 2. Avocado discrimination by variety: A: 3D separation using three independent root; and B: root 1 and root 3 separation in a 2D graph.
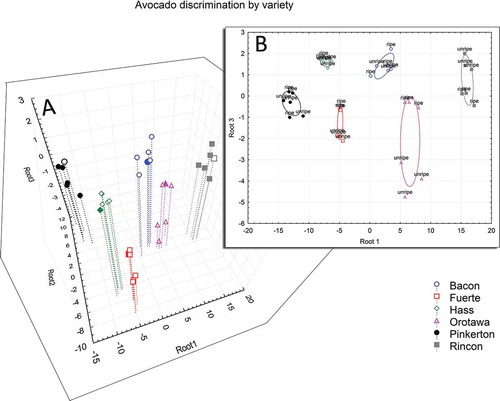
With a similar procedure it was possible to discriminate the two groups of fruit based on maturation stage, showing an average Mahalanobis distance of 118 between unripe (green) and ripe fruit. In this case, the canonical discriminant function after a stepwise analysis included just three variables: ferulic acid, gallic acid, and p-coumaric acid. Both the models were created using n-1 samples, leaving out one sample per cultivar to verify the reliability of the model created. In both cultivar and ripening discrimination, a full reliability of the model was obtained with 100% correct assignment of “in and out of the model” samples. The results of the latter statistical model are in agreement with previously published reports,[Citation21–Citation23] where p-coumaric acid and ferulic acid increased remarkably in ripe samples. Several authors have explained that these two metabolites are related to browning reactions of fruits and have observed similar trends in other fruits during ripening.
Conclusion
The results of this study indicate that the avocado fruit is an important source of phenolic compounds, especially at the ripe stage, that represent the stage at which the fruit is commonly consumed. We also found that the concentration of some metabolites (particularly gentisic and p-coumaric acid) shows a major increase during ripening. It has also been shown that different avocado cultivars present different phenolic profiles and total quantities and has been found that, among the tested cultivars, “Orotawa” avocados may provide the highest nutritional and health contribution to human diet. In a crowded market, those differences among cultivars may also be of interest to plant breeders in order to develop more healthy and appreciable products. In addition, the avocado trees grown in Sicily produced high quality avocados. It seems that these plants have found there an ideal micro-climate to produce fruits with high organoleptic properties. These fruits have a balanced phenolic content, thus contributing to an appropriate intake of elements useful for maintaining good health conditions, and especially for preventing hypertension and cardiovascular disease. Finally, the qualitative and quantitative differences among cultivars and maturation stages allowed for the individuation of a set of phenolic compounds that have a great potential in the discrimination and identification of different genetic groups.
Acknowledgment
The UHPLC-HESI-MS measurements were performed at the Laboratory of Mass Spectrometry, ATeN Center, University of Palermo.
References
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Cunsolo, V.; Muccilli, V.; Sforza, S.; Dossena, A.; Drahos, L.; Vékey, K. Applications of Liquid Chromatography–Mass Spectrometry for Food Analysis. Journal of Chromatography A 2012, 1259, 74–85.
- Nicosia, A.; Celi, M.; Vazzana, M.; Damiano, M.A.; Parrinello, N.; D’Agostino, F.; Avellone, G.; Indelicato, S.; Mazzola, S.; Cuttitta, A. Profiling the Physiological and Molecular Response to Sulfonamidic Drug in Procambarus Clarkii. Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology 2014, 166, 14–23.
- López, G.B.; García-Reyes, J.F.; Molina-Díaz, A. Sample Treatment and Determination of Pesticide Residues in Fatty Vegetable Matrices: A Review. Talanta 2009, 79(2), 109–128.
- Cháfer-Pericás, C.; Maquieira, Á.; Puchades, R. Fast Screening Methods to Detect Antibiotic Residues in Food Samples. TrAC Trends in Analytical Chemistry 2010, 29(9), 1038–1049.
- Alarcón-Flores, M.I.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Multiclass Determination of Phenolic Compounds in Different Varieties of Tomato and Lettuce by Ultra High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. International Journal of Food Properties 2016, 19, 494–507.
- Yeddes, N.; Kalthoum Chérifa, J.; Guyot, S.; Baron, A.; Trabelsi-Ayadi, M. Phenolic Profile of Tunisian Opuntia Ficus Indica Thornless Form Flowers Via Chromatographic and Spectral Analysis by Reversed Phase-High Performance Liquid Chromatography-UV-Photodiode Array and Electrospray Ionization-Mass Spectrometer. International Journal of Food Properties 2014, 17, 741–751.
- Marini, F.; Balestrieri, F.; Bucci, R.; Magrì, A.D.; Magrì, A.L.; Marini, D. Supervised Pattern Recognition to Authenticate Italian Extra Virgin Olive Oil Varieties. Chemometrics and Intelligent Laboratory Systems 2004, 73, 85–93.
- Krichene, D.; Taamalli, W.; Daoud, D.; Salvador, M.D.; Fregapane, G.; Zarrouk, M. Phenolic Compounds, Tocopherols and Other Minor Components in Virgin Olive Oils of Some Tunisian Varieties. Journal of Food Biochemistry 2007, 31(2), 179–194.
- Owen, R.W.; Haubner, R.; Würtele, G.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Olives and Olive Oil in Cancer Prevention. European Journal of Cancer Prevention 2004, 13, 4, 319–326.
- Morton, J. Avocado. In Fruits of Warm Climates; Morton, J.F.; Ed.; Miami, FL, 1987; 91–102 pp.
- Weschenfelder, C.; dos Santos, J.L.; de Souza, P.A.L.; de Campos, V.P.; Marcadenti, A. Avocado and Cardiovascular Health. Open Journal of Endocrine and Metabolic Diseases 2015, 5, 77–83.
- Yasir, M.; Das, S.; Kharya, M.D. The Phytochemical and Pharmacological Profile of Persea Americana Mill. Pharmacognosy Reviews 2010, 4, 77–84.
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estévez, M. Avocado (Persea Americana Mill.) Phenolics, in Vitro Antioxidant and Antimicrobial Activities and Inhibition of Lipid and Protein Oxidation in Porcine Patties. Journal of Agricultural and Food Chemistry 2011, 25, 5625–5635.
- Lu, Q.Y.; Arteaga, J.R.; Zhang, Q.; Huerta, S.; Go, V.L.; Heber, D. Inhibition of Prostate Cancer Cell Growth by an Avocado Extract: Role of Lipid-Soluble Bioactive Substances. The Journal of Nutritional Biochemistry 2005, 16, 23–30.
- Butt, A.J.; Roberts, C.G.; Seawright, A.A.; Oelrichs, P.B.; Macleod, J.K.; Liaw, T.Y. A Novel Plant Toxin, Persin, with in Vivo Activity in the Mammary Gland, Induces Bim-Dependent Apoptosis in Human Breast Cancer Cells. Molecular Cancer Therapeutics 2006, 5, 2300–2309.
- Kawagishi, H.; Fukumoto, Y.; Hatakeyama, M.; He, P.; Arimoto, H.; Matsuzawa, T. Liver Injury Suppressing Compounds from Avocado (Persea Americana). Journal of Agricultural and Food Chemistry 2001, 49, 2215–2221.
- De Sousa Galvão, M.; Narain, N.; Nigam N. Influence of Different Cultivars on Oil Quality and Chemical Characteristics of Avocado Fruit. Food Science and Technology (Campinas) 2014, 34, 539–546.
- Kassim, A.; Workneh, T.S.; Bezuidenhout, C.N. A Review on Postharvest Handling of Avocado Fruit. African Journal of Agricultural Research 2013, 8, 2385–2402.
- Agozzino, P.; Avellone, G.; Bongiorno, D.; Ceraulo, L.; Indelicato, S.; Indelicato, S.; Vèkey, K. Determination of the Cultivar and Aging of Sicilian Olive Oils Using HPLC-MS and Linear Discriminant Analysis. Journal of Mass Spectrometry 2010, 45(9), 989–995.
- Bucci, R.; Magrí, A.D.; Magrí, A.L.; Marini, D.; Marini, F. Chemical Authentication of Extra Virgin Olive Oil Varieties by Supervised Chemometric Procedures. Journal of Agricultural and Food Chemistry 2002, 50(3), 413–418.
- Hurtado-Fernández, E.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Profiling LC-DAD-ESI-TOF MS Method for the Determination of Phenolic Metabolites from Avocado (Persea Americana). Journal of Agricultural and Food Chemistry 2011, 59, 2255–2267.
- Hurtado-Fernández, E.; Bajoub, A.; Morales, J.C.; Fernández-Gutiérreza, A.; Carrasco-Pancorbo, A. Quantitative Characterization of Important Metabolites of Avocado Fruit by Gas Chromatography Coupled to Different Detectors (APCI-TOF MS and FID). Food Research International 2014, 62, 801–811.
- Contreras-Gutiérrez, P.K.; Hurtado-Fernández, E.; Gómez-Romero, M.; Ignacio Hormaza, J.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Determination of Changes in the Metabolic Profile of Avocado Fruits (Persea Americana) by Two CE-MS Approaches (Targeted and Non-Targeted). Electrophoresis 2013, 34, 2928–2942.
