ABSTRACT
The aim of this study was to investigate the influence of microwave vacuum drying on carotenoids in pumpkin (Cucurbita maxima L.) slices. Carotenoids were measured using the reverse-phase high-performance liquid chromatography technique. It was shown that compared with hot air drying, microwave vacuum drying inhibited color changes and significantly (p < 0.05) improved total carotenoid retention (89.1%) in pumpkin slices. During the microwave vacuum drying process, microwave power had an important effect on total carotenoid and all-trans carotenoids. As microwave power increased, the total carotenoid content significantly decreased (p < 0.05), and the levels of individual carotenoids, including all-trans-α-carotene, all-trans-β-carotene, and all-trans-lutein, generally decreased. However, there was an overall upward trend for the levels of 13-cis-β-carotene, 15-cis-β-carotene, 9-cis-β-carotene, and 9-cis-α-carotene. The trans carotenoid quality of the finished products was improved within a certain range of vacuum levels. In addition to the degradation induced by microwave energy, isomerization was considered to be responsible for the loss of all-trans carotenoids. These results indicated that inappropriate drying methods and conditions might result in high losses of all-trans carotenoids in pumpkins.
KEYWORDS:
Introduction
Pumpkin (Cucurbita maxima L.) is a gourd squash of the genus Cucurbita in the family Cucurbitaceae and is widely grown and consumed in many countries around the world.[Citation1] It is appreciated by consumers for its soft and sweet taste and its high nutritive value, it is an especially excellent source of carotenoids such as β-carotene, lutein, and zeaxanthin.[Citation2,Citation3] In the fresh mass of the fruit, the total carotenoid (TC) content ranges from 0.3 to 12 mg/g FW. In addition to pro-vitamin A activity, studies have indicated that consumption of the carotenoids in pumpkin lowers the risk of degenerative and cardiovascular diseases, cataracts, macular degeneration, and certain types of carcinomas.[Citation4–Citation7] The findings regarding the antioxidant activities and singlet oxygen-quenching and free radical-scavenging abilities of carotenoid compounds and their benefits to human health have increased research interest tremendously.[Citation8,Citation9]
Microwave vacuum drying (MVD) is one of the advanced methods used for drying fruits and vegetables.[Citation10] MVD has shown potential in improving process efficiency and the quality of dried products, showing an advantage related to carotenoid retention in pumpkin by speeding up the drying process at a comparatively low temperature. However, various microwave drying conditions, such as the applied microwave power, vacuum levels, and drying time, have significant effects on carotenoid contents.[Citation11,Citation12] Because much of the bioactivity of carotenoids can be lost due to the conversion of all-trans-isomers to cis-isomers,[Citation13,Citation14] it is necessary to identify optimized MVD conditions that have the least impact on carotenoid compounds for further processing. In addition, it would be desirable to accurately assess the characteristics and quantities of carotenoid isomers, rather than just the TC content without knowledge of its isomeric composition. Compared with conventional hot air drying, MVD can reduce the drying time by 70–90%, in addition to improving rehydration characteristics.[Citation15] However, no reports on the effect of MVD on carotenoids in dehydrated pumpkin slices are currently available. The objectives of this study were, therefore, to investigate the influence of MVD parameters on carotenoids in pumpkin slices and their degradation and cis-trans isomerization.
Materials
Fresh material
Ripe pumpkins (Cucurbita maxima L. “Benmi”) were obtained from the local market in Nanjing, China. Whole pumpkins showing uniformity of the raw material without any damage were selected. The moisture content of the pumpkins was 87.8 ± 0.6 g/100 g fresh weight (FW). Before each experiment, pumpkins were removed from the storage compartment (4°C), left to equilibrate at room temperature, and washed with tap water. Prior to drying, the pumpkins were peeled, their seeds were removed, and they were then cut into slices with thickness of 6 mm and placed in 100°C water for 40 s.
Chemicals and reagents
Lutein (95% pure, Sigma-Aldrich, catalogue no. C-9750) and β-carotene (≥95% pure, Sigma-Aldrich, catalogue no. C4582) were obtained from Sigma (St. Louis, MO). β-cryptoxanthin (97% pure, CaroteNature, catalogue no. 0055) was procured from CaroteNature (Lupsingen, Switzerland). Methyl tert-butyl ether (MTBE) and methanol were purchased from Tedia (Fairfield, USA). Hexane, anhydrous sodium sulphate, acetone, toluene, and ethanol were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All other chemicals and reagents were of analytical grade.
Methods
Drying treatment
Three drying methods were tested. (1) For MVD, in each experiment, approximately 100 g of sliced pumpkin was placed in a microwave-vacuum dryer (MVD-1, Nanjing Xiaoma Electrome-chanical Equipment Factory, Nanjing, China), and different microwave powers (4, 5, 6, 7, and 8 W/g) and pressure levels (–50, –60, –70, –80, and –90 KPa) were tested. The samples remained in the container for 6 min while drying took place and were then withdrawn to be freeze-dried until the moisture content was reduced to approximately 5.0%. (2) For freeze drying (FD), pumpkin slices were weighed and lyophilized (FD-1A-50, Beijing Boyikang Laboratory Instruments Co., Ltd, China) at –50°C (vacuum degree of 15 Pa) until a constant weight was obtained with an approximately 5.0% water content. (3) For hot air drying (HD), pumpkin slices were hot air dried at an air temperature of 60°C in a cross-flow type dryer with an air flow rates of 1.5 m/s. Air was heated electrically before entering the heater. Slices were spread in a single layer on the tray, and the samples were dried until the moisture content was reduced to approximately 5.0%.
Color measurement
Pumpkin color was measured with a colorimeter (WSC-S, Shanghai Precision & Scientific Instrument Co., Ltd., China) and reported as L*, a*, and b* values. The L* value represents lightness; the a* value represents redness (positive value) and greenness (negative value); and the b* value represents yellowness (positive value) and blueness (negative value). The chroma is an expression of the saturation or intensity of the color attained, and the Hue, or the observable color (e.g., red, blue, yellow), is an angular measurement, where 0° indicates a red hue, 90° a yellow hue, 180° a green hue, and 270° a blue hue. The color index chromatic attributes C* (chrome) and H* (metric angle) are calculated according to the following equations:
Total color differences (ΔE) are calculated using the following equation:
where L0, a0, and b0 are the control values for freeze-dried pumpkin.
Carotenoid extraction
Carotenoid extraction was carried out according to the method reported previously, with some modifications.[Citation16] Prior to analysis, the slice samples were ground to a powder using a grinder (FW100, Tianjin Taisite Instrument Co., Ltd., China) and sieved using 40 standard meshes to ensure symmetry of particle size. A 0.5-g sample of pumpkin powder was treated with 30 mL of a mixture of acetone-petroleum ether (2:1, v/v) in a 100-mL volumetric flask, followed by shaking for 4 h. To the contents, 2 mL of 10% methanolic potassium hydroxide (KOH) was added for saponification at 25°C in the dark under nitrogen gas for 12 h. After saponification, 30 mL of hexane was added for partitioning of carotenoids, followed by shaking for 1 min, after which a 10% sodium sulfate solution was added, and the sample was diluted to volume. The mixture was allowed to stand until two phases separated clearly. The upper layer containing lutein was collected, evaporated to dryness, dissolved in 10 mL of methanol, and filtered through a 0.45-µm membrane filter for high-performance liquid chromatography (HPLC) analysis. The whole extraction procedure was carried out under dimmed light, and nitrogen gas was flushed into vials to avoid isomerization or degradation of carotenoids.
High performance liquid chromatography-diode array detection-tandem mass spectrometry (HPLC-DAD-MS/MS) analysis
For the analysis of carotenoids, published procedures were adopted.[Citation17] The HPLC analysis was performed using an analytical-scale C30 reversed-phase column (250 mm × 4.6 mm i.d.) with a particle size of 5 µm (YMC, Wilmington, MA, USA). Eluent A consisted of methanol/MTBE/water (70: 25: 5, v /v), and eluent B was prepared by mixing MTBE/methanol/water (85: 10:5, v/v). Separation was performed at a column temperature of 25°C using gradient elution conditions within 30 min at a flow rate of 0.6 mL/min. Aliquots of 20 µL were used for HPLC. The solvent gradient elution program employed was as follows: 0–4.5 min, linear gradient 95%A–80%A; 4.5–12.5 min, linear gradient 80%A–50%A; 12.5–18 min, linear gradient 50%A–25%A; 18–24 min, linear gradient 25%A–5%A; and finally, a return to the initial conditions.
The MS was set as follows: Experiments were carried out on an Agilent 1290 Infinity LC/Agilent Technologies 6530 Q-TOF MS (Agilent Technologies, Santa Clara, CA, USA). The positive ion mode (APCI) was used to detect carotenoids, with a total ion current (TIC) scanning range of 80 to 1000 m/z, a corona current of 4 μA, capillary voltage of 2500 V, nitrogen as the nebuliser gas (purity 99.9% and flow rate 4 L/min) and a vapourizer temperature of 350°C. Quantification was performed using the external standards method, after the preparation of a 5-point external standard calibration curve for each available standard; standard calibration curve RCitation2 values were in the range of 0.9991 to 0.9995.
Statistical analysis
All experiments were carried out at least in duplicate. The data were subjected to analysis of variance and Duncan’s multiple range tests, performed with Statistical Analysis System (SAS) software (SAS institute, Cary, NC, USA), at the 95% confidence interval to determine any significant difference between the various treatments.
Results and discussion
Sensory evaluation
Pumpkin slices were dried separately through MVD, FD, and HD, resulting in pumpkin chips (). As shown in , among the dehydrated samples, the freeze-dried samples exhibited the highest L* values, indicating that the least light was reflected, whereas the hot air dried samples showed a significantly lower L* value (p < 0.05), and no significant difference was found between MVD and FD. Decreasing the L* value resulted in darker dried pumpkin products, indicating that the Hunter L* value was more influenced by HD. This result was in agreement with the results of Chen et al.[Citation18] related to dehydration of mango. Regarding a* and b* values, FD and MVD were not significantly different, but these samples were redder than the HD samples (p < 0.05). This result was correlated with greater loss of carotenoids in the HD pumpkin slices because the yellow and/or red color of pumpkin slices is largely attributed to the presence of various carotenoids. The ΔE values of the air dried samples were significantly higher than those of the microwave vacuum-dried samples (p < 0.05), which could be due to the shorter drying time and vacuum conditions of MVD. An improvement of color is also associated with a decreased pressure during the drying of potato.[Citation19] According to a previous report,[Citation20] the hue angle exhibits the most significant correlation with visual scores, and the chroma value (C) provides a good indication of the amount of color. The hue angles (H) of the HD samples were significantly decreased compared with the FD and MVD samples, while a comparatively high C value was observed, which indicates brown coloration of the processed fruits. The reason for this result may be that in addition to thermal pigment degradation, Maillard reactions might also be responsible for the formation of brown compounds.
Table 1. Color of pumpkin slices by different drying methods.
Figure 1. Dried pumpkin chips after A: freeze drying; B: hot air drying; and C: microwave vacuum drying.

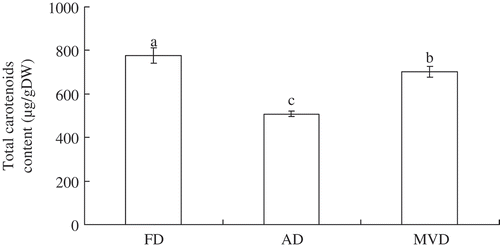
In , it can be observed that significant differences (p < 0.05) in the TC content were found between the three drying methods. FD resulted in the highest levels of TC (776 µg/g dry weight [DW]), followed by MVD and AD (509 µg/g DW). The drastic reduction in TC retention observed in AD compared with the FD or MVD method suggested an obvious detrimental effect of heat and oxygen on the stability of these pigments. Regier et al.[Citation21] compared the convectional drying of lycopene-rich carrots with MVD drying and concluded that the two dried products showed a similar carotenoid stability. Yan et al.[Citation22] also found that the highest carotenoid content of sweet potato dices was associated with microwave-spouted bed drying (MSBD) and MVD, reaching approximately 80% of the value in the FD product. For HD products, only approximately 40% retention was observed. These results could be explained by a reduction of the loss of TC during drying due to the rapid heating rate and depletion of oxygen associated with a vacuum microwave.
Effect of MVD conditions on carotenoid degradation
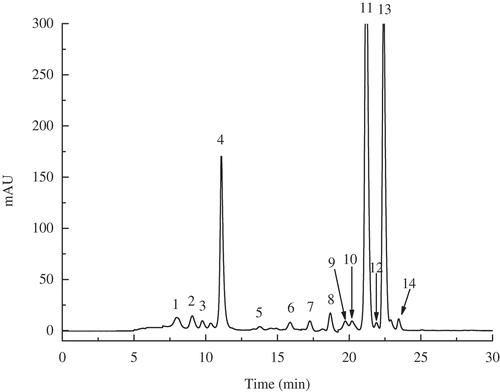
A typical HPLC chromatogram profile of carotenoids extracted from MVD pumpkin slices is shown in . Most of the peaks showed an adequate resolution, and 14 carotenoids were isolated and identified as (1) carotenes (all-trans-α-carotene, 9-cis-α-carotene, all-trans-β-carotene, 9-cis-β-carotene, 15-cis-β-carotene, and 13-cis-β-carotene); and (2) xanthophylls (α-cryptoxanthin, β-cryptoxanthin, β-carotene-5, 6-epoxide, violaxanthin, all-trans-lutein, neoxanthin, neochrome, and 9-cis-lutein). The identification of these carotenoids was accomplished by comparing retention times, ultraviolet (UV)-visible absorption maxima, electron ionization, and chemical ionisation mass spectroscopy (EIMS and CIMS) fragmentation patterns.[Citation23] The major carotenoids, including all-trans-β-carotene, all-trans-α-carotene, all-trans-lutein, and β-carotene isomers, were similar to the results reported by others.[Citation24] To further investigate the effect of different MVD conditions on the carotenoids in pumpkin slices, the carotenoid levels associated with various microwave powers and vacuum categories were individually assessed.
Figure 3. HPLC chromatograms of carotenoids in pumpkin slices under microwave vacuum drying (MVD). Peak identification: 1. neoxanthin, 2. neochrome, 3. violaxanthin, 4. all-trans-lutein, 5. 9-cis-lutein, 6. β-carotene-5, 6-epoxide, 7. α-cryptoxanthin, 8. β-cryptoxanthin, 9. 15-cis-β-carotene, 10. 13-cis-β-carotene, 11. all-trans-α-carotene, 12. 9-cis-α-carotene, 13. all-trans-β-carotene, and 14. 9-β-carotene.
Microwave power
It was found that microwave power significantly (p < 0.05) affected TC contents (). The TC value was lowest (564 µg/g DW) under a power level of 8 W/g. One of reasons for this result might be that higher microwave power caused a rapid increase in the temperature of the product, thus accelerating the decomposition of carotenoids. The observed results were in agreement with previous research conducted by the authors.[Citation25,Citation26] When the microwave power was increased from 4 to 8 W/g, a pronounced decline in trans carotenoids was observed, corresponding to 17 μg/g DW, 97 μg/g DW and 81 µg/g for all-trans-lutein, all-trans-β-carotene, and all-trans-α-carotene, respectively. The content of all-trans-β-carotene in pumpkin slices under a microwave power of 8 W/g decreased more rapidly than under a microwave power of 4 W/g, which indicated that all-trans-β-carotene was more sensitive to microwave power. Additionally, the levels of 13-cis-β-carotene and 15-cis- β-carotene significantly increased (p < 0.05) when the microwave power was increased to 8 W/g, up to contents of 17.0 and 23.1 µg/g DW, respectively. The contents of 9-cis-α-carotene and 9-cis-β-carotene showed the same trends, first decreasing and then increasing, but the 9-cis-β-carotene content was lower at all of the time points. It is well documented that isomerization may proceed during microwave heating of all-trans-α-carotene or all-trans-β-carotene, with both 9-cis and 13-cis-isomers being dominant, and the latter forms at higher levels than the former. A similar phenomenon was observed by Guo et al.,[Citation27] as the activation energy required for the isomerisation of all-trans-β-carotene to 13-cis-β-carotene was shown to be lower than that for other cis isomers. Isomerization is encouraged, but oxidation is also responsible for the loss of total all-trans carotenoids.
Table 2. Quantitative changes in the carotenoid composition of MVD pumpkin slices under different microwave powers (μg/gDW).
The results presented in show that there was a considerable increase in the content of β-carotene-5, 6-epoxide; over-heating all-trans-β-carotene in pumpkin slices might lead to the formation of β-carotene-5, 6-epoxide.[Citation28] This could be explained by the fact that during drying with microwaves, heat is not only transferred through the surface, but the generation of volumetric heating also causes rapid transfer of energy to the sample.[Citation29]
Vacuum
As shown in , vacuum did not have a significant (P>0.05) impact on the TC levels in pumpkin slices, but the retention rate of TCs was more than 80% among various vacuum levels and was higher compared with the equivalent microwave drying process without vacuum. Thus, within a certain range of vacuum, the TC quality of the finished products was improved. Part of the reason for this improvement may be that the boiling point of water in pumpkin slices is relatively higher at a certain vacuum level than in the normal atmosphere, causing the temperature of the material to rise faster and to a higher level than under high vacuum levels, which would readily lead to burning of the final products and affect the quality of their TCs.[Citation11] Another explanation may be that under vacuum, microwave drying reduces TC degradation due to the absence of oxidation. [Citation30] When the level of vacuum increased, the all trans-α-carotene content increased, and when the level of vacuum was –80 and –90 KPa, the all trans-α-carotene content was increased by 20 and 25.1 µg/g DW, respectively, compared with a vacuum level of –50 KPa. These results indicated that a higher vacuum level deterred oxidative degradation due to limited oxygen exposure. It also shortened the drying time through increasing the driving force for mass transfer and facilitating the evaporation and volatilization of water from the materials, thus greatly preventing loss. Lin et al.[Citation31] also demonstrated that the rapid heating rate and depletion of oxygen associated with a vacuum microwave reduced the loss of α-carotene in carrot slices during drying. Simultaneously, 13-cis-β-carotene, 15-cis-β-carotene, and 9-cis-β-carotene contents were decreased, while the contents of all-trans-lutein, all trans-β-carotene, and 9-cis-α-carotene were not significantly affected (p > 0.05). The reverse isomerization reaction may occur, and the conversion rate of β-carotene cis isomers to all-trans-β-carotene is faster than the isomerisation of all-trans-β-carotene.[Citation32]
Table 3. Quantitative changes in the carotenoid composition of MVD pumpkin slices under different vacuum levels (μg/g DW).
Conclusion
The present study demonstrated that compared with FD, the retention of TCs in pumpkin slices after MVD was significantly higher (p < 0.05) than after HD. Microwave power had a significant effect on TC. With an increasing microwave power, the TC content significantly decreased (p < 0.05). Moreover, the levels of individual carotenoids, including all-trans-α-carotene, all-trans-β-carotene, and all-trans-lutein, generally decreased during MVD. However, the contents of 13-cis-β-carotene, 15-cis-β-carotene, 9-cis-β-carotene, and 9-cis-α-carotene exhibited an overall upward trend. In addition to the degradation induced by microwave energy, isomerization was also considered to be responsible for the loss of these all-trans carotenoids. The results showed that inappropriate drying methods and conditions resulted in a large loss of all-trans carotenoids. The significant findings of this research can provide a technical basis for the drying of pumpkin and can serve as a reference for optimisation of the drying process for other fruits and vegetables.
Funding
The research was financially supported by the special fund for agro-scientific research in the public interest (No.201503142-5) and the basal research fund for the Jiangsu Academy of Agricultural Sciences (major cultivation project No. ZX(15)1008).
Additional information
Funding
References
- Paris, H.S.; Brown, R.N. The Genes of Pumpkin and Squash. HortScience 2005, 40, 1620–1630.
- Ben-Amotz, A.; Fishier, R. Analysis of Carotenoids with Emphasis on 9-cis β-carotene in Vegetables and Fruits Commonly Consumed in Israel. Food Chemistry 1998, 62, 515–520.
- Murkovic, M.; Piironen, V.; Lampi, A.M.; Kraushofer, T.; Sontag, G. Changes in Chemical Composition of Pumpkin Seeds During the Roasting Process for Production of Pumpkin Seed Oil (Part 1: Non-Volatile Compounds). Food Chemistry 2004, 84, 359–365.
- Sommerburg, O.; Keunen, J.E.; Bird, A.C.; Van Kuijk, F.J. Fruits and Vegetables That Are Sources for Lutein and Zeaxanthin: The Macular Pigment in Human Eyes. British Journal of Ophthalmology 1998, 82, 907–910.
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid Content in Different Varieties of Pumpkins. Journal of Food Composition and Analysis 2002, 15, 633–638.
- Adaramoye, O.A.; Achem, J.; Akintayo, O.O.; Fafunso, M.A. Hypolipidemic Effect of Telfairia Occidentalis (Fluted Pumpkin) in Rats Fed a Cholesterol-Rich Diet. Journal of Medicinal Food 2007, 10, 330–336.
- Aizawa, K.; Inakuma, T. Quantitation of Carotenoids in Commonly Consumed Vegetables in Japan. Food Science and Technology Research 2007, 13, 247–252.
- Paiva, S.A.; Russell, R.M. β-Carotene and Other Carotenoids as Antioxidants. Journal of the American College of Nutrition 1999, 18, 426–433.
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free Radicals and Antioxidants in Human Health: Current Status and Future Prospects. JAPI 2004, 52, 794–804.
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent Developments in High-Quality Drying of Vegetables, Fruits and Aquatic Products. Critical Reviews in Food Science and Nutrition 2015, DOI:10.1080/10408398.2014.979280
- Cui, Z.W.; Xu, S.Y.; Sun, D.W. Effect of Microwave-Vacuum Drying on the Carotenoids Retention of Carrot Slices and Chlorophyll Retention of Chinese Chive Leaves. Drying Technology 2004, 22, 563–575.
- Bechoff, A.; Westby, A.; Owori, C.; Menya, G.; Dhuique‐Mayer, C.; Dufour, D.; Tomlins, K. Effect of Drying and Storage on the Degradation of Total Carotenoids in Orange-Fleshed Sweetpotato Cultivars. Journal of the Science of Food and Agriculture 2010, 90, 622–629.
- Shi, J.; Le Maguer, M.; Kakuda, Y.; Liptay, A.; Niekamp, F. Lycopene Degradation and Isomerization in Tomato Dehydration. Food Research International 1999, 32, 15–21.
- Shi, J.; Wu, Y.; Bryan, M.; Le Maguer, M. Oxidation and Isomerization of Lycopene Under Thermal Treatment and Light Irradiation in Food Processing. Journal of Food Science and Nutrition 2002, 7, 179–183.
- Arévalo-Pinedo, A.; Murr, F.E. Kinetics of Vacuum Drying of Pumpkin (Cucurbita Maxima): Modeling with Shrinkage. Journal of Food Engineering 2006, 76, 562–567.
- Li, D.J.; Song, J.F.; Liu, C.Q. Kinetic Stability of Lutein in Freeze-Dried Sweet Corn Powder Stored under Different Conditions. Food Science and Technology Research 2014, 20, 65–70.
- Song, J.F.; Li, D.J.; He, M.J.; Chen, J.Q.; Liu, C.Q. Comparison of Carotenoid Composition in Immature and Mature Grains of Corn (Zea Mays L.) Varieties. International Journal of Food Properties 2016, 19, 351–358.
- Chen, J.P.; Tai, C.Y.; Chen, B.H. Effects of Different Drying Treatments on the Stability of Carotenoids in Taiwanese Mango (Mangifera Indica L.). Food Chemistry 2007, 100, 1005–1010.
- Bondaruk, J.; Markowski, M.; Błaszczak, W. Effect of Drying Conditions on the Quality of Vacuum-Microwave Dried Potato Cubes. Journal of Food Engineering 2007, 81, 306–312.
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Evolution of Colour and Anthocyanins in Cherries (Prunus Avium L.). Food Chemistry 2007, 103, 976–984.
- Regier, M.; Mayer-Miebach, E.; Behsnilian, D.; Neff, E.; Schuchmann, A. Influences of Drying and Storage of Lycopene-Rich Carrots on the Carotenoid Content. Drying Technology 2005, 23, 989–998.
- Yan, W.Q.; Zhang, M.I.N.; Huang, L.L.; Mujumdar, A.S.; Tang, J. Influence of Microwave Drying Method on the Characteristics of the Sweet Potato Dices. Journal of Food Processing and Preservation 2013, 37, 662–669.
- Song, J.F.; Li, D.J.; Pang, H.L.; Liu, C.Q. Effect of Ultrasonic Waves on the Stability of All-Trans Lutein and Its Degradation Kinetics. Ultrasonics Sonochemistry 2015, 27, 602–608.
- Kurz, C.; Carle, R.; Schieber, A. HPLC-DAD-MSn Characterisation of Carotenoids from Apricots and Pumpkins for the Evaluation of Fruit Product Authenticity. Food Chemistry 2008, 110, 522–530.
- Soysal, Y.; Ayhan, Z.; Eştürk, O.; Arıkan, M.F. Intermittent Microwave-Convective Drying of Red Pepper: Drying Kinetics, Physical (Colour and Texture) and Sensory Quality. Biosystems Engineering 2009, 103, 455–463.
- Bal, L.M.; Kar, A.; Satya, S.; Naik, S.N. Kinetics of Colour Change of Bamboo Shoot Slices During Microwave Drying. International Journal of Food Science & Technology 2011, 46, 827–833.
- Guo, W.H.; Tu, C.Y.; Hu, C.H. Cis-Trans Isomerizations of β-Carotene and Lycopene: A Theoretical Study. The Journal of Physical Chemistry B 2008, 112, 12158–12167.
- Rodriguez, E.B.; Rodriguez-Amaya, D.B. Formation of Apocarotenals and Epoxycarotenoids from β-Carotene by Chemical Reactions and by Autoxidation in Model Systems and Processed Foods. Food Chemistry 2007, 101, 563–572.
- Andres, A.; Bilbao, C.; Fito, P. Drying Kinetics of Apple Cylinders Under Combined Hot Air-Microwave Dehydration. Journal of Food Engineering 2004, 63, 71–78.
- Pérez-Gálvez, A.; Mínguez-Mosquera, M.I. Structure-Reactivity Relationship in the Oxidation of Carotenoid Pigments of the Pepper (Capsicum Annuum L.). Journal of Agricultural and Food Chemistry 2001, 49, 4864–4869.
- Lin, T.M.; Durance, T.D.; Scaman, C.H. Characterization of Vacuum Microwave, Air and Freeze Dried Carrot Slices. Food Research International 1998, 31, 111–117.
- Zepka, L.Q.; Mercadante, A.Z. Degradation Compounds of Carotenoids Formed During Heating of a Simulated Cashew Apple Juice. Food Chemistry 2009, 117, 28–34.