ABSTRACT
A study was conducted to evaluate the basic chemical composition, organic acids and volatile compound profiles of ultra-high-temperature milk samples sold in Turkey. The organic acids were determined by reverse-phase high-performance liquid chromatography method, and volatile compounds were analyzed by headspace solid phase micro-extraction/gas chromatography/mass spectrometry technique. A total of 43 volatile compounds including 4 aldehydes, 5 alcohols, 10 ketones, 9 acids, 9 aromatic hydrocarbons, 3 nitrogenous, 2 sulfur containing compounds, and 1 alkane hydrocarbon, were identified in the ultra-high-temperature milk samples. The main compounds were found to be oxime methoxy phenyl, 2-heptanone, 2-mercapto-4-phenylthiazole, 2-amino-5-ethoxycarbonyl benzophenone, acetic acid, 2,6,10,14-tetramethyl pentadecane, and 2-nonanone. The main organic acid in the ultra-high-temperature milk was citric acid a mean value of 133 mg/100 mL, followed by formic, lactic, succinic, oxalic, acetic, orotic, propionic, pyruvic, hippuric, and uric acids.
Introduction
In ultra-high-temperature (UHT) treatment, milk is heated at temperatures higher than 130°C (usually 138–145°C) for a holding time of 1–10 s (usually 3–5 s), followed by aseptic packaging.[Citation1] UHT process increases the shelf life of milk up to 6 months at room temperature by destroying spoilage bacteria and inactivating enzymes. However, UHT processing of milk and its subsequent storage leads to the chemical changes such as whey protein denaturation, isomerization of lactose (lactulose), Maillard browning, and the formation of various volatile compounds (VCs).[Citation2–Citation4] The extent of these changes depends on many factors such as the type of UHT heating, the combination heat and time, the quality of initial raw milk, and the duration and temperature storage.[Citation5–Citation8] In general, UHT milk is characterized by an increase in the amount of methyl ketones,[Citation9,Citation10] hydrocarbons,[Citation4] aldehydes,[Citation10,Citation11] and sulfides.[Citation12] The content and profiles of VCs identified in UHT milk are related to the fat content of milk, the intensity of heat treatment, the homogenization process (pressure/temperature) and the storage conditions.[Citation6,Citation7,Citation13,Citation14] VCs are not only responsible for the characteristic flavor of a food, but also for its off-flavors. Knowledge of VCs in UHT milk will be important to determine the acceptability of the product to the consumer. Even if UHT milk is within microbiologically and chemically acceptable limits, it has a limited shelf life.
During heating of milk, lactose undergoes reactions that have important consequences for the milk: results in compounds such as formic acid, acetic acid, pyruvic acid, hydroxyl methyl furfural, and furfuryl alcohol. Formic acid, organic acid (OA), is primarily responsible for the increased acidity of heated milk.[Citation5,Citation15] Formation of hydroxyl methyl furfural can indicate the severity of heat-treatment.[Citation2] Among currently available volatile extraction techniques, solid-phase micro-extraction (SPME) is the relatively new analytical technique for the extraction of flavor compounds. It is a simple, fast, and solvent-free technique.[Citation16] SPME-gas chromatography-mass spectrometry (GC/MS) technique has been used in analysis of volatiles of milk.[Citation4,Citation13,Citation17,Citation18]
In the present study, we sampled the UHT milks with the similar expiration date and fat content in order to elimination the effects of storage period and fat content on VCs. Therefore, we aimed to determine the changes in VCs and OA contents in the UHT milk samples with the similar basic nutrient components, packing materials, and the expiration date. OAs are minor constituents of milk, which have important physiological and/or technological roles. To our knowledge, no studies on the volatile organic compounds (VOCs) and OAs of UHT milk sold in Turkey have been published. Therefore, the objective of this study was to study the VOCs, OA contents and basic nutrient constituents of UHT cows’ whole milk samples obtained from the different brands.
Materials and methods
Sampling
The 11 samples of UHT milk with fat (≤3% and <3.3%) packed aseptically in 1000 mL Tetra Brik Cartons produced at different factories were collected on the markets, which are the most popular brands in Turkey. Three lots of each UHT milk sample were obtained from the same brand. The all UHT milk samples were analyzed within 90 days before their expiration date. The samples were immediately transferred to laboratory under cold chain after purchasing.
Gross-chemical analyses
The total solid content was determined by gravimetric method using an oven (Binder, FD115, Germany).[Citation19] Total nitrogen was measured by the micro-Kjeldahl method[Citation20] using the Gerhardt the Gerhardt KB 40S digestion and Vapotest distillation systems (C. Gerhardt, Bonn, Germany). The pH, specific gravity, and fat values of milk were determined according to the procedure reported in Turkish Standard Institution[Citation21,Citation22] using a pH meter (Orion, Thermo, Beverly, MA, USA), a lactodansimeter and Gerber centrifugate, respectively. The titratable acidity was determined by titrimetric method with 0.1 N NaOH as percentage of lactic acid.[Citation23] The ash content was quantitated by dry ashing the samples in a muffle furnace (Protherm, PLF 110/10, Turkey) at 550°C for 24 h. The total carbohydrate was calculated by the sum of lactose, glucose, and galactose. All gross-chemical analyses were performed in duplicates from each lot.
OA and sugar analysis
The analysis of OA and sugar was performed according to the procedure described by Fernandez-Garcia and McGregor[Citation24] with minor modifications. In order to extraction of OAs and sugars, UHT milk was dissolved in 10 mM sulfuric acid 4:1 (v/v) sample-to-solution ratio and centrifuged at 5697 × g for 7 min at 5°C. The upper layer was filtrated by paper (Whatman No. 1), and followed by filtration using 0.45-μm syringe type filter (Millex Polyvinylidene difluoride (PVDF) Millipore, Billerica, MA, USA). Separations were carried out in an automated high-performance liquid chromatography system (HPLC-20 AD Prominence, Shimadzu, Kyoto, Japan) using an ion exchange column (Aminex HPX-87 H, 300 × 7.8 mm, BIO-RAD, Hercules, CA, USA) according to the procedure reported by Guler.[Citation25] OA and sugar (lactose, glucose, and galactose) standards were purchased from Sigma-Aldrich GmbH (Steinheim, Germany) and Supelco (Bellefonte, PA, USA), respectively. OA and sugar solutions were prepared in distilled deionized water, filtered through a 0.45-μm syringe filter (PVDF, Millipore, Billerica, MA, USA) and injected into the HPLC system to provide standard lines based on the peak for each OA and sugar. Linear regression curve-based peak areas were calculated for the individual OA and sugar. The data points from calibration curves were subjected to a least square regression analysis. The coefficients of determination (RCitation2) obtained were 0.9998.
VC analysis
The VCs of UHT milk samples were extracted by using the headspace (HS)-SPME. The VCs extraction was performed in triplicate by using 50/30 µm DVB/CAR/PDMS (divinylbenzene/carboxen/polydimethyl siloxane) fiber (Supelco, Bellefonte PA, USA). Ten grams of the milk sample was immediately transferred in 20 mL HS vial containing 3.0 g NaCl, to inhibit any enzyme reaction (Agilent, Palo Alto, CA, USA). Vials sealed using crimp-top caps with PTFE/silicone HS septum (Agilent, Palo Alto, CA, USA). After preliminary trials, for extraction of VCs the vials were kept at 55°C for 30 min in a water bath then SPME fiber which was conditioned (260°C for 1 h) before being placed in sample HS was inserted to the HS vial and held for 20 min at constant temperature for adsorption process of VCs. When the fiber was inserted in injection port, temperature procedure was started. Thedesorption of VCs from fiber was performed in the injection port at 260°C for 5 min in splitless mode. The VCs were separated on a HP-INNOWAX capillary column (60 m × 0.25 mm id × 0.25 μm film thickness) connected to the coupled 6890 GC and 5973 N MS (Agilent, Palo Alto, CA, USA). Helium was used as the carrier gas at a flow rate of 1 mL min−Citation1. The oven temperature program was initially held at 50°C for 5 min and then programmed from 50°C by a ramp of 5°C min 1 up to 100°C and then at 10°C min−Citation1 to reach a final temperature of 230°C, which was held for 15 min. The mass spectrometry (MS) detector was operating in the scan mode within a mass range of 20 to 350 m z−Citation1at 1 scan s−Citation1, with electron energy of 70 eV. The interface line to MS was set at 250°C. The total analysis time was 56 min. VOCs were identified by computer-matching of their mass spectral data against the Wiley7n.1 and Nist 02.L. GC-MS libraries (Agilent).The identities of most VOCs were confirmed by their GC retention time (RT) and the ion spectra of authentic standards (Sigma-Aldrich, Milwaukee, WI, USA). Retention indices (RI) were also determined for all constituents using a homologous series (C5–C25 and C7–C40) of n-alkanes (Sigma-Aldrich, Milwaukee, WI, USA) at the same chromatography conditions. The results from the VOC analyses were expressed as the percentage composition of each compound, from its integrated peak area relative to the total integration of peaks of all compounds identified.
Results and discussion
Basic chemical composition
The chemical composition of the UHT milk samples is shown in . The mean values (as g/100 mL) for non-fat dry matter, fat, protein, total carbohydrate, ash, titratable acidity, pH, and specific gravity of the UHT milk samples collected were 8.14 ± 0.27, 3.12 ± 0.12, 2.92 ± 0.10, 4.01 ± 0.86, 0.72 ± 0.08, 0.18 ± 0.02 (as lactic acid), 6.67 ± 0.12, and 1.0316 ± 0.001, respectively. Non-fat dry matter, fat, protein, titratable acidity, and specific gravity values were consistent with those stated by the Turkish Food Codex for UHT milk and raw cow milk.[Citation26] The results obtained for fat and protein were in consistent with the values on package but total carbohydrate was markedly low. This could be attributed to the degradation of lactose depending on the severity of heat-treatment or the type of UHT process used since at both higher temperatures and indirectly heated UHT milk lactose reacts in the Maillard reaction with milk proteins or isomerizes into lactulose through the Lobry de Bruyn–Alberda van Ekenstein transformation.[Citation5,Citation27] The mean values for titratable acidity, pH, and ash were similar to those in UHT milk stored at 20°C for 3 months[Citation28] and at 5ºC for 2 months,[Citation29] but lactose was low. The fat and protein contents of UHT milk samples had a coefficient of variation at below 5% as being standardized to the values reported in legal regulation whereas titratable acidity, carbohydrate and ash values showed a coefficient of variation at above 5%. This is probably due to the different storage temperatures and heat-treatment norms along with initial raw milk quality since the storage conditions at above 20°C and severe heat-treatment caused a significant decrease in lactose and ash, and increase in titratable acidity.[Citation28]
Table 1. Nutritional composition (g/100 mL milk) of UHT milk samples.
OAs
OAs occur in raw milk as a result of normal animal metabolism. Animal- (genetics, stage of lactation, ruminal fermentations, and udder infections) and feed- (grain, dietary protein intake, seasonal, and regional effects) related factors may account for variations in OA in raw milk.[Citation30–Citation32] With these factors the raw milk keeping conditions and the technological process (the severity and method of heat-treatment and homogenization pressure and temperature) and subsequent the storage conditions affect the concentrations of OAs in UHT milk.[Citation3,Citation5,Citation33] There is no available data in literature on the variations and concentrations of OAs in UHT milk samples. The OAs in UHT milk samples are given in . Compared to basic nutrient components with exception total carbohydrate, the OAs showed a wide variation from milk to milk, with coefficient of variation ranging from 10.10 to 59.70%. Citric, formic, succinic, and lactic acids were determined in all milk samples as the most abundant OAs. Citric and succinic acids are intermediate products in tricarboxylic acid (TCA) cycle, which ranged from 94.74 to 203.26 mg/100 mL and from 23.03 to 71.70 mg/100 mL with a mean value of 132.99 and 44.69 mg/100 mL, respectively. The mean value of citric acid was slightly lower than the findings of Güler[Citation25] and Garnsworthy, Masson, Lock, and Mottram[Citation30] for raw cow milk, but was similar to that (1439 mg/L) in pasteurized milk.[Citation34] As reported by Igual, García-Martínez, Camacho, and Martínez-Navarrete[Citation35] the heat treatment might have been led to a decrease in citric acid. Changes in the concentration of succinic acid of milk have been relatively little studied. The mean value of succinic acid was slightly lower than that reported by Gadaga et al.[Citation36] for UHT milk, and was higher than those (265 and 104 mg/L) in raw milk and pasteurized milk.[Citation32,Citation33] Compared to citric acid, succinic acid showed a wide coefficient of variation (35 versus 23%) which could explain that succinic acid has been derived from the different sources such as the oxidation of fatty acids during heat-treatment[Citation33] and the fermentation of cellulose by cellulolytic bacteria in ruminants.[Citation37]
Table 2. Organic acids (mg/100 mL milk) identified in UHT milk samples.
As shown in , the mean values of lactic and formic acids were higher than the findings (588 and 402 mg/L, respectively) of Ruas-Madiedo et al.[Citation34] for cow milk pasteurized at 63°C for 30 min. This may be due to a more intense heat treatment since formic acid is known as advanced Maillard reaction product.[Citation5,Citation38] It was reported that formic acid increased 150 times in sterilized milk compared to pasteurized milk.[Citation33] Formic acid is primarily responsible for the drop in pH.[Citation31] This confirmed that UHT milk with the highest formic acid had the lowest pH value.
Interestingly, the coefficient of variation of lactic acid was close to that of titratable acidity ( and ). This may explain that lactic acid has a determinant effect on titratable acidity of milk. Acetic, pyruvic, propionic, and butyric acids were determined in all UHT milk samples. These acids as well as formic acid and lactic can derive from the degradation of lactose and amino acid depending on the severity of heat treatment.[Citation31] Besides, succinic, lactic, acetic, propionic, pyruvic, butyric, oxalic, and formic acids are fermentation products of energy metabolism in ruminants,[Citation37] which can increase depending on anaerobic fermentation in silage and poor environment conditions.[Citation39] Recently, formic acid has been used as a preservative and antibacterial agent in livestock feed, might have been passed from feed into milk.[Citation40]
Orotic, uric, and hippuric acids are non-protein nitrogen (NPN) compounds, which are partly intermediate products of the protein metabolism of the animal. The mean values of orotic, uric, and hippuric acid were within ranges reported by Indyk and Woollard,[Citation41] Larsen and Moyes,[Citation42] and Gadaga et al.[Citation36] for raw cow milk. Compared to uric acid, orotic acid showed a wide coefficient of variation (56 versus 18%). This could be attributed to the origin of NPN compounds since orotic acid is an intermediate of pyrimidine metabolism in mammalian tissues, whereas uric acid is a product of degradation of the purine nucleosides.[Citation41] Celestino et al.[Citation15] reported a significant increase in NPN of UHT during storage due to the decomposition of the proteins by reactivated proteolytic enzymes.
VCs
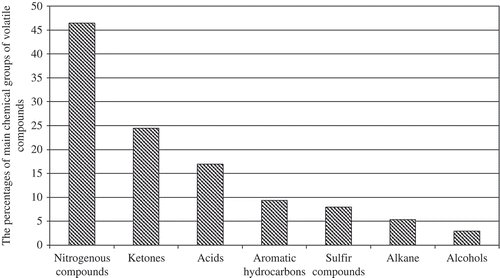
A total of 43 VCs were identified in UHT milk, including 4 aldehydes, 5 alcohols, 10 ketones, 9 acids, 9 aromatic hydrocarbons, 3 nitrogenous, 2 sulfur containing compound, and 1 alkane hydrocarbon (). The number of VOCs identified in UHT milk samples was greater than those in previous studies[Citation4,Citation8,Citation11,Citation13] where were ranged from 8 to 32. Nitrogenous containing compounds were the most abundant, accounting for 46.44% of total VOCs identified in all the UHT milk samples, followed by ketones (24.50%), acids (16.94%), aromatic hydrocarbons (9.35%), sulfur compounds (7.96%), alkane (5.37%), alcohols (2.94%), and aldehydes (0.99%) in decreasing order ().
Table 3. Volatile compounds identified in UHT milks according to chemical classes.
However, only 19 of 43 VOCs were found in the least nine UHT milk samples, which accounted for about 91.73% of total volatiles identified (). Among them, oxime methoxy phenyl (46.07%), 2-heptanone (16.75%), 2-mercapto-4-phenylthiazole (7.41%), 2-amino-5-ethoxycarbonyl benzophenone (6.11%), acetic acid (5.87%), 2,6,10,14-tetramethyl pentadecane (pristine; 5.37%), and 2-nonanone (4.29%) were the most abundant VOCs identified in UHT milk samples.
Table 4. The percentages of volatile compounds identified in most UHT milk samples.
The chemical groups of VOCs identified in UHT milk samples were similar to those previously determined by authors.[Citation4,Citation8,Citation11] As far as we know, 2-mercapto-4-phenylthiazole, 2-amino-5-ethoxycarbonyl benzophenone and 2,6,10,14-tetramethyl pentadecane (pristane) were identified in the volatile profile of UHT milk for the first time. The occurrence of these compounds may be associated to the severity of heat treatment applied to milk and the interaction between packaging material and milk.
As shown in , ketones were the major VOCs in all UHT milk samples, in terms of their number. In two UHT milk samples, are the most popular brands in Turkey, ketones were identified as the most abundant VOCs, in terms of their percentage composition. This result was in accordance with previous findings in conventional UHT milk.[Citation8–Citation11] Ketones 2-pentanone, 2-heptanone, 2-nonanone, 2-tridecanone, 2-undecanone, and δ-decalactone were identified in all UHT milk samples. As shown in , -heptanone was the most abundant ketone, ranged from 8 to 33%, which followed by 2-nonanone and 2-undecanone, ranged from 1.77 and 0.26% to 11.14 and 2.41%, respectively. 2-Heptanone and 2-nonanone have a major impact on the flavor of heat-treated milk,[Citation10] which are responsible for the development of a “ketone” and “stale” flavor in UHT milk. Ketones such as 2-heptanone and 2-nonanone having a higher carbon number can be formed as a consequence of heat treatment by both β-oxidation of fatty acids, followed by decarboxylation[Citation43] and the decarboxylation of a β-ketoacid, naturally biosynthesized in the mammary gland.[Citation9] Thus, methyl ketones are predominantly originated in the lipid fraction depending on the type of UHT process used. In previous studies,[Citation4,Citation9–Citation11] it has been reported that increase in methyl ketones progresses throughout the storage period following initiation of heat-induced oxidation with high homogenization pressure. In the present study, methyl ketones showed a wide variation among brands, ranged from 23.17 to 68.16%. This may be related to the differences in the severity of heat treatment and in the homogenization pressure and temperature among brands, and also the different storage temperatures since UHT milk samples analyzed had a similar expiration data and fat contents.
Nitrogenous-containing VOCs were dominant group in the nine UHT milk samples, in terms of their percent composition. Among their, oxime-methoxy phenyl was present in the highest concentration, ranged from 33.38 to 58.10%. There is little information on oxime-methoxy phenyl. This compound has previously been found in micro-filtered pasteurized milk as dominant VOC,[Citation18] and also in ultrapasteurized milk packaged in polyethylene terephthalate containers[Citation29] and reconstituted milk.[Citation44] Oximes are may be formed by reaction aldehydes or ketones with a nitrogen-containing reducing agent in a weakly acidic medium during the high heat treatment and/or homogenization since aldehydes were detected in UHT milk samples at trace levels and samples with low ketone percentages had a high level of oxime-methoxy phenyl.
From aromatic organic compounds consisting of a benzene ring, benzaldehyde and phenol were identified in all UHT milk samples at trace levels as previously reported.[Citation6] In UHT milk, benzaldehyde and phenol are described as heat-generated compounds.[Citation45] Two-amino-5-ethoxycarbonyl benzophenone was not found in one UHT milk sample only, and in the remaining samples its percentage ranged from 2.3 to 11.8%. This compound may be formed as a result of interaction between milk and packaging material since benzophenone, a main component of UV inks, is the most widely used both the outer surface of packaging materials as photoinitiator and the production of PE plastic.[Citation46] Benzophenone was previously found in UHT milk packed with cartons printed with ultraviolet (UV)-cured inks at high levels, which increased with increase in fat content of milk.[Citation46,Citation47] The problem of benzophenone migration in milk from packaging material printed with UV-cured inks is of great interest since several adverse and harmful effects are attributed to benzophenone.[Citation48] Nevertheless, no migration limit has yet been fixed by either European legislation or Turkish food codex.
Pristane, C14 isoprenoid alkane, was found in the nine of 11 UHT milk samples and its percentage ranged from 3.0 to 13%. Pristane has previously been detected in bovine milk,[Citation49] which is derived from pyrolysis or thermal degradation of α-tocophenol (vitamine E) as major source.[Citation50] The high pristane percentages in most milk samples may be due the high α-tocophenol, which was not analyzed.
As far as sulfur compounds are concerned, dimethyl disulfide (DMDS) and 2-mercapto-4-phenylthiazole (4-Phenylthiazole-2-thiol) were detected in our research. The both compounds were not found in two of 11 UHT milk samples. With a mean value of 7.40%, 2-mercapto-4-phenylthiazole was markedly higher than DMDS (). Thiazoles have previously been found in milk powder with “popcorn” flavor note.[Citation51] The high percentages of thiazole in most UHT milk samples may indicate an intense heat treatment since thiazoles are the secondary reaction products formed from amino acids phenyl alanine and methionine during the Maillard reaction.[Citation52] In previous studies,[Citation6,Citation11,Citation12] DMDS was found to be the most abundant sulfide in UHT milk, whereas it disappeared or decreased to trace level within 2 or 3 months during storage.[Citation6,Citation53] In our study, the low percentages of DMDS may be due to oxidation of the reducing sulfur compounds during storage or the detection technique used since MS may not be sensitive enough for compounds with sulfur.
Regarding on acids, acetic, butanoic, hexanoic, octanoic, and decanoic acids were identified in all milk samples, accounted for approximately 15.47% of VOCs identified in UHT milk samples. The majority of acids varied from the brand to brand. In most UHT milk samples, acetic acid was detected as major acid. Free fatty acids of short-chain length could be produced from hydrolysis of milk triglycerides by heat-resistant lipases or from lactose by heat treatment. Similarly, Vegas and Roussis[Citation13] reported an increase in butanoic, hexanoic, octanoic, and decanoic acids in pasteurized milk compared to raw cow milk. Acids identified in this study have previously been found in milk pasteurized by microfiltration and ultrapasteurized milk with dominance of hexanoic and octanoic acids, respectively,[Citation18,Citation29,Citation44] who indicated that pasteurization with microfiltration caused a greater increase in acids than pasteurization only. Although the fat content of commercial UHT milk samples analyzed is standardized to values of ≤3% and <3.5% as UHT whole milk, the values of free fatty acids showed a wide variation from brand to brand, ranging from 25.78 to 87.96%. This could be attributed to the different process technology, quality of initial raw milk and the high storage temperatures since the contents of free fatty acid are increased in UHT milk produced from the raw material with the largest number of psychrotrophic bacteria and stored at the high temperatures[Citation54] and also in milk pasteurized by microfiltration.[Citation18,Citation29,Citation44]
Although ethanol was found in all the samples at trace levels, 2-furan methanol, 2-pentanol, and 2-ethyl-1-hexanol were determined in some UHT milk samples as the major alcohols. These alcohols may be formed by the reduction of aldehydes, were previously identified in milk powder.[Citation55] Straight-chain-aldehydes such as pentanal, hexanal, heptanal, and nonanal were sporadically identified in UHT milk samples, are lipid oxidation products. The authors[Citation4,Citation11,Citation12] have been reported that aldehydes increase with high pressure and heat treatment at high temperature but decrease with the extended store. In the present study, when compared to the other VCs the high levels of alkane (pristine) and oxime–methoxy phenyl and the low levels of aldehydes may indicate the minimum level of free oxygen in milk samples.
Conclusions
This is one of the first reports for the VC profiles and OA contents in UHT whole milks sold in Turkey with a large number of VCs and the mixture of OAs using SPME-GC-MS and reverse-phase-HPLC, respectively. Compare to protein and fat, lactose, OAs and VCs showed a wide coefficient of variation in UHT milks. No definite conclusion can be drawn concerning these differences, because all the samples were collected from supermarkets. In general, UHT milk samples had the high percentages of oxime methoxy phenyl, 2-mercapto-4-phenylthiazole and 2-heptanone and also the high concentration of formic acid and the low levels of lactose which did not confirm to values on package. Due to the high benzophenone percentages in the most UHT milk samples, the attention of legislative authorities may be focused on the potential migrants from packaging inks into milk. Furthermore, a long-term goal of this research is to compare UHT milks with the different fat contents and to determine the correlations between individual aroma compound and flavor attributes in UHT milks stored at the same conditions.
References
- Deeth, H.C.; Datta, N. Heating Systems. In Encyclopedia of Dairy Science; Roginski, H.; Fuquay, J.W.; Fox, P.F.; Eds., Academic Press: Amsterdam, 2003; 2643–2652.
- Datta, N.; Elliott, A.J.; Perkins, M.L.; Deeth, H.C. Ultra-High-Temperature (UHT) Treatment of Milk: Comparison of Direct and Indirect Modes of Heating. Australian Journal of Dairy Technology 2002, 57, 211–227.
- Claeys, W.L.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; De Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; Vandenplas, Y.; Herman, L. Raw or Heated Cow Milk Consumption: Review of Risks and Benefits. Food Control 2013, 31, 251–262.
- Pereda, J.; Jaramillo, D.P.; Quevedo, J.M.; Ferragut, V.; Guamis, B.; Trujillo, A.J. Characterization of Volatile Compounds in Ultra-High-Pressure Homogenized Milk. International Dairy Journal 2008, 18, 826–834.
- Van Boekel, M.A.J.S. Effect of Heating on Maillard Reactions in Milk. Food Chemistry 1998, 62, 403–414.
- Valero, E.; Villamiel, M.; Miralles, B.; Sanz, J.; Martınez-Castro, I. Changes in Flavour and Volatile Components During Storage of Whole and Skimmed UHT Milk. Food Chemistry 2001, 2, 51–58.
- Hougaard, A.B.; Vestergaard, J.S.; Varming, C.; Bredie, W.L.P; Ipsen, R.H. Composition of Volatile Compounds in Bovine Milk Heat Treated by Instant Infusion Pasteurisation and Their Correlation to Sensory Analysis. International Journal of Dairy Technology 2011, 64, 34–44.
- Jansson, T.; Jensen, S.; Eggers, N.; Clausen, M.R.; Larsen, L.B; Ray, C.; Sundgren, A.; Andersen, H.J.; Bertram, H.C. Volatile Component Profiles of Conventional and Lactose-Hydrolyzed UHT Milk—A Dynamic Headspace Gas Chromatography-Mass Spectrometry Study. Dairy Science and Technology 2014, 94, 311–325.
- Contarini, G.; Povolo, M. Volatile Fraction of Milk: Comparison Between Purge and Trap and Solid Phase Microextraction Techniques. Journal of Agricultural and Food Chemistry 2002, 50, 7350–7355.
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative Determination of Thermally Derived Off-Flavor Compounds in Milk Using Solid-Phase Microextraction and Gas Chromatography. Journal of Dairy Science 2005, 88, 3764–3772.
- Contarini, G.; Povolo, M.; Leardi, R.; Toppino, P.M. Influence of Heat Treatment on the Volatile Compounds of Milk. Journal of Agricultural and Food Chemistry 1997, 45, 3171–3177.
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Quantification of Trace Volatile Sulfur Compounds in Milk by Solid-Phase Microextraction and Gas Chromatography-Pulsed Flame Photometric Detection. Journal of Dairy Science 2006, 89, 2919–2927.
- Vagenas, G.; Roussis, I.G. Fat-Derived Volatiles of Various Products of Cows’, Ewes’, and Goats’ Milk. International Journal of Food Properties 2012, 15, 665–682.
- Garcia-Risco, M.R.; Ramos, M.; Lopez-Fandino, R. Proteolysis, Protein Distribution and Stability of UHT Milk During Storage at Room Temperature. Journal of the Science of Food and Agriculture 1999, 79, 1171–1178.
- Celestino, E.L.; Iyer, M.; Roginski, H. Reconstituted UHT-Treated Milk: Effects of Raw Milk, Powder Quality and Storage Conditions of UHT Milk on Its Physico-Chemical Attributes and Flavour. International Dairy Journal 1997, 7, 129–140.
- Serrano, E.; Beltrán, J.; Hernández, F. Application of Multiple Headspace-Solid-Phase Microextraction Followed by Gas Chromatography-Mass Spectrometry to Quantitative Analysis of Tomato Aroma Components. Journal of Chromatography A 2009, 1216, 127–133.
- Valdivielso, I.; Bustamante, M.A.; Aldezabal, A.; Amores, G.; Virto, M.; Ruiz de Gordoa, J.C.; de Renobales, M.; Barron, L.J.R. Case Study of a Commercial Sheep Flock Under Extensive Mountain Grazing: Pasture Derived Lipid Compounds in Milk and Cheese. Food Chemistry 2016, 197, 622–633.
- Yue, J.; Zheng, Y.; Liu, Z.; Deng, Y.; Jing, Y.; Luo, Y.; Yu, W.; Zhao, Y. Characterization of Volatile Compounds in Microfiltered Pasteurized Milk Using Solid-Phase Microextraction and GC×GC-TOFMS. International Journal of Food Properties 2015, 18, 2193–2212.
- AOAC. AOAC Official Method 925.23: Solids (Total) in Milk. In Official Methods of Analysis of AOAC International (18th ed.); AOAC International: Gaithersburg, MD, 2005a.
- IDF. Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. IDF Standard 020-1. International Dairy Federation: Brussels, Belgium, 2014.
- TSI. Cow Milk-Raw. TS 1018. Turkish Standard Institution: Ankara, 2002.
- TSI. Milk-Determined of Fat Content. TS ISO 2446. Turkish Standard Institution: Ankara, 2015.
- AOAC. AOAC Official Method 947.05: Acidity of Milk, Titrimetric Method. In Official Methods of Analysis of AOAC International (18th ed.); AOAC International: Gaithersburg, MD, 2005b.
- Fernandez-García, E.; McGregor, J.U. Determination of Organic Acids during the Fermentation and Cold Storage of Yogurt. Journal of Dairy Science 1994, 77, 2934–2939.
- Güler, Z. Profiles of Organic Acid and Volatile Compounds in Acid-Type Cheeses Containing Herbs and Spices (Surk Cheese). International Journal of Food Properties 2014, 17, 1379–1392.
- Anonymous. Turkish Food Codex Regulation. Notification No. 2000/6 on raw and UHT milk. Official J., No. 23964, 14 February, 2000.
- Olano, A.; Martinez-Castro, I. Modification and Interactions of Lactose. Bulletin of the International Dairy Federation 1989, 238, 35–44.
- Rehman, Z.U.; Salariya, A.M. Effect of Storage Condition on Quality of UHT Processed Buffalo Milk. Journal of Chemical Society of Pakistan 2005, 27, 73–76.
- Solano Lopez, C.E.; Taehyun, J.; Alvarez, V.B. Volatile Compounds and Chemical Changes in Ultrapasteurized Milk Packaged in Polyethylene Terephthalate Containers. Journal of Food Science 2005, 70, 407–412.
- Garnsworthy, P.C.; Masson, L.L.; Lock, A.L.; Mottram, T.T. Variation of Milk Citrate with Stage of Lactation and De Novo Fatty Acid Synthesis in Dairy Cows. Journal of Dairy Science 2006, 89, 1604–1612.
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology, 2nd Ed; CRC, Taylor & Francis: New York, NY, 2006; 1–768.
- Islam, M.A.; Uddin, M.E.; Jahan, R.; Wadud, A.; Sarkar, M.M. Metabolites in the Milk of Buffalo, Holstein Cross, Indigenous and Red Chittagong Cattle of Bangladesh Bang. Journal of Animal Science 2013, 42, 152–157.
- Nishimura, J.; Makino, S.; Kimura, K.; Isogai, E.; Saito, T. Influence of Different Sterilization Conditions on the Growth Andexopolysaccharide of Streptococcus Thermophilus and Co-Cultivation with Lactobacillus Delbrueckii Subsp. Bulgaricus OLL1073R-1. Advances in Microbiology 2015, 5, 760–767.
- Ruas-Madiedo, P.; Alonso, L.; de Llano, D.G.; de los Reyes-GavilaAn, C.G. Growth and Metabolic Activity of a Cheese Starter in CO2-Acidified and Non-Acidified Refrigerated Milk. Z Lebensm Unters Forsch A 1998, 206, 179–183.
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of Thermal Treatment and Storage on the Stability of Organic Acids and the Functional Value of Grapefruit Juice. Food Chemistry 2010, 118, 291–299.
- Gadaga, T.H.; Mutukumira, A.N.; Narvhus, J.A. The Growth And İnteraction Of Yeasts And Lactic Acid Bacteria İsolated From Zimbabwean Naturally Fermented Milk in UHT Milk. International Journal of Food Microbiology 2001, 68, 21–32.
- Shirley, R.L. 1986. Nitrogen and Energy Nutrition of Ruminants. Academic Press Inc.: Orlanda, FL, 1986; 1–547.
- Nursten, H.E. Recent Developments in Studies of the Maillard Reaction. Food Chemistry 1981, 6, 263–277.
- Britt, D.G.; Huber, J.T.; Rogers, A.L. Fungal Growth and Acid Production During Fermentation and Refermentation of Organic Acid Treated Corn Silages. Journal of Dairy Science 1975, 58, 532–539.
- Reutemann, W.; Kieczka, H. “Formic Acid” in Ullmann’s Encyclopedia of Industrial Chemistry, 7th Ed; John Wiley and Sons Inc, Wiley-VCH Verlag GmbH: New York, NY, USA, 2005.
- Indyk, H.E.; Woollard, D.C.; Determination of Orotic Acid, Uric Acid, and Creatinine in Milk by Liquid Chromatography. Journal of AOAC International 2004, 87, 116–122.
- Larsen, T.; Moyes, K.M. Fluorometric Determination of Uric Acid in Bovine Milk. Journal of Dairy Research 2010, 77, 438–444.
- Nawar, W.W. Chemistry of Thermal Oxidation if Lipids. In Flavor Chemistry of Fats and Oils; Min, D.B; Smouse, T.H.; Eds.; American Oils Chemist’s Society: Champaign, Illinois, USA, 1995; 39–60.
- Wang, D.; Zheng, Y.; Liu, Z.; Hu, G.; Deng, Y. Impact of Microfiltration on Particle Size Distribution, Volatile Compounds and Protein Quality of Pasteurized Milk During Shelf Life. Journal of Food and Nutrition Research, 2014, 3, 26–33.
- Scanlan, R.A.; Lindsay, L.M.; Libbey, L.M.; Day, E.A. Heat Induced Volatile Compounds in Milk. Journal of Dairy Science 1968, 51, 1001–1007.
- Shen, D.X.; Lian, H.Z.; Ding, T.; Xu, J.Z.; Shen, C.Y. Determination of Low-Level Ink Photoinitiator Residues in Packaged Milk by Solid-Phase Extraction and LC-ESI/MS/MS Using Triple-Quadrupole Mass Analyzer. Analitical and Bioanalytical Chemistry 2009, 395, 2359–2370.
- Sanches-Silva, A.; Pastorelli, S.; Cruz, J.M.; Simoneau, C.; Castanheira, I.; Paseiro-Losada, P. Development of an Analytical Method for the Determination of Photoinitiators Used for Food Packaging Materials with Potential to Migrate Into Milk. Journal of Dairy Science 2008, 91, 900–909.
- Jickells, S. Chemical Migration from Secondary Packaging into Food. In Chemical Migration and Food Contact Materials. Barnes, K.; Sinclair, R.; Watson, D.; Eds.; Woodhead Publishing Limited and CRC Press: Cambridge, UK, 2007; 395–414, 448.
- Christie, W.W. Composition and Structure of Milk Lipids. In Advanced Dairy Chemistry Volume 2, Lipids; P.F. Fox Chapman & Hall: UK, 1994; 1–36, 443.
- Goossens, H.; Leeuw, JW.; Schenck, P.A.; Brassell, S.C. Tocopherols as Likely Precursors of Pristine in Ancient Sediments and Crude Oils. Nature 1984, 312, 440–442.
- Karagül-Yüzeer, Y.; Cadwallader, K.R.; Drake, M. Volatile Flavor Components of Stored Nonfat Dry Milk. Journal of Agricultural and Food Chemistry 2002, 50, 305–312.
- Belitz, H.D.; Grosch, W. Food Chemistry. Springer-Verlag: Berlin, 2009; 152–236.
- Gaafar, A.M. Chemical Changes in Ultra-Heat Treated Milk During Storage. 2. Production of Volatile Flavour Compounds. Milchwissenschaft 1991, 46, 233–235.
- Panfil-Kuncewicz, H.; Kuncewicz, A.; Juoekiewicz, M. Influence of Storage Conditions on Changes in the Fat Fraction of UHT Milk. Polısh Journal of Food and Nutrition Sciences 2005, 14, 341–348.
- Shiratsuchi, H.; Shimoda, M.; Imayoshi, K.; Noda, K.; Osajimat, Y. Volatile Flavor Compounds in Spray-Dried Skim Milk Powder. Journal of Agricultural and Food Chemistry 1994, 42, 984–988.