ABSTRACT
Vegetable oils are major lipid sources with high nutritional and calorific values for human diet. Specifically, virgin coconut oil and extra virgin olive oil are the functional oils widely used in food and pharmaceutical products, either as vehicles or main components. The quality of edible oils is determined by its contents and parameters inherent in vegetable oils. Infrared spectroscopy is an ideal technique for quantitative analysis of vegetable oils as well as for determination of oils parameters as the changes in infrared spectra can be associated with the changes of oils parameters. Infrared spectra in complex samples are difficult to interpret, as a consequence, spectroscopist uses additional tools called with chemometrics to analyse edible oils qualitatively and quantitatively. This article reviews the use of infrared spectroscopy combined with chemometrics (multivariate analysis) for quantitative analysis and determination of oil parameters of virgin coconut oil and extra virgin olive oil. Although infrared spectra for edible oils are similar, they exhibit some differences which enable spectroscopist to differentiate due to the nature property of infrared spectroscopy spectra as fingerprint spectra which can be understood that there are no different edible oils having the same infrared spectroscopy spectra.
Introduction
Chemically, edible oils are composed of 98% of triglycerides (TG; glycerol esterified with three fatty acids; FA) with different substitution patterns, lengths, and degrees of unsaturation of the chains, and some minor components (2%) such as sterols, phospholipids, carotenes, and lipid soluble vitamins (vitamin A, D, E, and K), sugars and more complex components which can be liberated using numerous hydrolytic reactions.[Citation1] Edible oils are good sources of nutrition for human diet, which are necessary for appropriate development of human tissues.[Citation2,Citation3] The FAs in TG composing edible oils can be saturated, monounsaturated, and poly-unsaturated FAs, which allow analyst to differentiate edible oils based on its FA composition.[Citation4]
The edible oils originate from plants. The largest sources of edible oils are the seeds of annual plants and oil-bearing trees.[Citation5] Among the valuable edible oils from plants reported to have some beneficial effects to human health are virgin coconut oil (VCO) and extra virgin olive oil (EVOO). VCO is an emerging edible oil in the food and pharmaceutical industries, as valuable as olive oil.[Citation6] VCO is oil produced from the fresh mature kernel of the coconut by mechanical or natural means, with or without use of heat and without undergoing chemical refining, bleaching, and deodorizing to produce refined-bleached-deodorized (RBD) oil.[Citation7] Unlike refined coconut oil which is produced through dry method from copra, VCO is produced through wet method, via coconut milk.[Citation8] listed some physicochemical properties of VCO.
Since VCO is produced differently from refined coconut oil, the oil obtained is slightly different in terms of its sensory characteristics. VCO is nearly colorless, with a slightly detectable acid aroma, sweet and salty taste, and is perceptible nutty aroma and flavor. On the other hand, RBD coconut oil is distinctively yellow, slightly salty, and has no perceptible aroma and flavor.[Citation7] VCO can be extracted in a straightforward manner from coconut under ambient temperature; therefore, the loss of minor components like pro-vitamin A, tocopherol, and phenolic compounds due to solar ultraviolet (UV) irradiation during coconut drying can be avoided. VCO may have more beneficial effects than copra oil, since it retains most of the unsaponifiable components.[Citation10] FA compositions of VCO are presented in . Lauric acid (C12:0) was the most dominant of FAs present in VCO. In addition, VCO contains less mono- and polyunsaturated FAs.
VCO exhibits some important biological activities such as antiviral and antibacterial. These effects may be attributed to the large amount of short-chain FAs like caproic, caprylic, and capric acids.[Citation7] Nevin and Rajamohan[Citation10] reported that VCO can lower triacylglycerol, phospholipids, total cholesterol, low density lipoprotein (LDL), and very low density lipoprotein (VLDL) cholesterol levels. VCO also increase high density lipoprotein (HDL) in tissues and serum. Phenolic content of VCO were able to prevent LDL oxidation in vitro with the formation of reduced level of carbonyl compounds. VCO did not change the lipid-related cardiovascular risk factors and events in those receiving standard medical care.[Citation11] In addition, fresh VCO has no detrimental effect on blood pressure, inflammatory biomarkers, and helps to reduce body weight in test animals.[Citation12]
EVOO
Olive oil is a fatty juice and may be consumed after proper processing of olives. Olive oil gained the popularity in recent years.[Citation13] It is consumed not only by the people in the Mediterranean countries but also worldwide because of its unique flavor. Olive oil contains high amounts of oleic acid (about 80%) and some minor components such as oleuroepein aglycon, oleuroepein, dimethyloleuroepein, ligstroside, hydroxytyrosol, and tochoperol which are important to biological activities. Due to its components, olive oil commands a high price in the market.[Citation14–Citation16]
Edible olive oils are graded into six categories, namely (1) EVOO with acidity up to 0.8%, calculated as oleic acid; (2) virgin olive oil (acidity about 2.0%); (3) refined olive oil with acidity of 0.3%; (4) regular olive oil, which is a mixture of refined olive oil and virgin olive oil with free acidity of 0.1%; (5) refined residue oil, and (6) olive residue oil, a blend of refined residue oil and virgin olive oil. [Citation14] EVOO is the highest quality of olive oil and accounts for only 10% of the produced oil. Its taste, aroma, and mouthfeel were used by experts to judge EVOO.[Citation17] The chemical properties and minor components present in EVOO are compiled in .
Table 2. Fatty acid composition and non-triacylglycerol fraction of EVOO.[Citation17]
García-González et al.[Citation18] reported that olive oil-rich diet can prevent cardiovascular diseases, reduce plasma triacylglycerol, increase HDL-cholesterol levels, improve the postprandial lipoprotein metabolism, and can reduce blood pressure and the risk of hypertension, as demonstrated through epidemiological studies. OO is reported to have anti-cancerogenic effects in animal models and in human cell lines,[Citation19] and also reveals anticlastogenesis due to the treatment of cisplatin (antineoplastic agents) during cancer therapy in Wistar rats. Besides, olive oil increases the lipolytic activity in adipose tissue, prevents age-related cognitive decline and dementia, and does not promote obesity.[Citation20]
The Food and Drug Administration (FDA) allows the claim related to the benefits of olive oil on the reduced risk of coronary heart disease due to the daily consumption of 23 g of olive oil attributed by the presence of monounsaturated FAs, especially oleic acid.[Citation21] In addition, the high levels of natural antioxidants found in OO provide health benefits to humans.[Citation16] The main antioxidants in OO are carotenoids and polyphenolic compounds, mainly secoiridoids (oleuroepein aglycon, oleuroepein, dimethyloleuroepein, ligstroside), hydroxytyrosol, tochoperol,[Citation17] some phenolic acids such as caffeic acid, vanillic acid, and syringic acid, and lignans.[Citation22]
Infrared (IR) spectroscopy and chemometrics
IR spectroscopy is one of vibrational spectroscopies intensively used for characterization of all aspects of edible oils.[Citation23] IR spectroscopy can be defined as the interaction between electromagnetic radiation in IR region in the form of scattering, reflection, absorption or transmission with substances analyzed (for example edible oils) as a function of wavenumbers.[Citation24] The frequencies or wavelengths, at which samples absorb IR radiation and their corresponding intensities (either transmittance or absorbance) are recorded into an IR spectrum.[Citation25]
IR spectroscopy can be taken into account as an ideal technique for analysis of edible oils because this technique is rapid, ease in sample presentation, non-destructive and non-invasive, meaning that the samples analyzed with IR spectroscopy can be analyzed using different instruments.[Citation26,Citation27] Today, the number of researchers using IR spectroscopy due to its capability to characterize the structure of different molecular species without destructing them. For a given sample, IR spectroscopy generates a “chemical fingerprint” which can be used for identification, characterization, qualitative, and quantitative analysis and monitoring the parameters of edible oils, including VCO and EVOO.[Citation28,Citation29]
Spectra IR can be obtained using dispersive and Fourier transformed IR (FTIR) spectrophotometer. The dispersive instrument has been scarcely used in edible oils due to the difficulties in sample handling technique and is not equipped with proper spectral acquisition and processing systems in order to give the valuable information for quantitative analysis,[Citation30] as a consequence, today, the FTIR spectrophotometer has replaced dispersive IR spectrometer.[Citation31] In addition, the FTIR spectrophotometer can be equipped with the advanced chemometrics software which able to handle the calibration development.[Citation32]
Chemometrics is the application of statistics and mathematics to extract the relevant information from the chemical data (IR spectra). Currently, the use of Chemometrics is very popular in all aspects of handling data. The IR spectra of edible oils in complex samples are difficult to interpret, as a consequence, spectroscopist use chemometrics in order to fasten the data assessment and to increases the precision of results.[Citation33] Some chemometrics techniques commonly used in FTIR spectra are: (1) data handling such as derivatization, normalizations, baseline corrections, standard normal variate (SNV), mean centering (MC), Savitzy-Golay derivatives, and multiplicative corrections; (2) classification techniques, which can be either supervised pattern recognition like discriminant analysis (DA) or unsupervised pattern recognition such as principal component analysis (PCA) and cluster analysis, as a function of the need (or not) for any a priori knowledge about the samples to be classified; and (3) multivariate calibration methods, in order to correlate between vibrational spectra to the quantifiable properties (concentration) of the analyte(s) of interest, namely stepwise multiple linear regression, principle component regression (PCR), partial least square (PLS) regression, multivariate curve resolution algorithms, genetic multivariate calibration or concentration residual augmented classical least squares.[Citation34–Citation36]
During analysis of edible oils using FTIR spectroscopy in combination with Chemometrics, some optimization was performed. Such optimization is typically subjected to frequency regions as well as data processing of FTIR spectra like smoothing and derivatization capable of providing the good correlation between the concentrations of analytes (edible oils) with spectral changes, expressed by correlation of coefficient. In addition, the type of multivariate calibration was also optimized to obtain the best calibration model with acceptable accuracy and precision.[Citation29]
The Use of FTIR spectroscopy for quantitative analysis of VCO and EVOO
Analysis of edible oils including VCO and EVOO is usually performed by determination of specific components present in VCO such as fatty acid methyl esters (FAME) using gas chromatography and triglyceride compositions with liquid chromatography tandem with mass spectrometry, rather than analysis of edible oils as a whole matter. For this reason, it is proposed that FTIR spectroscopy in combination with multivariate calibration can be used as an analytical method to overcome such problems.[Citation37] Analysis of VCO in the mixtures with other edible oils was relied on the additive nature of Beer’s law.[Citation38] In complex mixtures, one of the major difficulties is the spectral overlapping appear in FTIR spectra. As a consequence, multivariate calibration is employed for combatting this problem.[Citation36]
The analytical step for analysis of edible oils using FTIR spectroscopy and multivariate calibration includes preparation of standards or well characterized samples, the collection of FTIR spectra using certain condition, selection of calibration and validation sets from the standards or well-characterized samples previously prepared, calibration modeling from calibration datasets, validation the calibration models, and evaluation of the developed models in terms of its validation features (accuracy, precision, sensitivity), as well as its capability to predict the unknown samples.[Citation35,Citation39]
Among edible fats and oils, VCO has unique IR spectrum. revealed FTIR spectra of VCO and representative of other edible oils (olive oil and palm oil). In VCO spectrum, there is no peak at region near 3008 and 1654 cm−Citation1. Peaks at these regions correspond to unsaturated double bond (=CH; cis) and -C=C-(cis), respectively. These peaks can be used as an indicative for the unsaturation degree of triglyceride. VCO contained high level of lauric acid (about 50%) and very low level of unsaturated FA of oleic and linoleic acids, therefore, it is not surprising if VCO has no peak at region near 3008 and 1655 cm−Citation1. In addition, at region ranges of 1120–1090 cm−Citation1, due to C-O ester linkage vibration, VCO has one peak; meanwhile, almost other edible fats and oils showed two peaks. These differences can be exploited for quantitative analysis of VCO in the studied samples.[Citation40]
Figure 1. FTIR spectra of virgin coconut oil (VCO), olive oil (OO), and palm oil (PO) at wavenumber of 4000–650 cm−Citation1.
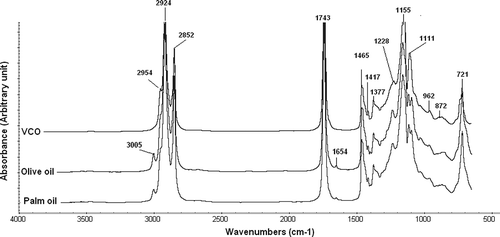
Rohman et al.[Citation41] analyzed VCO in binary mixture with palm oil and VCO in olive oil. In order to facilitate the quantification, two multivariate calibrations, namely PLS and PCR are optimized and compared. The other optimization step is the selection of frequency regions capable of providing high value of RCitation2 and low values of root mean square error of calibration (RMSEC), root mean square error of prediction (RMSEP), and root mean square error of cross validation (RMSECV). PLS at the combined frequency regions of 1120–1105 and 965–960 cm−Citation1 revealed the highest RCitation2 (0.9992) and the lowest RMSEC value of 0.756% for analysis of VCO in the mixture with olive oil. PLS calibration model was cross validated using “leave one out” technique. The values of RCitation2 and RMSECV obtained were 0.998 and 0.9911% (v/v), respectively; thus indicating that PLS model was well-suited for determination of VCO in OO.
PLS calibration model at two combined frequency regions of 1120–1105 and 965–960 cm−Citation1 offered a better model than PCR. PLS gives the highest value of RCitation2 (0.9996) and the lowest value of RMSEC (0.494%) for the relationship between actual value (x-axis) and FTIR predicted value (y-axis) of VCO in binary mixture with palm oil. The equation describing such relationship is y = 0.999x + 0.020. This result indicated that PLS regression model was able to estimate the VCO percentage in the mixture with palm oil. Cross validation using five factors revealed that RCitation2 and RMSECV values were 0.999 and 0.761%, respectively.[Citation41]
Rohman and Che Man[Citation40] have developed FTIR spectroscopy coupled with multivariate calibration (PLS and PCR) for analysis of VCO in ternary mixtures with olive oil and palm oil. PLS algorithm was well-suited for determination of VCO in the mixtures compared with PCR. Based on optimization, VCO in ternary mixtures is successfully determined at frequency region of 1200–1000 cm−Citation1 using second derivative FTIR spectra with RCitation2 and RMSEC values of 0.999 and 0.200%, respectively. The equation obtained for the relationship between actual (x-axis) and FTIR predicted value (y-axis) of VCO in ternary mixtures is y = 1.035x – 1.767, with RCitation2 of 0.996 and RMSEP value of 2.09 % (v/v).
FTIR spectroscopy and multivariate calibrations (PLS and PCR) allow someone to analyse edible oils simultaneously. Rohman and Che Man[Citation42] have used this combination for simultaneous quantitative analysis of VCO and EVOO. FTIR spectra were treated with several treatments including MC, derivatization, and SNV at the combined frequency regions of 3050–3000, 1660–1650, and 1200–900 cm−Citation1. Based on its capability to give the highest values of coefficient of correlation (R) for the relationship between actual value of EVOO/VCO and FTIR predicted value together with the lowest values of RMSEC, PLSR, with MC-first derivative spectra was chosen for simultaneous determination of EVOO and VCO. The R and RMSEP values obtained are 0.9998 and 2.47%, respectively. The equation describing the relationship between actual value of VCO (x-axis) and FTIR predicted value (y-axis) is y = 1.013x – 1.152.
In pharmaceutics products, FTIR spectroscopy combined with PLS is used for determination of VCO in cream cosmetics. In cosmetics formulation, VCO is good ingredient used as softener and skin moisturizer. The combined frequency regions of 924–930 and 725–730 cm−Citation1 which showed a great difference between VCO and the other components in cream cosmetics model is used proposed for modelling calibration and validation using PLS calibration. The difference between actual VCO value and FTIR predicted VCO either in calibration or validation is low, with RMSEC value of 0.826%, indicating that FTIR method is accurate enough for VCO analysis.[Citation43]
EVOO in ternary mixture with VCO and palm oil is quantified using FTIR spectroscopy and multivariate calibration of PLS and PCR. Some optimization in terms of the type of multivariate calibration and FTIR spectral treatment was performed. Finally, EVOO is best determined at 1200–1000 using normal FTIR spectra with RCitation2 and RMSEC values of 0.999 and 0.975%, respectively. The validation model revealed that the relationship between actual value of EVOO (x-axis) and FTIR predicted value (y-axis) has an equation of y = 1.003x + 0.274 with RCitation2 and RMSEP of 0.994 and 2.45%, respectively.[Citation40] EVOO in quaternary mixture with grape seed oil, rice bran oil, and walnut oil was also determined using FTIR spectroscopy and Chemometrics. FTIR spectra of studied samples were subjected to several treatments including MC, SNV, and spectra derivatives. [Citation44] The combined frequency regions of 1200–900 and 2949–2885 cm−Citation1 were used for determination of EVOO. FTIR normal spectra treated with MC combined with PLS model give the highest values of RCitation2 and the lowest values of RMSEC. The RCitation2 value obtained for the relationship between actual and FTIR predicted value of EVOO was 0.998. The RMSEC and RMSEP values obtained are 1.55 and 3.65% (v/v).
Evaluation of physicochemical properties of VCO and EVOO
FTIR spectra are an ideal technique for monitoring the oil parameters, as the changes of IR spectra can be correlated with the change of physico-chemical properties of oils. The oxidation of edible oils has been known as a major problem affecting the quality of edible oils,[Citation45] because it can change the flavor and nutritional quality of foods and produce toxic compounds, all of which can make the foods less acceptable or unacceptable to consumers. The oxidation products typically include primary oxidation products (peroxides) and secondary oxidation products, i.e., low molecular weight compounds that are volatile and possess off-flavor, primarily from the breakdown of unsaturated FAs during lipid autoxidation.[Citation46,Citation47] The primary oxidation products can be evaluated from its peroxide values; while, anisidine value, thiobarbituric acid reactive substances (TBARS) and volatile compounds can be used as an indicative for the development of secondary oxidation products.
FTIR spectroscopy is used for the assessment of peroxide value in VCO using attenuated total reflectance (ATR) as handling technique.[Citation48] The actual value of PV in VCO was determined using the standard method of American Oil Chemists’ Society (AOCS). VCO then is analyzed with FTIR spectroscopy to obtain predicted value. PLS calibration model was developed for calibration and validation modelling. PV is analysed at frequency region of 988 to 900 cm−Citation1. A linear calibration curve was obtained for the actual value (x-axis) of PV against FTIR predicted value of PV (y-axis) with an equation of y = 1.0398x + 0.0468. The RMSEP and RCitation2 values obtained are 0.9826 and 0.4978%, respectively.
FTIR spectroscopy has been used for stability of VCO during thermal oxidation. VCO samples were treated with the mixture of butylated hydroxyanisole and butylated hydroxytoluene of 200 mg/kg and citric acid of 100 mg/kg as antioxidants. The prominent peak change observed during thermal oxidation of VCO was at frequency 1739 cm−Citation1.[Citation49] These changes can be explained by the amount of carbonylic compounds such as aldehydes, esters, ketones, and lactones present in the oils formed during oxidation. The higher the intensities at 1739 cm−Citation1, the more carbonylic compounds present. Carbonylic compounds are the major secondary products during hydroperoxide decomposition.[Citation50] The evaluation of VCO stability during continuous/prolonged deep fat frying at 180 ± 5°C for 8 h using FTIR spectroscopy has been carried out by Srivastava and Semwal.[Citation51] FTIR spectra exhibit that VCO samples after treatment found to be stable and acceptable as there was no change occurred at 1739 cm−Citation1 corresponding to carbonylic compounds resulted from the hydroperoxide decompositions after 8 h of continuous frying.
During hydrolytic oxidation, free fatty acids (FFA) can be formed. FTIR spectroscopy combined with PLS is used for quantitative analysis of FFAs in VCO. Thirty training samples of VCO were prepared by spiking lauric acid to VCO samples covering a range of 0.1 to 5% FFA. The frequency region of 1730–1690 cm−Citation1 was exploited for determination of FFA. An equation used for describing the relationship between actual value of FFA (x-axis) as determined using method of AOCS (5a-40, 1996) and FTIR predicted value (y-axis) is y = 0.9744x + 0.0006. The RCitation2 and RMSEP values are 0.9281 and 0.1264%, respectively.[Citation52]
Navarra et al.[Citation53] have identified the spectral changes of EVOO during thermal oxidative process. Thermal treatment induced changes in the FTIR spectra in the wavenumbers region 3100–3600 cm−Citation1. The absorption profiles show an initial formation of hydroperoxides (as primary oxidation products) and a subsequent increase of secondary oxidation products such as alcohols, aldehydes, and ketones as evidenced by the modification of the spectral component at wavenumber 3530 cm−Citation1. IR spectroscopy in mid-infrared (MIR) and near-infrared (NIR) region is evaluated for its capability to monitor the oxidative degradation of olive oil. Some changes in peak intensities were observed. Wójcicki et al.[Citation54] reported that the most pronounced changes were observed in the fingerprint region, especially at wavenumbers of 968 and 986 cm−Citation1 corresponding to trans double bonds. In addition, peaks at 3006 and 710 cm−Citation1, due to cis double bond, were decreased. The authors stated that these peaks can be used as indicator of oxidation. Moreover, a characteristic intensity increase was observed at about 3500 cm−Citation1, corresponding to the formation of peroxides. While the most significant spectral changes in the NIR region due to oxidation were the absorbance increase at 4810 and 7068 cm−Citation1 due to formation of hydroperoxides.
Uncu and Ozen[Citation55] employed FTIR spectroscopy at wavenumbers of 4000–650 cm−Citation1 and PLS for determination of oxidative stability of olive oil. Using five principal components, 99% of the total variation can be explained. The coefficient of determination (RCitation2) values obtained during the correlation between actual value of oxidative stability as determined using Rancimat test (in hour) and FTIR predicted value 0.99 and 0.81, for calibration model and cross validation, respectively. The RMSEC and RMSECV values are 0.11 and 0.86%, with a slope value of the calibration curve is equal to 1 accounting for high reliability.
FFAs can be used as an indicative of olive oil deterioration. FTIR spectroscopy using PLS calibration at two wavenumber regions (1775–1689 and 1480–1050 cm−Citation1) and some several treatments including derivative spectra and SNV were optimized. The range of FFA contents of samples was extended by spiking oleic acid to several olive oils, ranging from 0.1 to 2.1%. FTIR spectra using first derivative treatment at 1775–1689 cm−Citation1 and PLS using three factors offer the best calibration and validation models with RMSEC and RMSEP values of 0.052 and 0.071%, respectively.[Citation56]
Conclusion
FTIR spectroscopy coupled with chemometrics is ideal technique for quantitative analysis and monitoring of oil parameters of VCO and EVOO because FTIR spectroscopy can measure the spectral changes due to specific treatments. FTIR spectroscopy for these purposes are regarded as fingerprint technique and green chemistry to minimum amount of chemical and reagents used.
References
- Christy, W.W. Lipid Analysis: Isolation, Separation, Identification, and Structural Analysis of Lipids, 2nd Ed; Pergamon Press: New York, NY, 1982.
- O’Brien, R.D. Fats and Oil: Formulating and Processing for Applications. Second Edition. CRC Press: London, UK, 2004.
- Moreno, M.M.C.M.; Olivares, M.D.; Lo´pez, A.F.J.; Adelantadoa, G.J.V.; Reig, B.F. Determination of Unsaturation Grade and Trans Isomers Generated During Thermal Oxidation of Edible Oils and Fats by FTIR. Journal of Molecular Structure 1999, 482–483, 551–556.
- Andrikopoulos, N.K. Triglyceride Species Compositions of Common Edible Vegetable Oils and Methods Used for Their Identification and Quantification. Food Reviews International 2002, 18(1), 71–102.
- Gunston, F.D. Oils and Fats in the Food Industry. Wiley-Blackwell Publishers: Oxford, 2008.
- Hamid, M.A.; Sarmidi, M.R.; Mokhtar, T.H.; Sulaiman, W.R.W.; Aziz, R.A. Innovative Integrated Wet Process for Virgin Coconut Oil Production. Journal of Applied Sciences 2011, 11(13), 2467–2469.
- Villarino, B.J.; Marsha, D.L.; Concepcion, M.; Lizada, C. Descriptive Sensory Evaluation of Virgin Coconut Oil and Refined, Bleached and Deodorized Coconut Oil. LWT–Food Science and Technology 2007, 40, 193–199.
- Marina, A.M.; Che Man, Y.B.; Amin, I. Virgin Coconut Oil: Emerging Functional Food Oil. Trends in Food Science and Technology 2009, 20, 481–487.
- Marina, A.M.; Che Man, Y.B., Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. Journal of the American Oil Chemists’ Society 2009, 86, 301–307.
- Nevin, K.G.; Rajamohan, T. Beneficial Effects of Virgin Coconut Oil on Lipid Parameters and in Vitro LDL Oxidation. Clinical Biochemistry 2004, 37, 830–835.
- Vijayakumar, M.; Vasudevan, D.M.; Sundaram, K.R.; Krishnan, S.; Vaidyanathan, K.; Nandakumar, S.; Chandrasekhar, R.; Mathew, N. A Randomized Study of Coconut Oil Versus Sunflower Oil on Cardiovascular Risk Factors in Patients with Stable Coronary Heart Disease. Indian Heart Journal 2016. http://dx.doi.org/10.1016/j.ihj.2015.10.384.
- Hamsi, M.A.; Othman, F.; Das, S.; Kamisah, Y.; Thent, Z.C.; Qodriyah, H.M.S.; Zakaria, Z.; Emran, A.; Subermaniam, K.; Jaarin, K. Effect of Consumption of Fresh and Heated Virgin Coconut Oil on the Blood Pressure and Inflammatory Biomarkers: An Experimental Study in Sprague Dawley Rats. Alexandria Journal of Medicine 2015, 51, 53–63.
- Arvanitoyannis, I.S.; Vlachos, A. Implementation of Physicochemical and Sensory Analysis in Conjunction with Multivariate Analysis Towards Assessing Olive Oil Authentication/Adulteration. Critical Reviews in Food Science and Nutrition 2007, 47, 441–498.
- Boskou, D. Culinary Applications of Olive Oil-Minor Constituents and Cooking. In Olive Oil-Minor Constituents and Health, 1st Ed., Boskou, D.; Ed; CRC Press: Boca Raton, FL, 2009; 1–6.
- Fomuso, L.B.; Akoh, C.C. Lipase-Catalyzed Acidolysis of Olive Oil and Caprylic Acid in a Bench-Scale Packed Bed Bioreactor. Food Research International 2002, 35, 15–21.
- Jansen, M.; Birch, J. Composition and Stability of Olive Oil Following Partial Crystallization. Food Research International 2009, 42, 826– 831.
- Huang, C.L.; Sumpio, B.E. Olive Oil, the Mediterranean Diet, and Cardiovascular Health. Journal of American College of Surgeons 2008, 207, 407–416.
- García-González, D.L.; Aparicio-Ruiz, R.; Aparicio, R. Review Article: Virgin Olive Oil—Chemical Implications on Quality and Health. European Journal of Lipid Science and Technology 2008, 110, 602–607.
- Hillestrøm, P.R.; Covas, M.-I.; Poulsen, H.E. Effect of Dietary Virgin Olive Oil on Urinary Excretion of Etheno–DNA Adducts. Free Radical Biology and Medicine 2006, 41, 1133–1138.
- Evangelista, C.M.W.; Antunes, L.M.G.; Francescato, H.D.C.; Bianchi, M.L.P. Effects of the Olive, Extra Virgin Olive and Canola Oils on Cisplatin-Induced Clastogenesis In Wistar Rat. Food and Chemical Toxicology 2004, 42, 1291–1297.
- Covas, M.-I. Review: Olive Oil and the Cardiovascular System. Pharmacological Research 2007, 55, 175–186.
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic Compounds in Olive Oil and Olive Pomace from Cilento (Campania, Italy) and Their Antioxidant Activity. Food Chemistry 2010, 121, 105–111.
- Yang, H.; Irudayaraj, J. Characterization of Semisolid Fats and Edible Oils by Fourier Transform Infrared Photoacoustic Spectroscopy. Journal of the American Oil Chemists’ Society 2000, 77, 291–295.
- Dufour, E. Principles of Infrared Spectroscopy. In Infrared Spectroscopy for Food Quality Analysis and Control, 1st Ed.; Sun, D.-W.; Ed; Elsevier: London, 2009; 3–27.
- Cadet, F.; de la Guardia, M. Quantitative Analysis, Infrared. In Encyclopedia of Analytical Chemistry; Meyers, R.A.; Ed; John Wiley & Sons, Inc.: New York, NY, 2001; 1–26.
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Fleschin, S. Vibrational Spectroscopy in Clinical Analysis. Applied Spectroscopy Reviews 2014, 50, 176–191.
- Prazeres, S.F.; Ruiz, C.G.; García, G.M. Vibrational Spectroscopy as a Promising Tool to Study Enzyme-Carrier Interactions: A Review. Applied Spectroscopy Reviews 2015, 50, 797–821.
- Guillen, M.D.; Cabo, N. Infrared Spectroscopy in the Study of Edible Oils and Fats. Journal of the Science of Food and Agriculture 1997, 75, 1–11.
- Rohman, A.; Che Man, Y.B. Application of Fourier Transform Infrared Spectroscopy for Authentication of Functional Food Oils. Applied Spectroscopy Reviews 2012, 47(1), 1–13.
- Guillen, M.D.; Cabo, N. Characterization of Edible Oils and Lard by Fourier Transform Infrared Spectroscopy: Relationships Between Composition and Frequency of Concrete Bands in the Fingerprint Region. Journal of the American Oil Chemists’ Society 1997, 74, 1281–1286.
- van de Voort, F.R.; Ghetler, A.; García-González, D.L.; Li, Y.D. Perspectives on Quantitative Mid-FTIR Spectroscopy in Relation to Edible Oil and Lubricant Analysis: Evolution and Integration of Analytical Methodologies. Food Analytical Methods 2008, 1, 153–163.
- Bendini, A.; Cerretani, L.; Di Virgilio, F.; Belloni, P.; Bonoli-Carbognin, M.; Lercker, G. Preliminary Evaluation of the Application of the FTIR Spectroscopy to Control the Geographic Origin and Quality of Virgin Olive Oils. Journal of Food Quality 2007, 30, 424–437.
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Methods and Techniques for Detecting Food Authenticity in Authenticity of Foods of Animal Origin; Arvanitoyannis, I.S.; Ed; CRC Press Taylor & Francis Group: Boca Raton, FL, 2016.
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th Ed.; Prentice Hall: Harlow, England, 2010.
- Moros, J.; Garrigues, S.; de la Guardia, M. Vibrational Spectroscopy Provides a Green Tool for Multi-Component Analysis. Trends in Analytical Chemistry 2010, 29, 578–591.
- Rohman, A. Application of FTIR Spectroscopy for Quality Control in Pharmaceutical Products: A Review. Indonesian Journal of Pharmacy 2012, 23(1), 1–8.
- Marigheto, N.A.; Kemsley, E.K.; Defernez, M.; Wilson, R.H. A Comparison of Mid-Infrared and Raman Spectroscopies for the Authentication of Edible Oils. Journal of the American Oil Chemists’ Society 1998, 75, 987–992.
- Wojciechowski, C.; Dupuy, N.; Ta, C.D.; Huvenneb, J.P.; Legrand, P. Quantitative Analysis of Water-Soluble Vitamins by ATR-FTIR Spectroscopy. Food Chemistry 1998, 63, 133–140.
- Duckworth, J.H. Spectroscopic Quantitative Analysis. In Applied Spectroscopy: A Compact Reference for Practicioners, Workman Jr., J.W.; Springsteen, A.; Ed; Academic Press: London, 1998; 1–71.
- Rohman, A.; Che Man, Y.B. Potential Use of FTIR-ATR Spectroscopic Method for Determination of Virgin Coconut Oil and Extra Virgin Olive Oil in Ternary Mixture Systems. Food Analytical Methods 2011, 4(2), 155–162.
- Rohman, A.; Che Man, Y.B.; Hashim, P.; Ismail, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy for Determination of Virgin Coconut Oil (VCO) in Binary Mixture with Palm Oil and Olive Oil. Journal of the American Oil Chemists’ Society 2010, 87, 601–606.
- Rohman, A.; Che Man, Y.B. Simultaneous Quantitative Analysis of Two Functional Food Oils, Extra Virgin Olive Oil and Virgin Coconut Oil Using FTIR Spectroscopy and Multivariate Calibration. International Food Research Journal 2011, 18(4), 1231–1235.
- Rohman, A.; Che Man, Y.B.; Sismindari. Quantitative Analysis of Virgin Coconut Oil (VCO) in Cream Cosmetics Preparations Using Fourier Transform Infrared (FTIR) Spectroscopy. Pakistan Journal of Pharmaceutical Sciences 2009, 22(4), 415–420.
- Rohman, A.; Che Man, Y.B. Determination of Extra Virgin Olive Oil in Quaternary Mixture Using FTIR Spectroscopy and Multivariate Calibration. Spectroscopy 2011, 26, 203–211.
- Velasco, J.; Dobarganes, C. Oxidative Stability of Virgin Olive Oil. European Journal of Lipid Science and Technology 2002, 104, 661–676.
- Min, D.B.; Boff, J.M. Lipid Oxidation of Edible Oil in Food. In Food Lipids: Chemistry, Nutrition, and Biotechnology, 2nd Ed.; Akoh, C.C.; Min, D.B.; Ed; Marcell Dekker: New York, NY, 2002; 335–365.
- Reische, D.W.; Lillard, D.A.; Eitenmiller, R.R. Antioxidants. In Food Lipids: Chemistry, Nutrition, and Biotechnology, 2nd Ed.; Akoh, C.C.; Min, D.B.; Eds; Marcell Dekker: New York, NY, 2002; 479–516.
- Marina, A.M.; Wan Rosli, W.I.; Noorhidayah, M. Quantitative Analysis of Peroxide Value in Virgin Coconut Oil by ATRFTIR Spectroscopy. The Open Conference Proceedings Journal 2013, 4, 53–56.
- Rohman, A.; Che Man, Y.B.; Ismail, A.; Hashim, P. Monitoring the Oxidative Stability of Virgin Coconut Oil During Oven Test Using Chemical Indexes and FTIR Spectroscopy. International Food Research Journal 2011, 18, 303–310.
- Smith, S.A.; King, R.E.; Min, D.B. Oxidative and Thermal Stabilities of Genetically Modified High Oleic Sunflower Oil. Food Chemistry 2007, 102, 1208–1213.
- Srivastava, Y.; Semwal, A.D. A Study on Monitoring of Frying Performance and Oxidative Stability of Virgin Coconut Oil (VCO) During Continuous/Prolonged Deep Fat Frying Process Using Chemical and FTIR Spectroscopy. Journal of Food Science and Technology 2015, 52(2), 984–991.
- Marina, A.M.; Wan Rosli, W.I.; Noorhidayah, M. Rapid Quantification of Free Fatty Acids in Virgin Coconut Oil by FTIR Spectroscopy. Malaysia Applied Biology 2015, 44(2), 45–49.
- Navarra, G.M.; Cannas, M.; D’Amico, M.; Giacomazza, D.; Militello, V.; Vaccaro, L.; Leone, M. Thermal Oxidative Process in Extra-Virgin Olive Oils Studied by FTIR, Rheology and Time-Resolved Luminescence. Food Chemistry 2015, 126, 1226–1231.
- Wójcicki, K.; Khmelinskii, I.; Sikorski, M.; Sikorska, E. Near and Mid Infrared Spectroscopy and Multivariate Data Analysis in Studies of Oxidation of Edible Oils. Food Chemistry 2015, 187, 416–423.
- Uncu, O.; Ozen, B. Prediction of Various Chemical Parameters of Olive Oils with Fourier Transform Infrared Spectroscopy. LWT–Food Science and Technology 2015, 63, 978–984.
- Bertran, E.; Blanco, M.; Coello, J.; Iturriaga, H.; Maspoch, S.; Montoliu, I. Determination of Olive Oil Free Fatty Acid by Fourier Transform Infrared Spectroscopy. Journal of the American Oil Chemists’ Society 1999, 76, 611–616.
