ABSTRACT
Effects of gutting on biogenic amine (putrescine, cadaverine, histamine, agmatine, tyramine, spermidine, and spermine) formation, biochemical (pH, TVBN, K value), microbial (APC, H2S producing, Coliform bacteria), and sensory quality of farmed monosex tilapia (Oreochromis niloticus) was studied during ice stored at 4 ± 2ºC. Spermine and spermidine levels were high with cadaverine reached 4.66 ppm in gutted sample. The other amines were detected at relatively low level. K value of both samples crossed 60% on 15th and 18th day, respectively. The total phenolic content exceeded 7 log cfu/g after sensory rejection. The shelf life of whole and gutted samples was estimated to be 24 and 21 days, respectively.
Introduction
Tilapia is one of the most important farmed species all over the world. Farming of fast growing monosex populations of tilapia produced by hormonal sex reversal of male tilapia, Oreochromis niloticus is getting popularized, as male tilapia grow nearly twice as fast as females and its commercial production is increasing worldwide. The most important producers of tilapia today are China, Egypt, Indonesia, and the Philippines. In the export industry, fresh or frozen fillets of tilapia are available in different sizes and packages, as skin-on, skin-off, deep skinned, individually quick-frozen, smoked, sashimi grade, and are treated by carbon monoxide or ozone. Tilapia in whole or gutted form is available in retail markets.
Effective preservation to meet the huge quantity of mononsex tilapia production is necessary to maintain the quality and safety during long-distance transporting and marketing. Freshness decides the quality of fish as food both for home cooking and commercial processing. As fish is a rich source of protein breaking up protein during spoilage yields peptides and amino acids which will further decay and result in forming biogenic amines. Histamine (HIM) forms from histidine, tyramine (TYM) from tyrosine, phenylethylamine (PEA) from phenylalanine, tryptamine (TRM) from tryptophane, cadaverine (CAD) from lysine, and putrescine (PUT) from arginine or ornitine. The amino acid content of fish, the presence of amino acid decarboxylases and favorable environmental conditions determines the formation of biogenic amines in fish and fishery products.[Citation1] The contaminating Enterobacteriaceae namely Salmonella, Shigella, Escherichia, Serratia, Yersinia, Morganella, and genera Pseudomonas usually possess amino acid decarboxylase enzymes.
Low concentrations of biogenic amines in food and drink (practically under 100 mg/kg) do not represent a significant risk for a healthy human.[Citation2] Biogenic amines above 100 mg/kg may induce undesirable psychoactive and vasoactive effects (hypotension or hypertension, headache, nausea, breathing problems, etc.). Biogenic amine formation can be used to assess the potential toxicity, as well as food quality.
Biochemical changes induced by endogenous enzymes can cause loss of freshness and are independent of bacterial deterioration. The loss of freshness can occur fast or slow and it depends on the species, the physiological condition of fish, microbial contamination, and temperature. Quality and shelf life are directly associated with protein and Adenosine tri phosphate (ATP) degradation, drop in pH, lipid oxidation, undesirable compounds such as trimethylamine (TMA-N) and total volatile bases (TVB-N), which are produced by bacterial action. Measurement of K value based on nucleotide degradation correlates well with the freshness of fish.[Citation3] ATP content in fish decreases rapidly in the first 24 h after death regardless of the species and muscle type. In fish muscle, ATP is metabolized as follows: ATP→ADP→AMP→IMP→HxR (inosine)→Hx (hypoxantine). K value is widely used to determine fish freshness and it is closely correlated with storage time.[Citation4]
Many freshwater and marine fish from temperate and tropical areas have been studied for quality changes during storage in ice. However, information regarding biogenic amine formation and quality changes of farmed monosex tilapia during iced storage is not available. Hence, the present study was undertaken to evaluate the changes in biogenic amine formation, biochemical, sensory, and microbial quality of whole and gutted monosex tilapia stored in iced condition.
Materials and methods
Chemicals and materials
Biogenic amine standards were purchased from Sigma-Aldrich (USA). Acetonitrile and methanol for use in liquid chromatography and mass spectrometry was purchased from J.T. Baker, USA. Filtered water was prepared by using Cascada LS water, Lab Water Technology (Pall Corporation).
Sample preparation
Samples of monosex tilapia were collected from a local fish farm in Kodungallore, Kerala, India. The samples were allowed to die naturally by retaining in the net in which it is caught. Samples had an average weight of 508 ± 2.3 g and average length of 23.29 ± 0.3 cm. Samples were immediately washed with potable water, iced, and packed in insulated boxes and transported to the laboratory. At the laboratory, the fish were deiced and segregated into two portions. One was kept in whole form, iced in 1:1 ratio in insulated boxes. The other lot was gutted, washed in potable water, and iced (1:1) in insulated boxes. Both lots were kept in a chilled room at 4 ± 2ºC. Fish were randomly chosen for each sampling and examined at 48 h intervals for changes in sensory, biogenic amine, biochemical, and microbiological characters in triplicate. For analytical purposes, three numbers of fish were taken, filleted, and fillets were homogenized in a blender maintained at 4 – 6 ± 1ºC. The homogenized meat was kept refrigerated pending analysis.
Biochemical analysis
The samples were analyzed in triplicate. The pH of the samples was measured using digital pH meter (Hanna instruments, HI 2221 pH/OHP meter) after blending 4 g of sample with 40 mL of distilled water.[Citation5] The TVB-N was determined by the micro diffusion method.[Citation6]
K value
K value was determined based on measurement of ATP degraded compounds.[Citation3] The analysis was carried out using high-performance liquid chromatography (HPLC; Merch Hitachi LaChrom) with ultraviolet (UV) detector (L-7400). A 20 µL sample was injected after filtration through a 0.45 µ filter. Nucleotides were separated by a 5 µm RP–C18 column 250–4 mm ID. 0.04 M potassium dihydrogen orthophosphate (KH2PO4) and 0.06 M dipotassium hydrogen orthophosphate (K2HPO4) dissolved in micro filtered water was the eluting solvent at a flow rate of 1–1.8 mL/min. The monitoring wavelength was set at 250 nm for ATP breakdown compounds.
where ATP = Adenosine 5’-triphosphate; ADP = Adenosine 5’-diphosphate; AMP = Adenosine 5’-monopho-sphate; IMP = Inosine 5’-monophosphate; HxR = Inosine; Hx = hypoxanthine.
Evaluation of biogenic amine content
Sample extraction
Five grams of homogenized sample was extracted with 25 mL of 6% trichloroacetic acid (TCA) for 1 min and centrifuged at 8000 rpm for 10 min at 4ºC. Filtered after centrifugation using Whatman no.1 filter paper and 0.45 µ filter. Dilution of the filtrate was carried out using methanol water (1:1). Samples were stored at –20ºC until further analysis.
LC MS MS analysis
Liquid chromatography mass spectrometry mass spectrometry (LC MS MS) analysis of biogenic amines without derivatisation was carried out with some modification to the method.[Citation7] LC separation was achieved by passing the sample (1 µL) through a column (water acquity BEH column RM 8) using a mobile phase 0.05% trifluroacetic acid containing water (A) and 0.05% trifluroacetic acid containing acetonitrile (B) at a flow rate of 0.3 mL/min. The gradient program was: 0 min 30% B and 70% A; 0–15 min 90% B and 10% A; 15–20 min 30% B and 70% A; 20–25 min 30% B and 70 % A. Analysis was done using LC MS MS (API 4000 Q Trap of AB Sciex, Canada). Instrument control, data acquisition and evaluation were done with the Analyst software (v. 1.5.2).
Microbiological analysis
A 25 g portion of fish was weighed aseptically and taken into a stomacher bag, 225 mL of sterile physiological saline (0.85%) was added, and the suspension was homogenized for 60 s in a stomacher (Lab Blender 400; Seward Medical, London, UK). The homogenized sample was serially diluted using 9 mL sterile saline solution. Aerobic plate count (APC) was determined in a plate count agar by the spread plate method.[Citation8] The inoculated plates were incubated at 30ºC for 48 h.
H2S producing bacteria were enumerated using iron agar (IA, Oxoid code CM 867) by pour plate method. After setting the plates were overlayed with same medium and incubated at 20ºC for 5 days; black colonies formed due to the production of H2S were enumerated.[Citation9] The most probable number (MPN) technique was used for the enumeration of total coliform bacteria using Macconkey broth.[Citation10].
Sensory analysis
Sensory analysis was carried out for whole and gutted samples during 24 days of iced storage. A sensory panel was constituted by five trained and experienced persons in the laboratory. The panel members were asked to rinse their mouth with water after tasting the sample to reduce bias in the judgement. In the case of both whole and gutted sample sensory scoring was done based on a 9-point hedonic scale.[Citation11] Assessment was based on appearance, color, odor, flavor, taste, and texture (like extremely = 9 to dislike extremely = 1). Samples with no off-flavor, bright color, firm and elastic structure were given a score of 9–7. Those samples with trace off-flavor, pale to colorless, and elastic structure were scored from 7 to 5. Samples with medium off-flavor, slight brownish color, and slight soft texture were given a score of 4–5. For the evaluation of cooked sample, the samples were cooked for 10 min in boiling brine (1.5% NaCl). Each panel member expressed the “overall acceptability” based on overall impression of the samples. An overall acceptability score was calculated based on the total score obtained for raw and cooked samples. An overall score below four was taken as “rejected.”
Statistical analysis
All the analyses were carried out in triplicate (n = 3). Results are given as mean ± standard deviation. Analysis of variance (ANOVA) was carried out using the statistical software SPSS.16 (SPSS Inc. Chicago). The statistical significance was identified at 95% confidence level (p < 0.05).
Results and discussion
Biochemical changes in quality
Variations in pH of whole and gutted samples are depicted in . The initial average pH of homogenized monosex tilapia meat was 6.34. The pH of both whole and gutted samples showed an increasing trend from 0th day to 9th day, then noticed slight fluctuations of increase and decrease of pH. A maximum value of 6.67 was observed in case of whole sample on 24th day of storage and the gutted sample showed a maximum value of 6.8 on 24th day of iced storage. Species, seasons, diet, level of activity or stress during the catch as well as type of muscle can lead to variations among the initial pH values.[Citation4] In the present study values of pH were significantly different (p < 0.05) during the entire period of storage. The pH of live fish muscle is close to the value 6.5–7. Post mortem pH can vary from 6.0 to 7.1 depending on season, species, and other factors.[Citation12] Generally it is noticed that, the pH is about 6.0–6.5 for fresh fish, and it increases during storage. The limit of acceptability is usually 6.8–7.0.
Table 1. Changes in pH, TVBN (mg%), and K value (%) of whole and gutted samples of monosex tilapia during iced storage.
TVB-N
The TVB-N in fish species consists mostly of TMA, dimethylamine, and ammonia. TVB-N content is mainly related to bacterial spoilage since both ammonia and TMA are produced by spoilage microorganisms. TVB-N was reported as the standard chemical indicator of seafood spoilage and are appropriate for advanced spoilage, but is an insufficient sign of quality during the initial stages of seafood spoilage.[Citation13] The TVB-N content of both whole and gutted fish is depicted in . There observed an average TVB-N value of 12.6 mg% in the case of fresh sample. A gradual increasing trend of TVB-N content was noticed and the values increased significantly (p < 0.05) in both the case of whole and gutted samples with the advancement of storage period. However, TVB-N values remained below the upper limit of acceptability throughout the storage period, both for the whole and gutted samples. TVB-N content in freshly caught fish is typically between 5 and 20 mg%.[Citation14] Content of TVB-N is regarded as an indicative of spoilage when it ranges between 25[Citation15] to 40 mg%.[Citation16] In this study, the average values of TVB-N content significantly increased (p < 0.05) from 12.6 to 19.56 mg% by 24 days of storage in ice, in the case of the whole sample and to 21 mg% in 21 days in the case of gutted samples. This increase may be due to ammonia production in the muscle during storage. TVB-N values of whole and gutted samples were showing similar trend in the case of monosex tilapia. Similar TVB-N values have been reported for whole fish,[Citation17] as well as for gutted fish.[Citation18] Increase in TVB-N with the lapse of storage, particularly, toward the end of storage period may be attributed to bacterial spoilage after the bacterial population has grown. The total Aerobic plate count (APC) was in the higher range in both whole and gutted fish () deriving the same point indicating the possibility of higher TVBN production during spoilage.
K value
The nucleotide degradation products have been found to contribute directly to the sensory quality of fish, with IMP showing a distinct taste-enhancing effect, particularly in combination with glutamic acid, and Hx contributing bitterness to chill-stored fish that is nearing the limit of acceptability. Quantification of ATP concentration and its degradation products was used as the basis to calculate the K value or freshness index, which is defined as the ratio (×100) of nonphosphorylated ATP breakdown products to the total ATP breakdown products, which has been used as a freshness measure in many species.[Citation18]
In the present study, K value of fresh sample on the 0th day was found to be 0.53%. K value increased linearly over the storage period of samples in ice (). The K value of the whole and gutted sample steadily increased during storage in iced condition and reached 90.88 and 94.45% respectively on the 24th day of storage. Statistically significant difference (p < 0.01) in K value of whole and gutted samples were noticed from 6th day onward. Fish products with K values lower than 20% are considered as very fresh ones, less than 50% as moderate fresh, and higher than 70% as not fresh.[Citation19] The K value of the whole and gutted sample crossed the 60% limit[Citation20] on the 15th and 18th day, respectively.The K value crossed 20% on the 6th day in the case of the whole sample and 9th day in the case of gutted sample. Iced gilthead sea bream was considered unacceptable by the members of the taste panel on day 17, when the K value was 39%.[Citation21] This indicator has been extensively used in a variety of freshwater species such as carp,[Citation22] catfish,[Citation23] or marine species such as ray fish.[Citation4] K value of Tilapia (Oreochromis niloticus) during refrigerated storage was evaluated and it is reported that whole, gutted, and filleted Tilapia crossed 60% K value on 12th, 10th, and 6th day, respectively.[Citation24] K value of Tilapia fillets (Oreochromis niloticus) during iced storage was evaluated and the values were 11.61% on the 0th day to a final value of 86.46% on the 18th day.[Citation25]
Formation of biogenic amines
Biogenic amines have been used as chemical indicators of seafood quality. Changes in biogenic amine formation during iced storage of whole and gutted monosex tilapia are given in and . Although seven biogenic amines were studied, namely, PUT, CAD, HIM, AGM, TYM, SPD and SPM, amines such as HIM and TYM were not detected at any time during the storage study. The other amines were detected at relatively low levels. Presence of PUT was not noticed in the case of both whole and gutted samples until the 18th day of storage and was detected on 21st and the 24th day of storage. Significant difference in (p < 0.01) PUT content of whole and gutted sample on 21st day of storage was noteiced. CAD was not detected in the whole samples in the entire storage period, but in the case of gutted samples it was detected at a maximum level of 4.66 ppm on 24th day. The process of gutting had a significant effect on formation of PUT and CAD during ice storage of monosex tilapia. Comparatively higher values of PUT and CAD in the gutted samples can be due to the higher microbial load in in gutted samples as the process of gutting itself is a source of microbial contamination. CAD and PUT due to their ability to potentiate the toxicity of HIM are very important in food, especially in fish and fish products. CAD and PUT producing bacteria can survive and multiply rapidly between 9 and 12 days of storage at 0ºC and contribute to the formation of amines during ice storage of fish and shrimp.[Citation16]
Table 2. Changes in biogenic amines of whole samples of monosex tilapia during iced storage.
Table 3. Changes in biogenic amines of gutted samples of monosex tilapia during iced storage.
The content of spermidine was very low initially, increasing and decreasing fluctuations in content during storage was observed and reached 2.03 ppm in case of whole sample and 1.71 ppm in case of gutted sample on 24th day of storage. Spermine is the only amine which is present in higher concentration during the entire storage. Spermine content reached a level of 15.83 ppm in whole sample and 18.78 ppm in gutted sample on the 24th day of storage. Both spermine and spermidine content reached a maximum value of 16.05 and 2.12 ppm, respectively, on 15th day of storage of gutted samples, followed by a decrease in content. No significant difference in spermidine and spermine content of whole and gutted sample on 24th day of storage. Similar patterns of biogenic amine formation were observed in case of ice-stored tilapia, rainbow trout and carp.[Citation26] Formation of PUT and CAD was evident when the APC crossed 6 log cfu/g. Biogenic amines in iced stored whole and gutted monosex tilapia do not represent any health hazard for individuals, as the contents of the most problematic amines, HIM and TYM, were not detected in the samples analyzed.
Microbiological quality
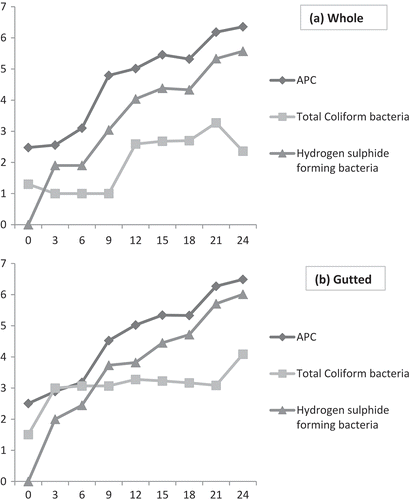
APC
The results given in and depicted that the APC increased with the increase in storage period of whole and gutted samples. In whole samples, the APC increased from initial load of 2.4 to 6.36 log cfu/g and in gutted samples from 2.5 to 6.49 log cfu/g after 24 days of iced storage. The upper limit of APC for fresh fish is 5 ×105 cfu/g.[Citation27] Initial mesophilic counts between 4 and 6 log cfu/g were reported for freshwater fish species such as tilapia, rainbow trout, silver perch by various workers.[Citation28,Citation29] Gutting procedure can influence the initial load of microorganisms by exposing the fish flesh area to environmental conditions. Bacterial population associated with gutted sample were higher than that of whole sample and were statistically significant (p < 0.05). In both the case of whole and gutted samples APC increased continuously along with iced storage and reached 106 and 105 cfu/g on the 21st and 12th day of storage, respectively, when the gutted and whole samples were deemed unfit for human consumption as per the sensory score on the 21st day and 24th day, respectively. APC of whole and gutted samples exceeded 7 log cfu/g, which is the maximum level of acceptability of freshwater and marine fish after the day of sensory rejection by the sensory panel members.
H2S forming bacteria
Offensive, fishy, rotten, and H2S-off-odors and off-flavors associated with the spoilage of marine, temperate water fish stored in melting ice is caused by sulphide producers, the predominant sulphide-producing bacterium being Shewanella putrefaciens. In warmer waters, Pseudomonas spp. can be the dominant spoilage bacteria.[Citation30] Counts of sulphide producers have been used as indicators of iced fish spoilage. On the initial day, H2S producing bacteria were absent both in the case of whole and gutted sample. H2S producing bacteria counts were higher for gutted than for whole samples throughout the entire storage period and reached a final level of 5.57 and 6.01 log cfu/g for the whole and gutted samples, respectively. Counts of sulphide producers in the range of log 6–6.7 are normally present in rejected fish from temperate and tropical waters.[Citation31]At the time of rejection of sea bass and sea bream during ice storage counts of H2S-producing bacteria of 5 log cfu/g after 14–15 days was noticed.[Citation17,Citation32] The result was in agreement with previous studies.[Citation33] On the contrary, lower counts of H2S producing bacteria have been reported for iced storage European sea bass at the spoilage time.[Citation17] It can be concluded that that the whole fish has a greater sensorial and microbiological quality than gutted and filleted fish.[Citation33,Citation34]
Total coliform bacteria
The total coliform count increased with the increase in storage time in both the whole and gutted samples. The coliform count in the gutted samples was higher than the whole samples in the entire study period and were statistically significant (p < 0.05). Total coliform counts were generally lower than total aerobic plate count throughout the period of the experiment. and revealed that in whole samples the coliform count was found to increase from 1.3 to 2.36 log cfu/g and in gutted samples from 1.5 to 4.08 log cfu/g along with storage. The higher incidence in gutted samples can be due to secondary contamination during handling and storage.[Citation35]
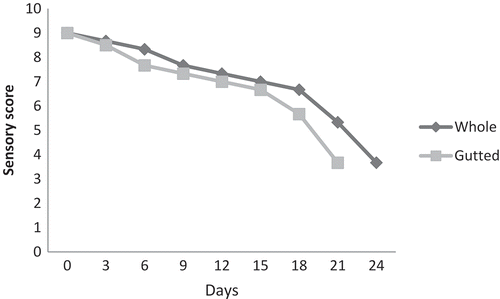
Sensory characteristics
Acceptability of fish and fishery products during storage is influenced by the changes in their sensory attributes. Changes in sensory characteristics of whole and gutted monosex tilapia during iced storage were given in . Significant differences were found in the acceptability score during the storage periods (p < 0.05). Whole samples got rejected by the sensory panel on the 24th day of storage. Gutted samples were considered to be acceptable for human consumption until the sensory score reached 4.0 based on 9-point hedonic scales11A significant (p < 0.05) decrease in the acceptability score was noticed along with storage. The gutted samples got rejected by the sensory panel on the 21st day of storage. The difference in sensory quality between whole and gutted samples can be attributed to the gutting procedure which can cause cross contamination of the fish from processing tables, knives, etc., or due to the higher fish flesh area exposed to environmental microbial contamination in the case of gutted fish.[Citation24] This study allowed us to describe the changes in indicators of biochemical and microbilogical action in farmed monosex tilapia during iced storage and its shelflife. We could evaluate the changes in the content of biogenic amines both in the case of whole and gutted samples during iced storage.
Conclusions
The biochemical indices, namely TVBN and pH, did not cross the acceptable limit during iced storage both in the case of whole and gutted samples. Changes in K value were agreeing with the sensory evaluation results. However, presence of PUT and CAD, sensory evaluation and microbial count determined the shelf life of 21 and 24 days for the gutted and whole samples respectively. Higher incidence of environmental microbial action due to increased flesh area after gutting might be contributed toward the shorter shelf life of gutted samples compared to the whole one. Adequate food handling practise and complete prevention of cross-contamination should be ensured in the gutting of fish as it is a sourse of contamination.
Acknowledgments
The authors would like to thank the Director, Indian Council of Agricultural Research-Central Institute of Fisheries Technology (ICAR-CIFT), Cochin for providing facilities to undertake this work.
Funding
This research work was completed with the support of Indian Council of Agricultural Research, New Delhi, India.
Additional information
Funding
References
- Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huisint Veld, J.H.J. Occurrence and Formation of Biologically Active Amines in Foods. International Journal of Food Microbiology 1990, 11, 73–84.
- Bunka, F.; Budinsky, P.; Zimakova, B.; Merhaut, M.; Flasarova, R.; Pachlova, V.; Kuban, V.; Bunkova, L. Biogenic Amines Occurrence in Fish Meat Sampled from Restaurants in Region of Czech Republic. Food Control 2013, 31, 49–52.
- Ryder, J.M. Determination of Adenosine Triphosphate and Its Breakdown Products in Fish Muscle by High Performance Liquid Chromatography. Journal of Agricultural Food Chemistry 1985, 33, 678–680.
- Ocaño-Higuera, V.M.; Maeda-Martinez, A.N.; Marquez-Rios, E.; Canizales-Rodriguez, D.F.; Castillo-Yanez, F.J. Freshness Assessment of Ray Fish Stored in Ice by Biochemical, Chemical and Physical Methods. Food Chemistry 2011, 125, 49–54.
- AOAC. Official Methods of Analysis, 15th Ed; Association of Official Analytical Chemists: Washington, DC, 1990.
- Conway, E.J. Micro-Diffusion Analysis and Volumetric Error. Crosby Lockwood and Son Ltd.: London, 1950.
- Sagratini, G.; Fernandez-Franzon, M.; De Berardinis, F.; Font, G.; Vittori, S.; Manes, J. Simultaneous Determination of Eight Underivatised Biogenic Amines in Fish by Solid Phase Extraction and Liquid Chromatography–Tandem Mass Spectrometry. Food Chemistry 2012, 132, 537–543.
- AOAC. Official Methods of Analysis. Ch. 17, Vol. 1.; Association of Official Analytical Chemists International: Gaithersburg, Arlington, Virginia, USA 2002; 4–5, 52 p.
- Gennari, M.; Campanini, R. Isolamento e Caratterizzazione di Shewanellaputrefaciens da Pesce Fresco e Alterato, Carnifresche e Alterate, Prodottilattiero-Caseari, Acqua e Suolo [Isolation and Characterization of Shewanellaputrefaciens from Fish Fresh and Altered, Carnifresche and Weathered, Prodottilattiero-Dairy Products, Water and Soil]. IndAlimentas 1991, 30, 965–976, 988.
- APHA. Standard Methods for the Examination of Water and Waste Water, 20th Ed; American Public Health Association: Washington, D.C., Part 9000-9221, 1998; 48–59 p.
- Amerine, M.A.; Pongborn, R.; Roescler, E.B. Principles of Sensory Evaluation of Food. Academic Press: New York, NY, 1965; 602 pp.
- Simeonidou, S.; Govaris, A.; Vareltzis, K. Quality Assessment of Seven Mediterranean Fish Species During Storage on Ice. Food Research International 1998, 30, 479–484.
- Tejada, M.; Huidobro, A. Quality of Farmed Gilthead Seabream (Sparusaurata) During Ice Storage Related to the Slaughter Method and Gutting. European Food Research Technology 2002, 215, 1–7.
- Connel, J.J. Control of Fish Quality, Fishing New Books. Blackwell Science Ltd.: Cambridge, London, 1995; 241 p.
- Ababouch, L.H.; Souibri, L.; Rhaliby, K.; Ouahdi, O.; Battal, M.; Busta, F.F. Quality Changes in Sardines (Sardinapilchardus) Stored in Ice and at Ambient Temperature. Food Microbiology1996, 13(2), 123–132.
- Lakshmanan, R.; Shakila, R.J.; Jeyasekaran, G. Survival of Amine-Forming Bacteria During the Ice Storage of Fish and Shrimp. Food Microbiology 2002, 19, 617–625.
- Kyrana, V.R.; Lougovois, V.P. Sensory, Chemical and Microbiological Assessment of Farm-Raised European Sea Bass (Dicentrarchuslabrax) Stored in Melting Ice. International Journal of Food Science and Technology 2002, 37, 319–328.
- Ehira, S.; Uchiyama, H. Determination of Fish Freshness Using the K Value and Comments on Some Other Biochemical Change in Relation to Freshness. In Seafood Quality Determination; Kramer, D.E.; Liston, J.; Eds.; Elsevier: New York, NY, 1987; 185–207 p.
- Saito, T.; Arai, T.; Mutsuyoshi, M. A new Method for Estimating the Freshness of Fish. Bulletin of Japanese Society of Scientific Fisheries 1959, 24, 749–750.
- Ehira, S.; Uchiyama, H. Freshness Lowering Rates of Cod and Seabream Viewed from Changes in Bacterial Count, Total Volatile Base and Trimethyl Amine Nitrogen and ATP Related Compounds. Bulletin of Japanese Society of Scientific Fisheries 1974, 40, 479–487.
- Alasalvar, C.; Taylor K.D.A.; Oksuz, A.; Garthwaite, T.; Alexis, M.N.; Grigorakis, K. Freshness Assessment of Cultured Sea Bream (Sparusaurata) by Chemical, Physical and Sensory Methods. Food Chemistry 2001, 72(1), 33–40.
- Icekson, I.; Pasteur, R.; Drabkin, V.; Lapidot, M.; Eizenberg, E.; Klinger, I.; Gelman, A. Prolonging Shelf‐Life of Carp by Combined Ionising Radiation and Refrigeration. Journal of Science Food and Agriculture 1996, 72, 353–358.
- Ozogul, F.; Kamari, N.; Kuley, E.; Ozogul, Y. The Effects of Ice Storage on Inosine Monophosphate, Inosine, Hypoxanthine and Biogenic Amine Formation in European Catfish (Silurusglanis) Fillets. International Journal of Food Science and Technology 2009, 44, 1966–1972.
- Rong, C.; Chang-hu, X.; Liu, Q.; Yin, B. Microbiological, Chemical and Sensory Assessment of (I) Whole Ungutted, (II) Whole Gutted and (III) Filleted Tilapia (Oreochromisniloticus) During Refrigerated Storage. International Journal of Food Science and Technology 2009, 44, 2243–2248.
- Castillo-Yanez, F.J.; Jimenez- Ruiz, E.I.; Canizales- Rodriguez, D.F.; Marquez-Rios, E.; Montoya-Camacho, N.; Ruiz-Cruz, S.; Ocano-Higuera, V.M. Postmortem Biochemical Changes and Evaluation of the Freshness in the Muscle of Tilapia (Oreochromisniloticus) during the Storage in Ice. Journal of Fisheries and Aquatic Sciences 2014, 9, 435–445.
- Kulawik, P.; Ozogul, F.; Glew, R.H. Quality Properties, Fatty Acids, and Biogenic Amines Profile of Fresh Tilapia Stored in Ice. Journal of Food Science 2013, 78(7):s1063–8. doi: 10.1111/1750-3841.12149
- ICMSF. International Commission on Microbiological Specification for Foods. Microorganisms in Foods. Sampling for Microbiological Analysis: Principles and Specific Applications, 2nd Ed; International Commission on Microbiological Specifications for Foods, 1986.
- Gelman, A.; Glatman, L.; Drabkin, V.; Harpaz, S. Effects of Storage Temperature and Preservative Treatment on Shelf Life of the Pond-Raised Freshwater Fish, Silver Perch (Bidyanusbidyanus). Journal of Food Protection 2001, 64, 1584–1591.
- Savvaidis, I.N.; Skandamis, P.N.; Riganakos, K.A.; Panagiotakis, N.; Kontominas, M.G. Control of Natural Microbial Flora and Listeria Monocytogenes in Vacuum-Packaged Trout at 4 and 101C Using Irradiation. Journal of Food Protection 2002, 65, 515–522.
- Koutsoumanis, K.; Nychas, G.J.E. Chemical and Sensory Changes Associated with Microbial Flora of Mediterranean Boque (Boopsboops) Stored Aerobically at 0, 3, 7 and 10ºC. Applied Environmental Microbiology 1999, 65, 698–706.
- Hanna, J. Rapid Microbial Methods and Fresh Fish Quality Assessment. In Fish Processing Technology; Hall, G.M. Ed.; Blackie Academic & Professional: London, UK, 1992, 252–254 p.
- Lougovois, V.P.; Kyranas, E.R.; Kyrana, V.R. Comporasion of Selected Methods of Assessing Freshness Quality and Remaining Storage Life of Iced Gilthead Sea Bream (Sparusaurata). Food Research International 2003, 36, 551–560.
- Paleologos, E.K.; Savvaidis, I.N.; Kontominas, M.G. Biogenic Amines Formation and Its Relation to Microbiological and Sensory Attributes in Ice-Stored Whole, Gutted and Filleted Mediterranean Sea Bass (Dicentrarchuslabrax). Food Microbiology 2004, 21, 549–557.
- Chytiri, S.; Chouliara, I.; Savvaidis, I.N.; Kontominas, M.G. Microbiological, Chemical and Sensory Assessment of Iced Whole and Filleted Aquacultured Rainbow Trout. Food Microbiology 2004, 21, 157–165.
- Mandal, S.C.; Hasan, M.; Rahman, M.S. Coliform Bacteria in Nile Tilapia, Oreochromisniloticus of Shrimp-Gher, Pond And Fish Market. World Journal of Fish and Marine Sciences 2009, 3, 160–166.