ABSTRACT
The effects of fatty acid, monoacylglycerol, and polyglycerol fatty acid ester with varying chain length in their acyl chains on the extent of complex formation (complex index) and in vitro enzymatic digestibility of gelatinized potato starch were investigated. The complex index increased with increase in the concentration of the ligands (fatty acid, monoacylglycerol, and polyglycerol fatty acid ester), with the plateau in the complex index value depending on the type of ligands. In comparison of complex index among fatty acid-samples, the complex index maximum increased as the chain length increased up to octanoic acid and then decreased. In comparison of complex index among fatty acid-, monoacylglycerol-, and polyglycerol fatty acid ester-samples at each acyl chain, the complex index maximum followed the order polyglycerol fatty acid ester > monoacylglycerol > fatty acid. Fatty acid, monoacylglycerol, and polyglycerol fatty acid ester with long acyl chains greatly reduced the enzymatic hydrolysis of starch. Polyglycerol fatty acid ester with palmitic acid chains was the strongest inhibitor of starch hydrolysis, suggesting that further complex formation may occur during the hydrolysis of gelatinized starch (enzyme-annealing).
Introduction
Starch is composed of amylopectin, amylose, and minor components such as fatty acid (FA) and phosphate, depending on the botanical origin of the starch.[Citation1,Citation2] Amylose can form helical inclusion complexes with FA and emulsifiers containing acyl chains. Cereal starch containing FA essentially includes the complex.[Citation3] In addition, the complexes can be formed in gelatinized starch when amorphous amylose leaks from starch granules and then contacts FA and/or emulsifiers.[Citation4,Citation5] The formation of these complexes has attracted much attention for many years because of their impact on rheological properties,[Citation6–Citation8] retrogradation,[Citation9] and in vivo[Citation10] and in vitro digestibility[Citation4,Citation11] of gelatinized starch. Furthermore, the molecular structures of these complexes have been extensively studied.[Citation12–Citation18]
We previously studied the effects of long-chain FAs, including lauric (C12), myristic (C14), palmitic (C16), stearic (C18), oleic (C18:1), and linoleic (C18:2) acids, on the in vitro digestibility of gelatinized potato starch, and it was demonstrated that C12 and C18:1 caused the largest reduction in hydrolyzed starch content among the long-chain FAs.[Citation19] This result could be related to the extent and thermal stability of the complex. In addition, there was a possibility that further complex formation occurred during the hydrolysis of gelatinized starch, when FA existed as a liquid in partially hydrolyzed starch. Since the viscosity reflecting a large molecular interaction is diminished during the hydrolysis of gelatinized starch, the complex formation between partially hydrolyzed amylose and the FA would be promoted temporarily. In addition, when the gelatinized amylopectin was partially hydrolyzed, the steric hindrance between the branches of amylopectin would loosen, and thus further complex formation between partially hydrolyzed amylopectin and FA could be expected. This effect had been explained as “enzyme-annealing.”[Citation12,Citation13] Although the total energy content of the gelatinized starch is increased by the addition of FAs to gelatinized starch, the reduced hydrolysis rate of gelatinized starch will be useful for controlling glycemic response.
The previous observations suggested that the in vitro enzymatic digestibility of starch could be reduced further by using other FAs and emulsifiers. For example, short- and medium-chain FAs have higher water solubility than long-chain FAs and, therefore, will promote complex formation in gelatinized starch.[Citation20] In addition, these shorter chain FAs can exist in the liquid state in gelatinized starch solution, thus likely promoting the enzyme-annealing effect.[Citation12,Citation13] A disadvantage of using short- and medium-chain FAs is that the thermal stability of the complex is lower than long-chain FAs, because the resulting complexes have shorter helical complex.[Citation20] This disadvantage is eliminated by using emulsifiers with high water solubility, despite their long acyl chain. Indeed, it was reported that resistance to enzymatic hydrolysis of complexes formed between gelatinized starch and monoacylglycerol (MG) increased as the number of carbons in the MG increased.[Citation21,Citation22] Furthermore, a comparison of 30 emulsifiers showed that a decaglycerol monosterate-based emulsifier was most effective in reducing the in vitro starch digestibility.[Citation4]
Despite many reports on the effect of complex formation on in vitro starch digestibility, the data are insufficient to permit a systematic understanding of the characteristics, in part because different research groups use different assays to measure the in vitro enzymatic digestibility of starch. This was addressed in the present study by systematically investigating the effects of various types and amounts of short- and medium-chain FAs, MG, and polyglycerol fatty acid ester (PG) on the in vitro enzymatic digestibility of gelatinized potato starch.
Materials and methods
Sample preparation
Potato starch was purchased from Kanto Chemical Co., Inc., Japan. It is known that amylose content of potato starch is approximately 20% and that FA and/or complex are not contained essentially in the starch granules.[Citation1,Citation23,Citation24] Analytical grade acetic acid (C2), hexanoic acid (C6), octanoic acid (C8), decanoic acid (C10), glyceryl monolaurate (MG-C12), glyceryl monomyristate (MG-C14), glyceryl monopalmitate (MG-C16), and glyceryl monostearate (MG-C18) were purchased from Kanto Chemical Co., Inc., Tokyo, Japan. Polyglycerol lauric acid ester (PG-C12), polyglycerol myristic acid ester (PG-C14), polyglycerol palmitic acid ester (PG-C16), and polyglycerol oleic acid ester (PG-C18:1) were provided by Mitsubishi-Kagaku Foods Co., Tokyo, Japan. The product information given by the provider stated that the PG materials were mainly composed of decaglycerol; in addition, the hydrophilic-lipophilic balance (HLB) and esterification ratios were 17.5 and 5.8% for PG-C12, 17.1 and 5.8% for PG-C14, 16.3 and 6.7% for PG-C16, and 13.3 and 12.5% for PG-C18:1, respectively.
A suspension of potato starch in water (2%, w/w) was prepared in a glass vial, then stirred for 20 min in boiling water (above 90ºC) using a magnetic stirrer. The gelatinized starch (5 g) was placed in a glass vial, then short- and medium-chain FAs and MGs (0.15–2 mmol/g-starch) and PGs (45–600 mg/g-starch) were added to separate gelatinized samples. The molecular weights of the PGs could not be defined precisely, thus PGs were added on the basis of weight, similar to the addition of MGs (41–717 mg/g-starch). Each sample was hermetically sealed and mixed for 15 min using a magnetic stirrer at a temperature slightly higher than the melting temperature (Tm) of the ligand and intermittently subjected to vortex-mixing.
Complex index (CI)
The extent of complex formation was probed by investigating the reduction in the iodine binding capacity of starch.[Citation4–Citation6] The sample (5 g) was mixed with 25 mL of deionized water for 2 min by vortexing and centrifuged at approximately 1500 × g. The supernatant (0.4 mL) was mixed with 8.6 mL of deionized water and 1 mL of Lugol’s iodine solution (2.0% [w/w] potassium iodide and 1.3% [w/w] iodine aqueous mixture). The absorbance (ABS) of the sample and a reference (gelatinized starch alone) was measured at 690 nm, and the complex index (CI) was calculated using the following equation:
All tests were performed in triplicate and the results averaged.
Melting temperature
The thermal stability of the complexes was evaluated by measuring the Tm using a DSC60 (Shimadzu, Co., Tokyo, Japan). Only the Tm values of PG-samples were measured because those of FA-[Citation20] and MG-samples[Citation21] have previously been published. PG-samples (150 mg/g-starch) were rapidly frozen in liquid nitrogen and freeze-dried for 72 h, and the obtained solid was ground. Sample (2–4 mg) was placed in an aluminum DSC pan and water was added to a water content of more than 70%. The DSC pan was hermetically sealed, and a heating scan was performed at 5ºC/min between 25–120ºC. All tests were performed in triplicate and the results were averaged.
In vitro starch digestibility
Measurement of in vitro enzymatic digestibility of starch was performed according to previous studies with modifications.[Citation25–Citation27] In brief, 4 mL of a mixture of pancreatic α-amylase (30 U/mL) and amyloglucosidase (3 U/mL) in 0.1 M sodium maleate buffer solution (pH 6.0) was added to 5 g samples in 15 mL tubes. The contents were mixed using a seesaw shaker at 30 strokes/min at 37°C for 20 min or 120 min, 4 mL of ethanol was added, and the tubes were centrifuged at approximately 1500 × g for 10 min. The supernatant was removed and the precipitate was stirred with 2 mL of 2 M potassium hydroxide for 20 min, then 8 mL of 1.2 M sodium acetate buffer solution (pH 3.8) and 0.1 mL of amyloglucosidase in 50% glycerol aqueous solution (3300 U/mL) were added and the mixture was stirred at 50°C for 30 min. The mixture was centrifuged at approximately 1500 × g for 10 min, and the amount of glucose in the supernatant was analyzed quantitatively according to the glucose oxidase/peroxidase (GOPOD) method, allowing the amount of starch hydrolyzed in 20 min (HS20) or 120 min (HS120) to be evaluated. According to the classification proposed by Englyst et al.,[Citation25] the starch contents hydrolyzed within 20 min and between 20 and 120 min will be related to “rapidly digested starch” and “slowly digested starch,” respectively. However, the experimental procedure did not completely agree among both of them. The results of this study were shown as hydrolyzed starch content at the given condition. All tests were performed in triplicate and the results were averaged.
Statistical analysis
Analysis of variance (Tukey’s HSD test) was carried out using Kaleida Graph (Version 3.6, Synergy software, Reading, PA, USA).
Results and discussion
FAs
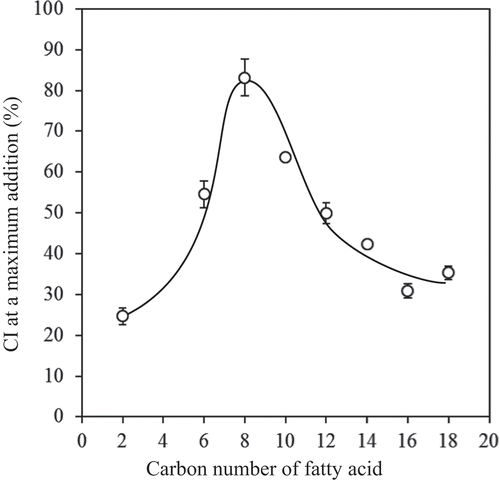
The effect of short- and medium-chain FAs on the CI is shown in . The CI values increased as the amount of FA increased, and the maximum CI observed at the maximum concentration of FA added (2 mmol/g starch) depended on the type of FA. This behavior agreed with the previous report on long-chain FAs.[Citation19] The results for the various types of FAs were compared by plotting maximum CI against the chain length of FAs, as shown in . The CI increased as the chain length increased up to C8 and then decreased. The complex was formed by hydrophobic interactions between acyl chain and amylose in an aqueous environment, with the polar group forming the surface of the complex.[Citation28] Thus, long-chain FAs have stronger thermodynamic driving force to form complexes than short-chain FAs. On the other hand, FAs have to disperse in the gelatinized starch in order to contact amylose. Short-chain FAs have better dispersity than long-chain FAs. From these reasons, it is thought that medium-chain FA (C8) exhibited the highest CI among the FAs. There is a minor effect of amylopectin on the complex formation because of its short glucose chain lengths and steric hindrance between the branches in amylopectin.[Citation4,Citation29] Short-chain FAs, however, may have formed complex with amylopectin because they can form complex with short glucose chains. Similar suggestions are also given to some aroma compounds.[Citation30–Citation32]
Figure 1. Effect of short- and medium-chain FAs on the CI (circle: C10; triangle: C8; square: C6; diamond: C2). The values are expressed as mean ± SD (n = 3). Values with the same letter at each addition are not significantly different at p < 0.05.
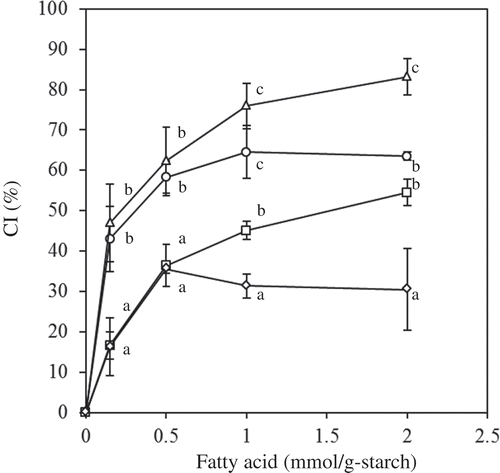
Figure 2. Effect of chain length in FAs on the CI observed at the maximum concentration tested (2 mmol/g starch). The values are expressed as mean ± SD (n = 3).
The effect of short- and medium-chain FAs (0.15 mmol/g-starch) on HS20 and HS120 was negligible (data not shown). There were no significant differences in HS20 (94–97%) and HS120 (98%) among the samples, except for C10, which showed a significant lower but minor difference in HS20 between the control and the sample. There was no reduction of in vitro starch digestibility even at higher concentrations of the FAs. For example, 2 mmol/g starch C8 had the highest CI among the FA-samples ( and ), but HS20 and HS120 were equivalent to those for 0.15 mmol/g-starch C8 (data not shown). In contrast, long-chain FAs reduced HS20 (C12 = 86%, C14 = 88%, C16 = 89%, C18 = 90%, C18:1 = 82%, C18:2 = 88%) even at a concentration of 0.15 mmol/g starch.[Citation19] These results indicated that complexes formed using short- and medium-chain FAs had negligible effect on the in vitro enzymatic digestibility of starch. Thermal stability is dependent on the helical order of the complex which increases with an increase in carbon chain length of the FA, and the helical order of the complex can be characterized by Tm. For example, Tufvesson et al.[Citation20] investigated the Tm of complexes formed by starch and FAs (C10–C18) and reported that the Tm increased linearly with increase in chain length. This suggests that the chain length of FAs has an inverse relationship: the higher the chain length of the FA, the greater the thermal stability, but the extent of complex formation decreases. Previous report showed that C12 resulted in the greatest reduction in the in vitro enzymatic digestibility of starch of the long-chain saturated FAs,[Citation19] suggesting that C12 is the optimum chain length for reducing the in vitro enzymatic digestibility of starch.
MG
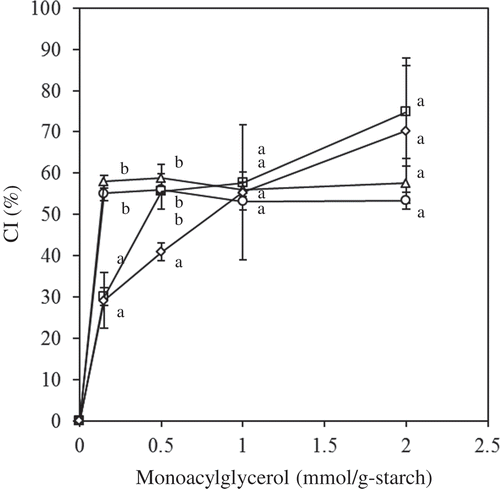
The effect of MGs on the CI () revealed large deviations, especially at high concentration. MGs are hydrophobic emulsifiers, and therefore micelles may have formed during mixing at high MG concentrations.[Citation5] The CI of MG-C12 and MG-C14 plateaued at 0.15 mmol/g starch, whereas the CI of MG-C16 and MG-C18 gradually increased as the concentration increased. There was no significant difference in the CI observed at the maximum concentration (2 mmol/g starch) among the samples, and the values ranged from 53–75%. MG also produced much higher CI values than the long chain FA samples (CI = 31–50%). MGs are more hydrophilic than FAs and would, thus, disperse in gelatinized starch, affording many opportunities to contact starch molecules and form complexes with amylose.
Figure 3. Effect of MGs on the CI (circle: MG-C12; triangle: MG-C14; square: MG-C16; open diamond: MG-C18). The values are expressed as mean ± SD (n = 3). Values with the same letter at each addition are not significantly different at p < 0.05.
The effect of MGs (0.15 mmol/g starch) on HS20 and HS120 is shown in . The HS20 and HS120 of the MG samples were 74–85% and 84–95%, respectively, and were significantly lower than the control. MG-C12 and MG-C14 provided significantly lower HS20 and HS120 than MG-C16 and MG-C18. This tendency agreed with the results previously reported for sago starch-MG mixtures; degree of hydrolysis of starch was higher in the order of MG-C18 > MG-C16 > MG-C14.[Citation22] The MG-samples provided significantly lower HS20 and HS120 than the FA samples (0.15 mmol/g starch C12–C18) with the corresponding chain length, with the exception of HS20 for MG-C18 and HS120 for MG-C14 and MG-C18.
Figure 4. HS20 (white) and HS120 (gray) for MG-samples (0.15 mmol/g starch). The values are expressed as mean ± SD (n = 3). Values with the same letter at each hydrolyzing condition are not significantly different at p < 0.05.
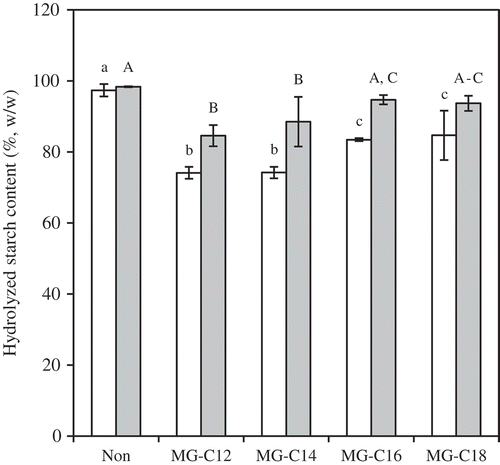
As stated previously, the reduction in the in vitro starch digestibility was likely related to the extent and thermal stability of the complex.[Citation19] The thermal stability of the complex can be characterized by Tm. The Tm (peak apex) of the complex formed with MG was reported to be 85ºC for MG-C12, 90ºC for MG-C14, 98ºC for MG-C16, and 103ºC for MG-C18.[Citation25] These were equivalent to or slightly lower than FA samples (93ºC for C12, 99ºC for C14, 98ºC for C16, and 109ºC for C18).[Citation20] Thus, the larger reduction of in vitro starch digestibility for MG-samples than FA-samples will be explained by CI reflecting the extent of complex. In fact, the CI for MG-samples (29–58%) was much higher than that of the FA-samples (12–38%) at a concentration of 0.15 mmol/g starch. Similarly, the CI values of MG-C12 and MG-C14 were significantly higher than the CI values of MG-C16 and MG-C18 at a concentration of 0.15 mmol/g starch (). The CI values of the MG-C16 and MG-C18 samples increased as the concentration of MG increased, thus likely reducing HS20 and HS120. For example, the HS20 (66%) and HS120 (78%) of 2 mmol/g starch MG-C16 were much lower than those of 0.15 mmol/g starch MG-C16 (data not shown).
PG
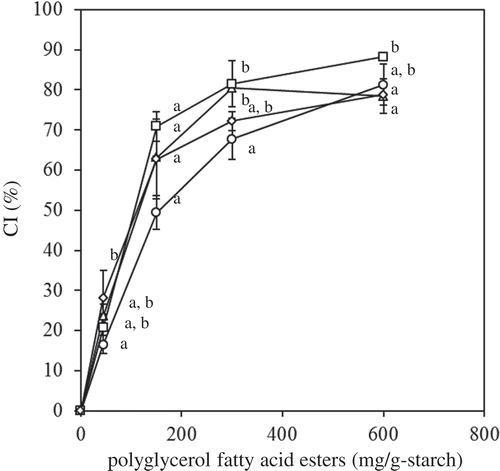
The effect of PGs on the CI is shown in . The CI values increased as the concentration of PG increased, reaching 79–88% at 600 mg/g starch, which was slightly higher than that observed for MG-samples (53–75% at 549–717 mg/g starch): PG-C12 versus MG-C12 and PG-C14 versus MG-C14 were significant, whereas PG-C16 versus MG-C16 and PG-C18:1 versus MG-C18 were not significant difference. PGs can dissolve completely in gelatinized starch solution because of their high HLB (13.3–17.5) and, thus will be readily available to form complexes with amylose. A comparison of the CIs of PG-C16 and PG-C18:1 indicated that the CI of PG-C16 was slightly but significantly higher than that of PG-C18:1. We previously reported that the maximum CI of FAs tended to increase as the number of double-bonds in the FA increased (C18:0 = 35 ± 2%, C18:1 = 37 ± 1%, and C18:2 = 48 ± 3%).[Citation19] Since the acyl chain of an unsaturated FA is bent at the double bond, the number of carbons participating in complex formation will be lower than in a saturated FA with the same number of carbons in the acyl chain. In contrast, the maximum CI of FAs tended to decrease as the number of carbons increased.[Citation5,Citation6,Citation19] A comparison of the CI of PG-C16 and PG-C18:1 indicates that the chain length has a greater effect on CI than the number of double bonds.
Figure 5. Effect of PGs on the CI value (circle: PG-C12; triangle: PG-C14; square: PG-C16; diamond: PG-C18:1). The values are expressed as mean ± SD (n = 3). Values with the same letter at each addition are not significantly different at p < 0.05.
The Tm (onset) values of PG-samples were determined to be 76 ± 1ºC for PG-C12, 80 ± 2ºC for PG-C14, 85 ± 1ºC for PG-C16, and 81 ± 1ºC for PG-C18:1 (n = 3), showing that the Tm of the PG samples increased as the carbon chain length increased. The exception is PG-C18:1, in which the bend in the oleic acid chain caused by the double bond effectively decreases the number of carbons involved in complex formation compared to that with palmitic acid.
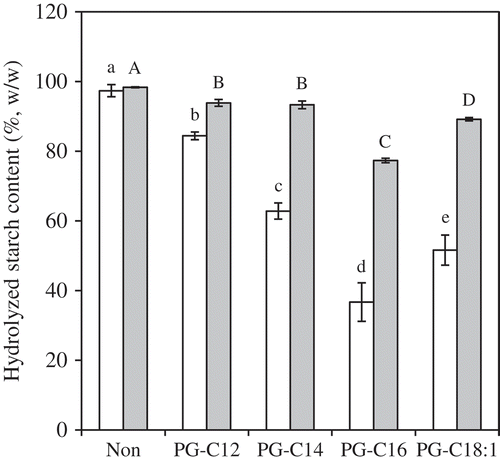
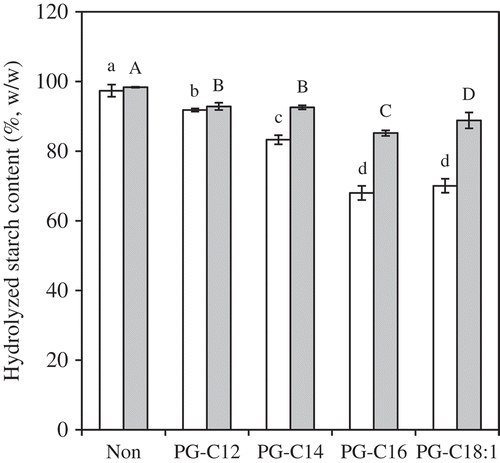
The effect of PGs (45 mg/g starch) on HS20 and HS120 are shown in . The 45 mg/g starch PG-samples have approximately equivalent concentrations of PG as the MG concentration in the 0.15 mmol/g starch MG samples (41–54 mg/g-starch). The HS20 and HS120 values for the PG-samples were significantly lower than to those of the MG-samples (HS20 = 74–85% and HS120 = 85–95%), except for the HS120 of PG-C14 versus MG-C14, and PG-C18:1 versus MG-C18. The high CI values of the MG- and PG-samples, despite their long carbon chains, would greatly reduce the HS20 and HS120. The HS20 and HS120 values of the PG-samples tended to decrease with an increase in the chain length, and PG-C16 provided the lowest HS20 and HS120 values of the PG-samples. The minor differences in the CI values between the PG-samples indicate that the differences in HS20 and HS120 arise from the thermal stability of the complexes. As mentioned previously, the Tm increases linearly with increase in chain length.[Citation20] On the other hand, unsaturated FA reduces the Tm because the bend in the FA chain caused by the double-bond reduces the number of carbons involved in complex formation.[Citation19,Citation20] Thus, it is expected that PG-C16 provided the highest Tm of the PG-starch complexes tested.
Figure 6. HS20 (white) and HS120 (gray) for PG-samples (45 mg/g starch). The values are expressed as mean ± SD (n = 3). Values with the same letter at each hydrolyzing condition are not significantly different at p < 0.05.
The effect of a higher concentration (150 mg/g starch PGs) on the in vitro enzymatic digestibility of starch was investigated () and resulted in a significant reduction in HS20 and HS120. Consistent with the results obtained using 45 mg/g starch PG-samples, PG-C16 provided the lowest HS20 (37%) and HS120 (78%) values among the 150 mg/g starch PG-samples. A comparison with 2 mmol/g starch (661 mg/g starch) MG-C16 (HS20 = 68% and HS120 = 85%) showed that HS20 and HS120 for PG-C16 were significantly lower. As mentioned above, there was no significant difference in CI between PG-C16 and MG-C16, and the thermal stability of the complexes would be comparable because the chains have the same chain length. Therefore, the reduction in HS20 and HS120 of the PG-samples is likely due at least in part to enzyme-annealing. The short glucose chain lengths of amylopectin make them poor at forming complexes, and steric hindrance between the branches in amylopectin further inhibits complex formation.[Citation4] However, potato starch has amylopectin with long glucose chains, and steric hindrance between the branches in amylopectin is loosened by hydrolysis.[Citation33,Citation34] Free (non-complexed) ligands are trapped in the packed area formed by the complexes and/or into the amorphous network area in gelatinized starch,[Citation13] and may leak during hydrolysis of complexes or gelatinized starch. PGs are water soluble and thus, exist in the amorphous (liquid) phase. Consequently, complex formation may occur during hydrolysis. The enzyme-annealing effect is unlikely in long-chain FAs and MGs due to their much lower water solubility than PG at the hydrolysis temperature. Thus, the results suggest that PG-C16 has high potential for reducing the enzymatic digestibility of starch.
Conclusion
The effects of various types and amounts of FA, MG, and PG on the degree of complex formation and the in vitro enzymatic starch digestibility of gelatinized potato starch were understood systematically. The reduction in in vitro enzymatic starch digestibility could be described by four factors: the CI, the numbers of carbons and double-bonds, and enzyme-annealing effect. Direct demonstration of the enzyme annealing effect will require further experiments with other techniques (e.g., FT-IR and X-ray diffraction).
Nomenclature
| FA | = | Fatty acid |
| C2 | = | Acetic acid |
| C6 | = | Hexanoic acid |
| C8 | = | Octanoic acid |
| C10 | = | Decanoic acid |
| C12 | = | Lauric acid |
| C14 | = | Myristic acid |
| C16 | = | Palmitic acid |
| C18 | = | Stearic acid |
| C18:1 | = | Oleic acid |
| C18:2 | = | Linoleic acid |
| MG | = | Monoacylglycerol |
| MG-C12 | = | Glyceryl monolaurate |
| MG-C14 | = | Glyceryl monomyristate |
| MG-C16 | = | Glyceryl monopalmitate |
| MG-C18 | = | Glyceryl monostearate |
| PG | = | Polyglycerol fatty acid ester |
| PG-C12 | = | Polyglycerol lauric acid ester |
| PG-C14 | = | Polyglycerol myristic acid ester |
| PG-C16 | = | Polyglycerol palmitic acid ester |
| PG-C18:1 | = | Polyglycerol oleic acid ester |
| HLB | = | Hydrophilic-lipophilic balance |
| ABS | = | Absorbance at 690 nm |
| CI | = | Complex index (%) |
| Tm | = | Melting temperature (ºC) |
| HS20 | = | The amount of starch hydrolyzed in 20 min (%, w/w) |
| HS120 | = | The amount of starch hydrolyzed in 120 min (%, w/w) |
Acknowledgments
Mitsubishi-Kagaku Foods Co. is acknowledged for providing the polyglycerol fatty acid esters. The authors also thank Mr. Makoto Soga for his technical support.
References
- Tester, R.F.; Karkalas, J.; Qi, X. Starch-Composition, Fine Structure and Architecture. Journal of Cereal Science 2004, 39, 151–165.
- Jacobs, H.; Delcour, J.A. Hydrothermal Modifications of Granular Starch, with Retention of the Granular Structure: A Review. Journal of Agricultural and Food Chemistry 1998, 46, 2895–2905.
- Morrison, W.R. Lipids in Cereal Starches: A Review. Journal of Cereal Science 1988, 8, 1–15.
- Guraya, H.S.; Kadan, R.S.; Champagne, E.T. Effect of Rice Starch–Lipid Complexes on in Vitro Digestibility, Complexing Index, and Viscosity. Cereal Chemistry 1997, 74, 561–565.
- Tang, M.C.; Copeland, L. Analysis of Complexes Between Lipids and Wheat Starch. Carbohydrate Polymers 2007, 67, 80–85.
- Kaur, K.; Singh, N. Amylose-Lipid Complex Formation During Cooking of Rice Flour. Food Chemistry 2000, 71, 511–517.
- Gelders, G.G.; Goesaert, H.; Delcour, J.A. Amylose-Lipid Complexes as Controlled Lipid Release Agents During Starch Gelatinization and Pasting. Journal of Agricultural and Food Chemistry 2006, 54, 1493–1499.
- Mira, I.; Persson, K.; Villwock, V.K. On the Effect of Surface Active Agents and Their Structure on the Temperature-Induced Changes of Normal and Waxy Wheat Starch in Aqueous Suspension. Part 1. Pasting and Calorimetric Studies. Carbohydrate Polymers 2007, 68, 665–678.
- Tufvesson, F.; Skrabanja, V.; Björck, I.; Elmståhl, H.L.; Eliasson, A.C. Digestibility of Starch Systems Containing Amylose-Glycerol Monopalmitin Complexes. Lebensmittel-Wissenschaft und-Technologie 2001, 34, 131–139.
- Holm, J.; Björck, I.; Ostrowska, S.; Eliasson, A.C.; Asp, N.G.; Larsson, K.; Lundquist, I. Digestibility of Amylose–Lipid Complexes in-Vitro and in-Vivo. Starch/Stärke 1983, 35, 294–297.
- Crowe, T.C.; Seligman, S.A.; Copeland, L. Inhibition of Enzymic Digestion of Amylose by Free Fatty Acids in Vitro Contributes to Resistant Starch Formation. Journal of Nutrition 2000, 130, 2006–2008.
- Heinemann, C.; Zinsli, M.; Renggli, A.; Escher, F.; Conde-Petit, B. Influence of Amylose-Flavor Complexation on Build-up and Breakdown of Starch Structures in Aqueous Food Model Systems. LWT–Food Science and Technology 2005, 38, 885–894.
- Biais, B.; Le Bail, P.; Robert, P.; Pontoire, B.; Buléon, A. Structural and Stoichiometric Studies of Complexes Between Aroma Compounds and Amylose. Polymorphic Transitions and Quantification in Amorphous and Crystalline Areas. Carbohydrate Polymers 2006, 66, 306–315.
- Conde-Petit, B.; Escher, F.; Nuessli, J. Structural Features of Starch-Flavor Complexation in Food Model Systems. Trends in Food Science & Technology 2006, 17, 227–235.
- Lesmes, U.; Cohen, S.H.; Shener, Y.; Shimoni, E. Effects of Long Chain Fatty Acid Unsaturation on the Structure and Controlled Release Properties of Amylose Complexes. Food Hydrocolloids 2009, 23, 667–675.
- Lesmes, U.; Barchechath, J.; Shimoni, E. Continuous Dual Feed Homogenization for the Production of Starch Inclusion Complexes for Controlled Release of Nutrients. Innovative Food Science & Emerging Technologies 2008, 9, 507–515.
- Zabar, S.; Lesmes, U.; Katz, I.; Shimoni, E.; Bianco-Peled, H. Studying Different Dimensions of Amylose–Long Chain Fatty Acid Complexes: Molecular, Nano and Micro Level Characteristics. Food Hydrocolloids 2009, 23, 1918–1925.
- Zabar, S.; Lesmes, U.; Katz, I.; Shimoni, E.; Bianco-Peled, H. Structural Characterization of Amylose-Long Chain Fatty Acid Complexes Produced Via the Acidification Method. Food Hydrocolloids 2010, 24, 347–357.
- Kawai, K.; Takato, S.; Sasaki, T.; Kajiwara, K. Complex Formation, Thermal Properties, and in-Vitro Digestibility of Gelatinized Potato Starch–Fatty Acid Mixtures. Food Hydrocolloids 2012, 27, 228–234.
- Tufvesson, F.; Wahlgren, M.; Eliasson, A.C. Formation of Amylase-Lipid Complexes and Effects of Temperature Treatment. Part 2. Fatty Acids. Starch/Stärke 2003, 55, 138–149.
- Eliasson, A.C.; Krog, N. Physical Properties of Amylose–Monoglyceride Complexes. Journal of Cereal Science 1985, 3, 239–248.
- Cui, R.; Oates, C.G. The Effect of Amylose-Lipid Complex Formation on Enzyme Susceptibility of Sago Starch. Food Chemistry 1999, 65, 417–425.
- Kaur, A.; Singh, N.; Ezekiel, R.; Sodhi, N.S. Properties of Starches Separated from Potatoes Stored under Different Conditions. Food Chemistry 2009, 114, 1396–1404.
- Eliasson, A.C. Interactions Between Starch and Lipids Studied by DSC. Thermochimica Acta 1994, 246, 343–356.
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. European Journal of Clinical Nutrition 1992, 46(2), S33–S50.
- Champ, M. Determination of Resistant Starch in Foods and Food Products: Interlaboratory Study. European Journal of Clinical Nutrition 1992, 46(2), S51–S62.
- McCleary, B.V.; McNally, M.; Rossiter, P. Measurement of Resistant Starch by Enzymatic Digestion in Starch and Selected Plant Materials: Collaborative Study. Journal of AOAC International 2002, 85, 1103–1111.
- Godet, M.C.; Buléon, A.; Tran, V.; Colonna, P. Structural Features of Fatty Acid-Amyloe Complexes. Carbohydrate Polymers 1993, 21, 91–95.
- Bertoft, E.; Annor, G.A.; Shen, X.; Rumpagaporn, P.; Seetharaman, K.; Hamaker, B.R. Small Differences in Amylopectin Fine Structure May Explain Large Functional Differences of Starch. Carbohydrate Polymers 2016, 140, 113–121.
- Arvisenet, G.; Le Bail, P.; Voilley, A.; Cayot, N. Influence of Physicochemical Interactions Between Amylose and Aroma Compounds on the Retention of Aroma in Food-Like Matrices. Journal of Agricultural and Food Chemistry 2002, 50, 7088–7093.
- Jouquand, C.; Ducruet, V.; Le Bail, P. Formation of Amylose Complexes with C6-Aroma Compounds in Starch Dispersions and Its Impact on Retention. Food Chemistry 2006, 96, 461–470.
- Heinemann, C.; Escher, F.; Conde-Petit, B. Structural Features of Starch–Lactone Inclusion Complexes in Aqueous Potato Starch Dispersions: The Role of Amylose and Amylopectin. Carbohydrate Polymers 2003, 51, 159–168.
- Hizukuri, S. Relationship Between the Distribution of the Chain Length of Amylopectin and the Crystalline Structure of Starch Granules. Carbohydrate Polymers 1985, 141, 295–306.
- Hizukuri, S. Polymodal Distribution of the Chain Lengths of Amylopectins, and Its Significance. Carbohydrate Polymers 1986, 147, 342–347.