ABSTRACT
Modified yam starch and dual-modified yam starch were produced with propylene oxide, sodium trimetaphosphate and sodium tripolyphosphate. Gelatinization temperature and final viscosity of native yam starch were 79.2 ± 0.4°C and 5702 ± 3 cP. Results showed that the molar substitution and degree of substitution were increased with the volume fraction of propylene oxide from 6–12%, the highest of molar substitution and degree of substitution were 0.0445 ± 0.0003 and 0.0065 ± 0.0006, the final viscosity and setback of dual-modified yam starch were also similar. However, the gelatinization parameters showed an inverse trend. Starch modified with a mixture of sodium trimetaphosphate and sodium tripolyphosphate had higher phosphorus content and increased viscosity compared to starch modified with sodium trimetaphosphate. The peak viscosity of starch modified with propylene oxide was higher than that of native yam starch and the highest was HP12. The granular surface of modified yam starch and dual-modified yam starch appeared significantly embossed and indented, while. Modified yam starch film treated with 12% propylene oxide showed a more homogeneous fractured surface. The tensile strength and elongation at break (E) of starch films were affected by crosslinking reagents and propylene oxide, respectively. The best transparence and E were demonstrated in starch film that was modified with 12% propylene oxide. However, the best tensile strength was demonstrated in starch film that was modified with 8% propylene oxide, sodium trimetaphosphate, and sodium tripolyphosphate. The final viscosity of HP6C1 and HP6C2 was 27 ± 7 and 45 ± 9 cP, which was too low to form film.
Introduction
Chinese Yam, namely Rhizoma Dioscoreas Oppositae, is one of the more well-known edible and pharmaceutical foods in China and contains many active ingredients such as polysaccharides, flavonoids, allantoin, and trace elements.[Citation1–Citation3] Chinese Yam is composed of a higher starch content of between 20~30% of which 19.4% is amylose.[Citation4] Yam starch is non-conventional starch and isolated from tuber and root. Shujun indicated that yam starch is a typical C-type starch, i.e., a mixture of A-type and B-type starches.[Citation5] Much research has focused on studying the capacity and activities of active ingredients. However, there are few studies about preparing modified yam starch (MYS).
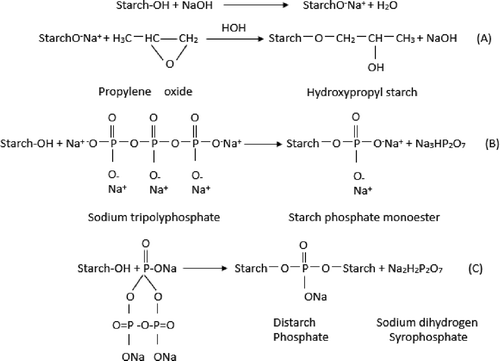
Primary physical methods to produce modified starch usually involve heat or moisture while chemical treatment involve the introduction of functional groups into the starch molecule using reactions of derivatization (etherification, esterification, and crosslinking) or decomposition (acid or enzymatic hydrolysis and oxidation).[Citation6] Hydroxypropylation and crosslinking are also widely used to produce modified starch. Hydroxypropylated starch produced by etherifing with propylene oxide has showed a lower gelatinization temperature, higher paste viscosity and greater paste clarity than native starch.[Citation7] Crosslinking reinforces the hydrogen bonds in the granules with chemical bonds acting as a bridge between the starch molecules.[Citation8] Crosslinking has provided lower paste viscosity, paste temperature, acid stability, and shear stability.[Citation8] Sodium trimetaphosphate (STMP) and sodium tripolyphosphate (STPP) are common crosslinking agents, which have been used to crosslink starch.[Citation9]
Biodegradable films made from natural materials and renewable resources do not contribute to significant environmental pollution compared to chemically synthesized plastic. Among many biopolymers, starch is being investigated as a potential material for biodegradable film.[Citation10] However, brittleness and the poor mechanical strength of starch film may restrict its application for packaging.[Citation11] An alternative to mitigate these drawbacks is the application of modified starch to film-forming matrices.
The objective of this study was to produce MYS and dual-modified yam starch (DMYS). The adding amount of propylene oxide, STMP, and STPP was based on dry weight of starch. MYS was etherified with 6–12% propylene oxide (HP) or crosslinked with 2%STMP (C1) or a mixture of 2% of STMP and 5% of STPP (C2). DMYS was etherified with 6–12% propylene oxide and crosslinked with 2% STMP (HPC1) or a mixture of 2% of STMP and 5% of STPP (HPC2). The film-forming properties and characteristics of NYS, MYS, and DMYS were explored.
Materials and methods
Materials
Chinese yam used in this study was purchased from Wenxian County Huai land planting professional cooperatives, Henan, China. Propylene oxide, STMP, and STPP were purchased from Xiya Chemical Reagent Corporation, Chengdu, China.
Starch isolation
The fresh yam was rinsed with water, peeled, and cut into pieces, and then smashed with a solid–liquid ratio of 1:11. Then 0.5M sodium hydroxide solution was added to adjust the suspension to pH 9.5. Starch extraction was accomplished in a magnetic stirring water bath at 45C° for 1 h. The starch suspension was cooled and precipitated temperature. After precipitation, the supernatant was removed by dumping, precipitating, and resuspending seven times. Finally, the starch layer was resuspended in absolute ethyl alcohol and dried at 45°C for 24 h and the dried starch was then passed through a 120 micron mesh sieve.
Preparation of modified starch
MYS and DMYS were prepared according to previous methods.[Citation12–Citation14] Twenty grams of yam starch (equivalent to 30% starch solid in suspension) was added to a 60 mL 5% sodium sulphate solution. Then 5% sodium hydroxide solution was added to adjust the suspension to pH 11.5. Propylene oxide 6–12% (volume by weight of starch solid, HP) was added and stirred at 40℃ for 24 h, the suspensions were cooled to room temperature and adjusted to pH 5.5–6.0 or 11 with 10% hydrochloric acid solution. The suspension pH was pH to 5.5–6.0 to terminate the reaction. 2% STMP (w/wt. of dry starch, HPC1) or a mixture of 2% STMP and 5% STPP (w/wt. of dry starch, HPC2) was added the suspension with pH 11 and stirred at 50℃ for 3 h, thereafter, the suspension was cooled to room temperature and adjusted to pH 5.5–6.0. The starch layer was washed with distilled water until free phosphorus disappeared in the supernatant.[Citation15] The starch was dried at 40℃ for 24 h and sieved (120 mesh).
Hydroxypropyl group and molar substitution (MS)
The hydroxypropyl group was determined according to the previously described procedure.[Citation16] MYS and DMYS (100 mg) were dispersed in 25 mL of 0.5 M sulfuric acid and transferred into 100-mL volumetric flasks and heated in a boiling water bath until the solution became clear. The solution was then cooled to room temperature and made up to 100 mL with distilled water. One milliliter of the solution was placed into 25 mL test tube with glass stopper and immersed into an ice bath, 8 mL of concentrated sulfuric acid was added. After thorough shaking, the tubes were placed in a boiling water bath for exactly 3 min, and immediately transferred to an ice bath until the solution was chilled. Two percent ninhydrin reagent (0.6 mL) was added and run carefully down the walls of the test tubes. The solution was immediately mixed well and placed at room temperature for 100 min. The volume was adjusted to 25 mL with concentrated sulfuric acid and mixed by inverting the tubes several times. The solution was immediately transferred to the cuvette and the absorbance was measured at 590 nm, using the starch blank as a reference. A calibration curve was prepared with an aliquot (1 mL) of standard aqueous solution, containing 10, 20, 30, 40, and 50 mg of propylene glycol per mL. The hydroxypropyl groups (%HP) were calculated by the following equation:
where C is amount of propylene glycol in the sample solution read from the calibration curve (mg/mL), F is the dilution factor and W is the weight of the sample (mg). The MS of MYS and DMYS was calculated using the following equation:[Citation13,Citation17]
Phosphorus content and degree of substitution (DS)
The phosphorus content was determined according to a procedure as described by the AOAC.[Citation15] Samples (1 g) were heated in a muffle furnace at 600°C for 4 h and cooled to room temperature. Then 10 mL of 5 M hydrochloric acid was added in the ash. NYS was prepared using the same protocol. The solution was diluted to 100 mL with distilled water and 10 mL of the solution was placed into 25 mL test tubes, 1 mL 2% vitamin C solution and 2 mL 4% vanadate-molybdate reagent were added in order, the solution was immediately mixed well. Then the absorbance of the samples was measured at 470 nm. A calibration curve was prepared from 0, 2.5, 5, 10, 20, 30, 40, and 50 mL of potassium dihydrogen phosphate (KH2PO4), using the deionized water as a reference. The phosphorous content (%P) was calculated from the calibration curve using the following equation:
where P is the phosphorous content (mg/100 mL) from the calibration curve, V is the dilution volume (mL), W is the weight of the sample (mg). The degree of substitution (DS) was calculated according to Morrison using the following equation:[Citation17]
Morphological analyses of starches and films
Scanning electron micrographs (SEM) of starches and films were obtained with a SU-1510 electron microscope (Hitachi, Japan). Film samples were frozen in liquid nitrogen and then fractured. Samples were applied onto an aluminum stub using double-sided adhesive tape and gold-coated at 20 mA/15 kV for 10 s before SEM analysis.
Gelatinization properties (DSC)
Thermal properties of starches were determined using a DSC 200 PC Phoxinstrument (Netzsch, Selb, Germany). Starch samples (2 mg) were weighed directly into a stainless steel pans and 8 uL of distilled water was added. The pan was hermetically sealed and equilibrated for 18 h at 25°C. The scanning temperature and the heating rates were 20–110°C and 10°C/min, respectively.
Pasting viscosity
The pasting properties of the starches were examined in a rapid visco analyzer (RVA). Starch samples (3 g) were weighed directly into aluminum canisters, followed by addition of distilled water to achieve a final weight of 25.0 g. The starch slurry was pasted with a temperature range from 50 to 95°C beginning with an initial hold 50°C for 1 min, and then a linear increase to 95°C for 3.8 min, a further hold 95°C for 2.5 min, linear cooling to 50°C for 3.8 min, and a final hold at 50°C for 2.5 min.
Starch films testing
Film preparation
Starch-based films were prepared by a casting method. Glycerol (0.7 g) and starch (2 g) were dispersed in 50 mL distilled water and mixed well with continuous stirring. The starch suspension was gelatinized in a boiling water bath for 1 h, cooled to 45°C and degassed with ultrasound. The mixtures were casted onto glass plates and dried at 50°C. The films were peeled off from the plates, after cooling to room temperature and stored at 25°C and 50% relative humidity (RH) prior to test.
Mechanical properties
Prior to the measurement of the mechanical properties, the thickness of films was measured by a micrometer at five random positions at each film and averaged. The tensile strength (TS) and elongation at break (E) were determined using a texture analyzer (TA). TX2i Stable Micro Systems TA (Godalming, UK) in accordance with the previously described method Intl, A.S. T. M.[Citation18] Samples (2 × 5 cm) were preconditioned at 25°C and 50 RH in a desiccator for at least 2 d prior to analysis. The tests were carried out with three replicates for each type of films, and mean values were acquired.
Transparence of starch films
Film samples were cut into rectangles and placed on the internal side of a spectrophotometer cell, using the blank spectrophotometer cell as a reference. The transmittance of each film samples was measured by a T6 spectrophotometer (Purkinje General Instrument Co., Beijing, China) at 600 nm and calculated as follows:
where T600 is the fractional transmittance at 600 nm and x is the film thickness (mm). The greater value represents lower transparency of the film.
Results and discussion
Hydroxypropyl groups, MS, phosphorus content, and DS
Chemical reactions generally occur randomly with the primary hydroxyls, the secondary hydroxyls, the aldehydic reducing end groupsand theglycol groups. was the mechanism of starch with propylene oxide, STMP, and STPP.[Citation19,Citation20] The hydroxypropyl groups, MS, phosphorus content, and DS of starches are shown in . Corresponding increases of MS and DS were observed as the propylene oxide was raised from 6 to 12%; however, it was found that MS values showed a lower rate of increase when propylene oxide increased from 10 to 12%. We propose that the starch reacted with 10% propylene oxide to saturation which limited any further increases in MS values. It was also observed that the HPC2 had higher DS values than HPC1. The highest phosphorus content and hydroxypropyl group content were 0.125 ± 0.009% and 1.397 ± 0.006%, lower than the maximum level (0.4%) allowed by the U.S. regulations (7.0%) stipulated by the Food and Drug Administration (FDA).[Citation9,Citation21] In previous studies, Pal reported that increasing the volume of propylene oxide added to corn, amaranth, canna, and maize starches produced progressive increases in MS.[Citation22] Chuenkamol have also reported that increasing the volume of propylene oxide added to dual-modified potato starch resulted in increased DS values.[Citation23] It has also been reported that DMYS produced with hydroxypropylation and crosslinking showed higher phosphorus content than MYS produced only with crosslinking. One explanation is hydroxypropylation may weaken the bonding of starch molecules and increase the swelling powder thus allowing more crosslinking reagents to react with the starch molecules.[Citation9,Citation24,Citation25]
Table 1. Hydroxypropyl groups, MS, phosphorus content, and DS of NYS, MYS, and DMYS.
SEM analysis of starches
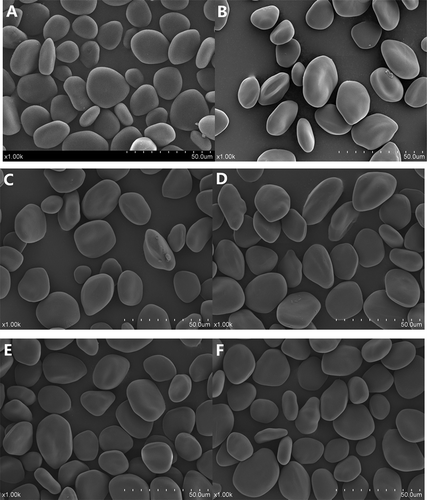
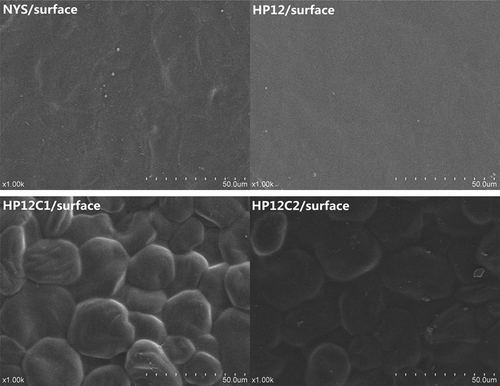
The morphology of the samples was studied by SEM as illustrated in . Unmodified starch granules were mainly oval or elliptical in shape and the surface of the granules was smooth and had no fissures. No obvious damage appeared on the surface of the native starch granules, which suggested that the method of extraction did not cause significant damage. The granule size of NYS ranged from 5 to 50 μmin agreement with previous studies.[Citation5] Compared the unmodified starch, the surface of the modified starch granules and dual-modified starch granules appeared significantly embossed and indented. Changes in the appearance of starch granules a following hydroxypropylation have been reported for potato starches.[Citation26] In this study, no obvious differences in surface characteristics were observed by SEM analysis of modified starches and dual-modified starch granules with various levels of propylene oxide (6–12%).
Gelatinization properties
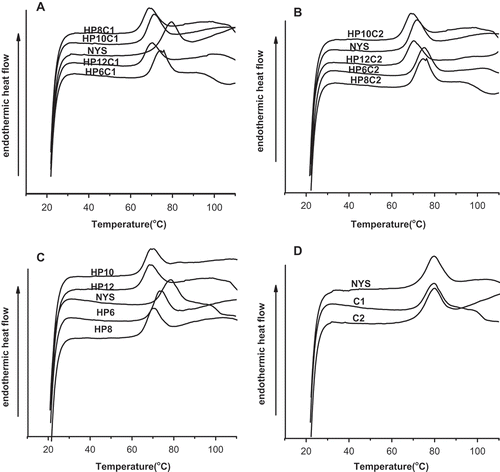
The gelatinization characteristics of starches determined by DSC are shown in . The NYS, MYS, and DMYS exhibited similar profiles on the DSC thermograms. It was observed that the transition temperatures (To, Tp, and Tc) of hydroxypropylated starches were lower than those of the NYS and were reduced when MS was increased. The gelatinization temperature of HP was in the range 74.2–68.8°C. Similar observations have been reported in rice and plantain starches.[Citation7,Citation27] The lowest gelatinization temperature was HP12. Hydroxypropylation also caused the granular structure to be looser and thus lowered the transition temperatures.
The gelatinization temperature of HPC1 and HPC2 was in the range 75.1–70.4°C and 75.9–69.2°C, respectively. The transition temperatures of DMYS with different crosslinking reagents did not show significant changes. Kaur reported that the addition of propylene oxide into the starch polymer backbone contributed to a higher flexibility which reduced the melting temperature.[Citation26]
Pasting viscosity
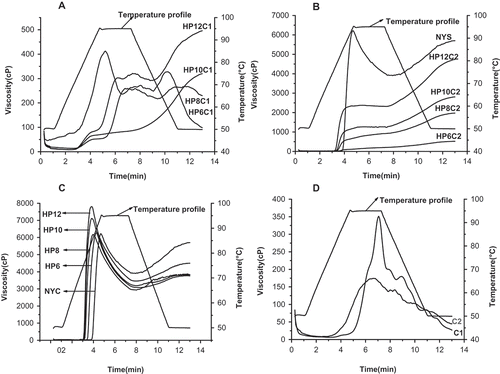
Pasting viscosity profiles of starches investigated by using the RVA are shown in . NYS, MYS and DMYS exhibited typical pasting viscosity profiles. Final viscosity of NYS were 79.2 ± 0.4°C, which was higher than rice and corn starches, modified starches crosslinked with phosphate salt revealed lower viscosities (peak, breakdown, setbacks, and final viscosity) than native yam starches (NYSs) and final viscosity tended to be increased when the volume of propylene oxide was increased. The final viscosity of HPC1, HPC2, and HP was in the range 99–496, 523–4100, and 4499–3624 cP, respectively. However, the setback of NYS was 1800 cP, which was higher than MYS and DMYS. Yook reported that the setbacks in modified rice starches decreased and it has also been shown that modified starches with propylene oxide are easier to form pasting than NYSs.[Citation28] Gunaratne and Corke have reported that the structures of the starch granules after hydroxypropylation are weaker than unmodified granules.[Citation24]
The effects of different crosslinking reagents on the pasting temperature and viscosity of starches are shown in . The pasting temperature of HPC1, HPC2, and HP was in the range 91.2–94.9, 81.3–76.6, and 78.3–74.3°C, respectively. However, the pasting temperatures of C1, C2 and NYS was 95.2±1.4°C, 94.9±1.5°C and 84.2±1.8°C. Significant differences were found in the pasting temperatures with HPC1, C1, and C2 exhibiting higher pasting temperatures than NYS; however, HP and HPC2 had lower pasting temperatures than NYS. Crosslinking could produce strong internal bonding forces which contribute to lower swelling and higher pasting temperatures in gelatinization.[Citation24] The final viscosity of HPC2 was higher than HPC1. STMP has been reported to be a strongly effective crosslinking reagent and starches crosslinked with STMP exhibit di-starch phosphates.[Citation29] Starches crosslinked with a mixture of phosphate 2% STMP and 5% STPP produced mono-phosphates.[Citation8,Citation30] After modification with phosphate salt, the starch granules were stronger and more difficult to breakdown. It has been reported that mono-phosphates from wheat and corn starches modified by STPP showed a lower pasting temperature but higher pasting peaks than starches modified with STMP.[Citation30]
SEM analysis of films
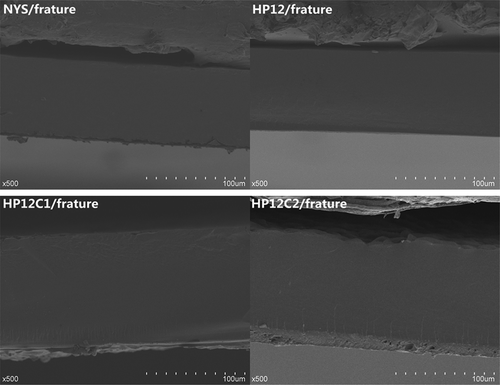
The surface and fracture characteristics of films were investigated by SEM and , respectively. HP12 showed the most homogeneous surface, followed by NYS, while HPC1 and HPC2 demonstrated a rough surface and starch granules that were not broken. DMYS heated to 100°C was not sufficient to breakdown the granular structure and so higher temperatures maybe needed. The fracture of HP12 and NYS was no pores or cracks and homogeneous. However, the fracture of HPC1 and HPC2 showed some tiny cracks and did not uniform. These results corresponded to the pasting properties of starches. Starches were hydroxypropylated with propylene oxide, the granular structures were weakened and water was allowed accessibility.[Citation9] Thus, these starches were easier to gelatinize and form uniform mucilage. Di-starch phosphate and mono-phosphates which were produced by crosslinking with STMP and a mixture of 2% STMP and 5% STPP, have shown a granular structure of di-starch phosphate and mono-phosphate which were strong and hard to break.[Citation31]
Mechanical properties and transparence of films
Mechanical properties and transparence are important parameters to evaluate polymer films. shows the TS, percent elongation at break (E), and transparency values of NYS, MYS, and DMYS films. The TS values of HP, HPC1, HPC2, and NYS were in the range 5.32–2.50, 16.51–11.47, 19.81–14.03, and 6.22 ± 0.71 MPa, respectively. Compared to the HP film, higher TS was present in NYS and the highest TS was measured in DMYS. The TS values of MYS and DMYS films tended to reduce with addition of propylene oxide. The TS of the crosslinked starch films was higher than that of the native starch films.[Citation32] In starch crosslinked with STMP and STPP, the starch granules were stronger and more difficult to breakdown. These results indicated that crosslinking had a predominant influence on TS. The E values of HP, HPC1, HPC2, and NYS were in the range 57.28–172.12, 4.34–3.69, 4.41–3.79, and 28.74 ± 0.91%, respectively. The E value of polymeric materials depends on the flexibility of molecular chains and the E and transparence of HP films was higher than that of NYS and DMYS films. It was also apparent that the E of HP films increased with increasing propylene oxide, while the E of DMYS film was reduced with increased propylene oxide. One explanation for this might be that the hydroxypropylation causes a weakening of the hydrogen bonding between starch chains. The propylene oxide may then act like a plasticizer in the films and induces the formation of starch-plasticizer interactions resulting in a higher E.[Citation9]
Table 2. Tensile strength (TS), elongation at break (%E), and transparence of films.
In our results, the effects of crosslinking reagents and propylene oxide resulted in higher TS and E, respectively. Gutiérrez have reported that photo-crosslinked rice starch shows an increase in TS but a decrease in E.[Citation33] Our results also indicate that the TS and E of HPC2 films demonstrated higher values than those of HPC1 films with the same content of propylene oxide, possibly due to the mixture of 2% STMP and 5% STPP resulting in a combination of mono-phosphates (STPP) and di-starch phosphates (STMP).
Transparence is a vital property of film and films transparence can be affected by film thickness and the molecular regularity, It was report that greater transparency values showed lower transparency in the film.[Citation34,Citation35] The influence of propylene oxide and crosslinking agents on film transparency was shown in . The transparency values of HP, HPC1, HPC2, and NYS were in the range 0.682–0.432, 2.176–2.550, 1.873–2.477, and 0.752 ± 0.055 mm−Citation1, respectively. Compared to NYS films, higher transparency values were present in HPC1 and HPC2 films with transparency values of HP films being lower than NYS films. It was also observed that increasing the level of propylene oxide tended to decrease the transparency value of HP films. However, transparency values of DMYS films were increased with the increase of the content of propylene oxide. HPC1 films demonstrated higher transparency values than the HPC2 films at the same level of propylene oxide. These results may be due to the bulk of mono-phosphate in the HPC2 leading to a higher transmittance of light than in the HPC1 films. The best transparence and E were demonstrated in starch film that was modified with 12% propylene oxide. However, the best TS was demonstrated in starch film that was modified with 8% propylene oxide, STMP and STPP.
Conclusions
Yam starch showed a higher amylose content of 19.4% in its composition, gelatinization temperature and final viscosity of NYS were 79.2 ± 0.4°C and 5702 ± 3cP. Yam starch was subjected to etherification with propylene oxide and esterification with STMP and STPP. The gelatinization temperature and final viscosity of HPC1, HPC2, and HP were in the range 75.1–70.4°C, 99–496 cP, 75.9–69.2°C, 523–4100 cP, 74.2–68.8°C, 4499–3624 cP, which were lower than NYS. However, peak viscosity of HP was in the range 6279–7803 cP, which was higher than NYS. The final viscosity of HPC1, HPC2 was increased with the volume fraction of propylene oxide from 6–12%. However, the final viscosity of HP showed an inverse trend. The gelatinization temperature of hydroxypropylated starches was reduced when MS was increased. The final viscosity of HP6C1 and HP6C2 was 27 ± 7 and 45 ± 9 cP, which was too low for film formation. The gelatinization temperature of C1 and C2 was 72.6 ± 0.1°C and 72 ± 1°C, which was similar to NYS. The TS, E and transparence of starch films were affected by crosslinking reagents and propylene oxide, respectively. Compared to the HP film, higher TS was present in NYS and the highest TS was measured in DMYS. However, compared to the HP film, lower E was present in NYS and the lowest E was measured in DMYS. Transparence of HP films was higher than that of NYS and DMYS films. The best TS, E and transparence were present in HP8C2, HP12, and HP12, respectively.
Funding
This work was financially supported with funds provided by Hebei Province and School Science and Technology Cooperation Development Fund Project.
Additional information
Funding
References
- Shujun, W.; Jinglin, Y.; Wenyuan, G.; Hongyan, L.; Peigen, X. New Starches from Traditional Chinese Medicine (TCM)—Chinese Yam (Dioscorea Opposita Thunb) Cultivars. Carbohydrate Research 2006, 341(2), 289–293.
- Zhao, G.; Li, Z.; Chen, Z. Structural Analysis and Antitumor Activity of RDPS-I Polysaccharide from Chinese Yam. Acta Pharmaceutica Sinica 2002, 38(1), 37–41.
- Zhao, G.; Kan, J.; Li, Z.; Chen, Z. Structural Features and Immunological Activity of a Polysaccharide from Dioscorea Opposita Thunb Roots. Carbohydrate Polymers 2005, 61(2), 125–131.
- Wang, L.X.; Li, B.; Cao, J.L. Detection of Amylose and Amylopectin from Different Yam (D Opposita Thunb) Cultivars by Capillary Electrophoresis. In Advanced Materials Research 2014, 955, 791–796.
- Shujun, W.; Jinglin, Y.; Hongyan, L.; Weiping, C. Characterisation and Preliminary Lipid-Lowering Evaluation of Starch from Chinese Yam. Food Chemistry 2008, 108(1), 176–181.
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors Influencing the Physico-Chemical, Morphological, Thermal and Rheological Properties of Some Chemically Modified Starches for Food Applications—A Review. Food Hydrocolloids 2007, 21(1), 1–22.
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and Characteristics of Dual-Modified Rice Starch Based Biodegradable Films. International Journal of Biological Macromolecules 2014, 67, 490–502.
- Mahmut, S.; Hasan, S.; Murat, O.; Milford, A.H. Cross‐Linking of Starch with Reactive Extrusion and Expansion of Extrudates. International Journal of Food Properties 2007, 6(3), 473–480.
- Liu, Y.; Chen, W.; Chen, C.; Zhang, J. Physicochemical Property of Starch-Soluble Dietary Fiber Conjugates and Their Resistance to Enzymatic Hydrolysis. International Journal of Food Properties 2015, 18(11), 2457–2471.
- Averous, L.; Moro, L.; Dole, P.; Fringant, C. Properties of Thermoplastic Blends. Starch–Polycaprolactone Polymer 2000, 41(11), 4157–4167.
- Ashwar, B.A.; Shah, A.; Gani, A. Rice Starch Active Packaging Films Loaded with Antioxidants—Development and Characterization. Starc/Stärke 2015, 67(3–4), 294–302.
- Van, H.P.; Morita, N. Physicochemical Properties of Hydroxypropylated and Cross-Linked Starches from A-Type and B-Type Wheat Starch Granules. Carbohydrate Polymers 2005, 59(2), 239–246.
- Suwanliwong, S. Preparation and characterisation of hydroxypropylated crosslinked sago starch for application in acidic, frozen and canned foods. Doctoral Dissertation, Universiti Putra Malaysia, 1998.
- Wattanachant, S.; Muhammad, K.M.A.T.; Hashim, D.M.; Rahman, R.A. Effect of Crosslinking Reagents and Hydroxypropylation Levels on Dual-Modified Sago Starch Properties. Food Chemistry 2003, 80(4), 463–471.
- Saberi, B.; Majzoobi, M.; Farahnaky, A.; Khaneghah, A.M. Effects of hydroxypropylation on rheological, morphological and thermal properties of oat starch. FAO/WHO Expert Committee. Joint FAO/WHO Expert Committee on Food Additives. In FAO Food and Nutrition Paper; 2001, 1–22.
- Morrison, W.R.; Milligan, T.P.; Azudin, M.N. A Relationship Between the Amylose and Lipid Contents of Starches from Diploid Cereals. Journal of Cereal Science 1984, 2, 257–271.
- Horwitz, W.; Senzel, A.; Reynolds, H.; Park, D.L. Official Methods of Analysis of the Association of Official Analytical Chemists, ed., Washington, D.C., 1975.
- Intl, A.S.T.M. Standard Test Method for Tensile Properties of Thin Plastic Sheeting D 882-01 Annual book of ASTM Standards. ASTM Intl.: Philadelphia, PA, 2001; 162–170 pp.
- Cui, S.W. Food Carbohydrates. Chemistry, Physical Properties, and Applications. CRC Press, Crc Pr I Llc 2005; 357–406 pp.
- Mega, T.L.; Etten, R.L.V. Oxygen Exchange and Bond Cleavage Reactions of Carbohydrates Studied Using the 18o Isotope Shift in 13c nmr Spectroscopy. Basic Life Sciences 1990, 56, 85–93.
- Dias, F.F.; Tekchandani, H.K. Mehta, D. Modified Starches and Their Use by Food Industry. Indian Food Industry 1997, 16(4), 33–39.
- Pal, J.; Singhal, R.S.; Kulkarni, P.R. Physicochemical Properties of Hydroxypropyl Derivative from Corn and Amaranth Starch. Carbohydrate Polymers 2002, 48(1), 49–53.
- Chun, S.Y.; Yoo, B. Effect of Molar Substitution on Rheological Properties of Hydroxypropylated Rice Starch Pastes. Starch/Stärke 2007, 59(7), 334–341.
- Gunaratne, A.; Corke, H. Functional Properties of Hydroxypropylated, Cross-Linked, and Hydroxypropylated Cross-Linked Tuber and Root Starches. Cereal Chemistry 2007, 84(1), 30–37.
- Chuenkamol, B.; Puttanlek, C.; Rungsardthong, V.; Uttapap, D. Characterization of Low-Substituted Hydroxypropylated Canna Starch. Food Hydrocolloids 2007, 21(7), 1123–1132.
- Kaur, L.; Singh, N.; Singh, J. Factors Influencing the Properties of Hydroxypropylated Potato Starches. Carbohydrate Polymers 2004, 55(2), 211–223.
- Gutierrez, T.; Perez, E.; Guzman, R.; Tapia, M.S.; Fama, L. Physicochemical and Functional Properties of Native and Modified by Crosslinking, Dark-Cush-Cush Yam (Dioscorea Trifida) and Cassava (Manihot Esculenta) Starch. Journal of Polymer & Biopolymer Physics Chemistry 2014, 2(1), 1–5.
- Yook, C.; Pek, U.H.; Park, K.H. Gelatinization and Retrogradation Characteristics of Hydroxypropylated and Cross‐Linked Rices. Journal of Food Science 1993, 58(2), 405–407.
- Woo, K.; Seib, P.A. Cross-Linking of Wheat Starch and Hydroxypropylated Wheat Starch in Alkaline Slurry with Sodium Trimetaphosphate. Carbohydrate Polymers 1997, 33(4), 263–271.
- Lim, S.; Seib, P.A. Preparation and Pasting Properties of Wheat and Corn Starch Phosphates. Cereal Chemistry 1993, 70, 137–137.
- Liu, H.; Ramsden, L.; Corke, H. Physical Properties and Enzymatic Digestibility of Hydroxypropylated ae, wx, and Normal Maize Starch. Carbohydrate Polymers 1999, 40(3), 175–182.
- Kim, M.; Lee, S.J. Characteristics of Cross-Linked Potato Starch and Starch-Filled Linear Low-Density Polyethylene Films. Carbohydrate Polymers 2002, 50(4), 331–337.
- Gutiérrez, T.J.; Tapia, M.S.; Pérez, E.; Famá, L. Structural and Mechanical Properties of Edible Films Made from Native and Modified Cush-Cush Yam and Cassava Starch. Food Hydrocolloids 2015, 45, 211–217.
- Spiridon, I.; Teaca, C.A.; Bodirlau, R. Preparation and Characterization of Adipic Acid-Modified Starch Microparticles/Plasticized Starch Composite Films Reinforced by Lignin. Journal of Materials Science 2011, 46(10), 3241–3251.
- Espinoza Acosta, J.L.; Torres Chávez, P.I.; Ramírez‐Wong, B. Mechanical, Thermal, and Antioxidant Properties of Composite Films Prepared from Durum Wheat Starch and Lignin. Starch/Stärke 2015, 67(5–6), 502–511.