ABSTRACT
The effects of microwave heating for 3, 6, and 9 min at a frequency of 2450 MHz on fatty acid composition, tocopherols, iodine value, free fatty acids (%), peroxide value, conjugated dienes and trienes, and hexanal contents of refined hazelnut, soybean, sunflower, and virgin olive oils were investigated. A significant (p < 0.05) decrease was observed in linoleic and linolenic acids contents of soybean oil during exposure to microwave heating. Tocopherol contents of oil samples significantly decreased (p < 0.05) during microwave heating. Free fatty acids of the samples slightly increased and iodine value showed reduction throughout the process. Conjugated dienes contents of samples showed an increasing trend up to the 6 min, followed by a reduction at 9 min. Conjugated triene fatty acids of all the samples significantly increased (p < 0.05) throughout the application. While peroxide value showed increasing trend up to the 3 min and sharply decreased at 9 min, hexanal contents of refined hazelnut, virgin olive, soybean, and sunflower oils increased 63, 28, 55, and 389 fold, respectively, after 9 min exposure to microwave heating. Kinetic analysis of data showed that the reaction orders for peroxide and hexanal formation were zero and first order, respectively, and in the tested oils the reaction rate followed the order: soybean oil ˃ sunflower oil ˃ hazelnut oil ˃ virgin olive oil for peroxide, and sunflower oil ˃ soybean oil ˃ hazelnut oil ˃ virgin olive oil for hexanal formation. It was concluded that hexanal could be considered as a parameter for evaluation of the quality of oils exposed to microwave heating.
Introduction
Ease and convenience of use and energy, time, and cost saving characteristics make microwave heating an attractive alternative method for food preparation and cooking. Microwave ovens are widely used at homes and restaurants for reheating and cooking purposes. Microwave energy has potential applications in industry such as heating, thawing, defrosting (or tempering), dehydration, baking, rendering of fats, blanching, pasteurization, sterilization, synthesis, and extraction.[Citation1,Citation2] The popularity of the microwave applicable foods which have been flooded to the market leads researchers to investigate the effects of microwave heating on food constituents. Among the numerous reactions that take place during food processing, lipid oxidation has a determining role for the quality and safety of food.[Citation2] Oxidation is a complex reaction that involves series and parallel reactions which are affected by several factors. Lampi et al.[Citation3] indicated that the amount of unsaturated fatty acids and their degree of unsaturation are the most important factors affecting oxidation and other factors, such as the position of unsaturated fatty acids in the triacylglycerols and the presence of anti- and pro-oxidants controlling oxidizability. Some researchers investigated the effects of microwave heating directly applied on oils[Citation4–Citation10] and others evaluated the effects of microwave application on oils extracted from microwaved seeds.[Citation11–Citation16] Jittrepotch et al.[Citation11] indicated that 0, 2.5, 3.5, 4.4, 5.5, and 6.5 min exposure to microwave heating significantly increased (p < 0.05) the peroxide value (PV), anisidine, and free fatty acid (FFA) of the peanut seeds oil. Lukesova et al.[Citation7] reported that the PV, conjugated dienes, and trienes of rapeseed oil, corn oil, soybean oil (SBO) and sunflower oil (SFO) increased gradually as microwave heating was progressing. Anjum et al.[Citation15] reported that microwave roasting of sunflower seed for 10 and 15 min significantly increased the FFA, p-anisidine, saponification, conjugated dienes and trienes, density, and color, and decreased the iodine value (IV) and tocopherol contents of oils extracted from exposured seeds.
Generally, the effects of microwave heating on oils and fats have been evaluated by determining the acid value which presented hydrolytic reactions; PV, conjugated diene and triene acids as indicators of primary oxidative reactions; and anisidine and thiobarbituric acid reactive substances (TBARS) as indices of secondary oxidative reactions. Recently, hexanal has been used as an important indicator of lipid oxidative deterioration.[Citation17–Citation19] Hexanal is a secondary metabolite of lipid oxidation, formed from the breakdown of linoleic acid hydroperoxides.[Citation18] Chitsamphandhvej et al.[Citation20] reported that hexanal is the major product of fat oxidation that increases in content during storage. Garcia-Martinez et al.[Citation21] noted hexanal as the major volatile oxidation compound found in oil and triacylglycerols rich in linoleic acid. Hexanal is also related to oxidative off-flavor and due to its low threshold (5 ppb) hexanal is easily detected.[Citation22] Besides of considering hexanal as indicator of oil oxidation and oxidative off-flavor, hexanal and heptanal are regarded as parameters for diagnosing of lung cancer[Citation23] and hexanal, 1-octen-3-ol, and octane are reported as biomarkers of liver cancer.[Citation24] The objective of this study was to determine the effects of microwave heating on the fatty acid composition and tocopherol contents, and levels of oxidation of different vegetable oils by evaluating PV, diene and triene conjugation, and hexanal contents.
Materials and methods
Material
Refined hazelnut oil (HO), SBO, SFO, and virgin olive oil (OO) were obtained from local markets in Van, Turkey. A mixture of 37 fatty acid methyl esters (FAME; C4-C24) and a 2 cm solid phase microextraction (SPME) fiber coated with 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane were purchased from Supelco Co. (Bellefonte, PA, USA). Hexanal, n-hexane, iso-propanol, 2-methyl-3-heptanone, tocopherols, and tocotrienols were obtained from Sigma-Aldrich Chem. Co. (St. Louis, MO, USA). The common chemicals were of analytical reagent grade.
Sample preparation
A domestic microwave oven (Arçelik, Model 2450 MHz, Turkey) operated at 600 W was used in this study. Two samples of each oil (10.0 g) and its replicates were weighed in 20 mL amber screw-top headspace glass vial with secure seal, one vial for chemical analysis (FAME, IV, tocopherol, FFAs, PV, diene and triene conjugated contents) and the other for hexanal determination. Oil samples were then heated in the microwave oven in the center of the rotated plate 27 cm in diameter simultanously for each time periods (3, 6, and 9 min). At the defined intervals, the samples were taken out of the oven, cooled rapidly, and stored at –18ºC until the analysis. Seperate samples were used for different heating times. Two independent series of experiments were carried out under the same conditions and the analysis was duplicated. As control groups, same analyses were applied to untreated oil samples.
Fatty acid composition
The FAMEs were prepared according to IUPAC methods[Citation25] and analyzed using a Shimadzu GC-2010 model gas chromatograph (Kyoto, Japan) equipped with a flame ionization dedector (FID) and a DB23 column (60 m, 0.25 mm i.d., 0.25 mm film thickness; J&W Scientific, Folsom, CA, USA). Injector, column, and detector temperatures were 230, 190, and 240ºC, respectively. The split ratio was 1:80. The carrier gas was helium at a flow rate of 1.5 mL/min. IV of the oil samples was calculated from fatty acid composition according to AOCS Official Method Cd 1c-85.[Citation26]
Tocopherol analysis
The samples were prepared by dissolving 1 g of each oil in 9 g of hexane and injected into a normal phase high-performance liquid chromatography (HPLC) to analyze tocols[Citation27] using a Shimadzu SCL-10A HPLC system (Kyoto, Japan). The chromatographic separation was done with a Lichrosorb Si60-5 (250 × 4.6 mm id, particle size S-5µm; Hichrom, Reading, UK). The column temperature was maintained at 25ºC. Separation of the tocols was based on isocratic elution with n-hexane (99%) and iso-propanol (1%) at 1 mL/min. Fluorescence detection utilized excitation and emission wavelengths of 295 and 330 nm, respectively. Tocols were quantified based on peak areas compared with standard solutions of tocopherols and tocotrienols.
Oxidation measurements
FFA, PV, conjugated dienes (K232) and trienes (K270) fatty acids were determined according to AOCS Official Method Ca 5a-40, Cd 8-53, and Ch 5-91, respectively.[Citation26] A 2 cm SPME fiber was used for the extraction of hexanal. The SPME fiber was conditioned in a gas chromatograph injection port at 270ºC for 1 h before use. The 10 g of each sample which was already weighed and microwaved was devided into two 5 g portions for duplication of SPME analysis for each treatment. Ten microliters of internal standard solution, 2-methyl-3-heptanone (1.37 ppm w/w) and a stirring bar were added to each 20 mL amber screw-top headspace glass vial with secure seal that contained 5 g sample. Five min equilibration at 40ºC with constant stirring by using a digital hot plate (Heidolph Instruments Gmbh and Co. KG, Schwabach, Germany) was applied to sample then the SPME fiber was exposed for 30 min to the headspace of the vial for hexanal extraction at the same temperature. After the extraction, desorption was performed in the injection port of an Agilent 6890 series GC (Agilent Technologies, Palo Alto, CA). The injector temperature was set at 250ºC and splitless injection mode was used for 5 min. The samples were analyzed on a HP-Innowax column (30 m, 0.25 mm i.d., 0.25 µm film thickness, J&W Scientific, Folsom, CA, USA). Helium was used as the carrier gas at a constant flow rate of 2 mL/min. The oven temperature was programmed at 35ºC for a hold of 5 min, and increased to 75ºC at a rate of 8ºC/min, then increased to 220ºC at a rate of 40ºC/min and hold at the final temperature for 5 min. The FID detector temperature was 270ºC. Samples were analyzed in duplicate, and the same fiber was used for the entire experiment.[Citation28] The concentrations of hexanal in tested samples were calculated based on the calibration curve. Calibration curves for the hexanal were constructed by applying linear regression analysis on the concentration ratio (concentration of compound/concentration of internal standard) and peak area ratio (area of compound/area of internal standard).[Citation29]
Statistical analysis
Data for each sample were recorded as means ± standard deviation and analyzed by SPSS (SPSS Inc., Chicago, IL) for Windows (ver.16.0). One-way analysis of variance (ANOVA) and Duncan’s multiple range test[Citation30] was performed to test any significant differences between samples. Significance level was established at p < 0.05.
Data analysis
It is well known that oxidation is a mixed reaction that involves series and parallel reactions.[Citation31] In this study, the kinetic data analyses were done according to the kinetic model suggested by Basturk et al.[Citation32] In this model, the orders of the reactions were not restricted and the kinetic expression of the autoxidation was written as Eq. (1):
where (meq O2/kg oil), t is time, k1 and k2 are the autocatalytic and decomposition rate constants, respectively, and α and β are the orders of the oxidation and decomposition reactions, respectively. Also, the increasing rate of Hexanal (H) that represents the content of secondary oxidation products was used to solve Eq. (1). It was assumed that the decomposition rate of PV is equal the production rate of H (Eq. 2):
The differential method was used to solve the Eq. (2) and the numerical method was used to determine the values from raw data. After the linearization of Eq. (2), the slope and the intercept of the line were determined as β and Ln (k2), respectively.
Thereafter, Eq. (1) was rewritten as Eq. (3);
The differential method was also used to solve the Eq. (3) and the numerical method was used to determine the values from raw data again. After the linearization of Eq. (3), the slope and the intercept of the line were determined as α and Ln (k1), respectively.
Results and discussion
Fatty acid composition and IV
The most abundant fatty acid in HO and OO was oleic acid and the major fatty acid of SBO and SFO was linoleic acid (). Linolenic acid was found in relatively high amount in SBO. Microwave heating for 9 min did not remarkably affect the fatty acid composition of examined oils, however, a slight but significant decrease (p < 0.05) was observed in linoleic and linolenic acids contents of SBO. No formation of trans fatty acids was observed. Dostalova et al.[Citation6] reported slight changes in fatty acid composition of pork lard, sunflower, zero-erucic rapeseed, peanut, and high-oleic peanut oils after 40 min microwave heating. Anjum et al.[Citation15] noted that 15 min microwave roasting of sunflower seed increased the oleic acid content, and decreased the linoleic acid content and IVs of extracted oil. The reduction in IV () is probably due to decrease in polyunsaturated fatty acids (PUFA) of microwaved oils.
Table 1. Fatty acid compositions of hazelnut (HO), olive (OO), soybean (SBO), and sunflower (SFO) oils (methyl esters %).
Table 2. Iodine values and free fatty acids % of hazelnut (HO), olive (OO), soybean (SBO), and sunflower (SFO) oils.
FFAs
FFA of all the samples exposured to microwave heating showed slight but significantly increasing (p < 0.05) trend during treatment (). OO showed higher initial and final acidity than the other oils, because it did not go through refining processes. Jittrepotch et al.[Citation11] noted that FFA contents of peanut oil significantly increased (p < 0.05) during 6.5 min exposure to microwave heating (from 0.45 to 0.72%). Dostalova et al.[Citation6] reported that the acid value changes were negligible in pork lard, sunflower, zero-erucic rapeseed, peanut, and high-oleic peanut oil samples microwaved for 40 min. Vieira et al.[Citation4] found that FFA showed little, but significant increase during microwave heating, indicating hydrolytic alteration under heating in canola oil, corn oil, and SBO. FFA content of SBO (0.07–0.11%) were in good agreement with the results of Vieira and Regitano-D’arce[Citation4] after 10 min microwave heating. The increase in FFA is due to the splitting of ester linkages of triacylglyceride molecules as a result of heating.[Citation10]
Tocopherols
HO, OO, SBO, and SFO showed different tocopherol contents (). While α-tocopherol was the major tocopherol of HO and SFO (236.16 and 513.01 ppm, respectively), γ-tocopherol (240.86 ppm) was the most abundant tocopherol in SBO, followed by β-tocopherol (176.05 ppm) and δ-tocopherol (78.71 ppm). α-tocopherol (48.91 ppm) was the only tocol found in OO. In most of the samples tocopherol contents significantly decreased (p < 0.05) during heating by microwave energy. After 9 min of microwave heating OO, HO, SFO, and SBO lost 41.24, 37.0, 17.18, and 14.54% of their total tocopherol contents, respectively. Yoshida et al.[Citation14] noted that more than 85% of tocopherols remained after 20 min of microwave roasting of pumpkin seeds. They also indicated that with a few exceptions, the exposure of sunflower seeds to microwaves for 12 min caused no significant (p > 0.05) changes in the content of tocopherols and PUFA in the kernels.[Citation12] Anjum et al.[Citation15] reported that after 15 min of microwave roasting of KI-39 and FH-330 sunflower varieties the amount of α-tocopherol homologs was still over 76 and 81% of the original levels, respectively; however, in the same time period the level of δ-tocopherol fell to zero. Tocopherol destruction during microwave heating is caused not only by thermal oxidative degradation but also by the non-volatile compounds such as FFAs accumulating in the oil.[Citation10]
Table 3. Tocopherol contents (ppm) of hazelnut (HO), olive (OO), soybean (SBO), and sunflower (SFO) oils.
PV
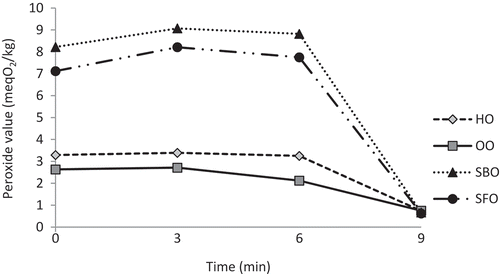
PVs of all the samples slightly but significantly (p < 0.05) increased after 3 min exposure to microwave heating, then showed decreasing trend up to the 6 min and sharply decreased at 9 min (). The initial PVs of HO, OO, SBO, and SFO were 3.29, 2.63, 8.22, and 7.12 meq O2/kg oil, respectively, and the final PVs of samples ranged between 0.62 and 0.75 meq O2/kg oil. HO and OO showed lower PVs than SBO and SFO because of their lower PUFA contents. Dostalova et al.[Citation6] noted that fats and oils (pork lard and peanut, respectively) with lower polyenoic fatty acids showed lower PVs than oils with high polyenoic acid contents (i.e., SFO) during microwave heating. PV is a reliable parameter to evaluate the oxidative stability of oils and fats kept at ambient temperatures because hydroperoxides are unstable on heating at high temperatures. Microwave heating promotes rapid transformation to secondary products, so monitoring PV during microwave heating is considered to be a questionable analytical procedure.[Citation4] The sharp decreases in PVs of tested samples at 9 min of microwave heating was probably a result of degradation of hydroperoxides to secondary oxidation products (i.e., hexanal). Vieira and Regitano-D’arce[Citation4] indicated that corn oil and SBO exposed to microwave heating showed maximum PV at 4 and 6 min of heating, and canola oil showed a decrease in PV after 6 min of heating, followed by an increase (to 5.74 meq O2/kg oil) at 20 min and stabilization until 36 min. Lukesova et al.[Citation7] noted that PV of rapeseed, SFO, SBO, and corn oil increased gradually as microwave heating was progressing until they reached to the highest values. They also indicated that the PV changes in oils during microwave heating showed different behavior according to their fatty acid composition.
Conjugated fatty acids
Conjugated diene contents of SBO and SFO significantly increased (p < 0.05) up to the 6 min, and then followed a decrease up to the 9 min of microwave heating (). After some increases at 3 min, conjugated diene contents of HO and OO showed decreasing trend up to the 9 min. SBO and SFO with higher linoleic acid contents showed higher initial and final conjugated diene contents than HO and OO. The conjugated triene contents of HO, SBO, and SFO significantly increased (p < 0.05) throughout the 9 min microwave heating (). SBO showed the highest initial and final conjugated triene contents, probably due to its higher linolenic acid content (6.52%). OO with the lowest conjugated triene content showed significant increase (p < 0.05) at 9 min. El-Moneim et al.[Citation33] reported a significant increase in absorptivity at 270 nm of extra virgin OO exposed to microwave heating started from 6 min heating. Vieira and Regitano-D’arce[Citation4] found that absorptivity at 232 nm of canola oil, corn oil, and SBO increased gradually after 12 min of microwave exposure, and absorptivity at 270 nm significantly increased after 4 min of heating. The absorptivity at 270 nm reported by Vieira and Regitano-D’arce[Citation4] for canola oil, corn oil and SBO increased from 0.762, 1.587, and 3.324 to 1.885, 2.833, and 3.894, respectively, after 36 min microwave heating. Molecular friction during heating could promote the formation of trienes and unsaturated ketones or aldehydes; secondary products of oxidation.[Citation4] Lukesova et al.[Citation7] reported that the PV, conjugated dienes, and trienes of rapeseed oil, corn oil, SBO, and SFO increased gradually as microwave heating was progressing. They also noted that conjugated dienes were formed at higher levels than conjugated trienes. Vieira and Regitano-D’arce[Citation4] and Lukesova et al.[Citation7] proposed that the absorbance at 232 nm, due to formation of conjugated dienes was a good index for measuring the degradation of microwave heated oils. Dostalova et al.[Citation6] noted that conjugated dienes were better markers of oxidative deterioration than hydroperoxides, especially at later stages of microwave heating.
Table 4. Conjugated diene (K232) and triene (K270) contents of hazelnut (HO), olive (OO), soybean (SBO), and sunflower (SFO) oils.
Hexanal
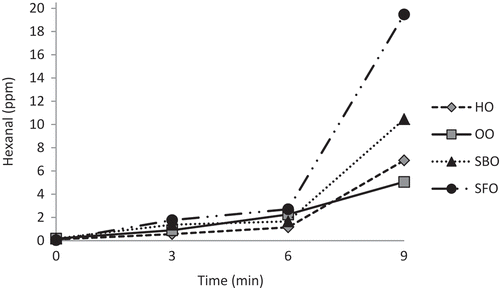
Hexanal contents of oil samples significantly increased (p < 0.05) throughout the microwave heating with a remarkable increase at 9 min of exposure (). Hexanal contents of HO, OO, SBO, and SFO increased 63-, 28-, 55-, and 389-fold, respectively, after 9 min exposure to microwave heating. Interestingly, the increase in hexanal contents at 9 min of exposure to microwave heating accompanied with a sharp decrease in PVs of the samples (). There have been very few reports related to hexanal contents of vegetable oils. Recently Kiralan and Kiralan[Citation34] reported that the hexanal content of HO increased from a initial value of 5.58 to 7.08 area unit after 8 min to microwave heating. As the secondary oxidation products, researchers mostly evaluated the changes in p-anisidine and TBARS values of oils during microwave heating. Megahed[Citation35] reported that p-anisidine value of linseed oil increased from 2.63 to 58.52 after 5 min microwave heating. The p-anisidine and TBARS values of oils extracted from peanut seeds exposed to microwave heating for 0–6.5 min changed from 0.58 to 0.90 and 0.02 to 0.03 mg/kg, respectively.[Citation11] Microwave heating promotes rapid transformation of hydroperoxides which are unstable under heating to secondary products.[Citation4] SBO with higher linolenic acid content was expected to show higher hexanal content than SFO. The lower hexanal content of SBO may be due to its higher β-, γ-, and δ-tocopherol contents than SFO. At frying conditions the antioxidative potential of tocols increased in the following order: α < γ < δ for tocopherols and α < β < γ < δ for tocotrienols.[Citation36] Lee et al.[Citation37] indicated that the induction period of SFO, SBO, and OO at 40 and 60ºC were 15.3, 22.4, 35.3, and 3.6, 5.3, and 7.5 days, respectively. They noted that SFO was more sensitive to oxidation than SBO, and OO was the most stable. OO showed the lowest PV at 6 min as well as the lowest hexanal content at 9 min. This is due to its lower PUFA content. Cossignani et al.[Citation38] indicated that changes of OO due to microwave heating were only small because of the low contents of polyenoic acids and relatively high contents of natural antioxidants, especially in OO. Hexanal was the only oxidation product that continously increased throughout the microwave heating in all oil samples. Garcia-Martinez et al.[Citation21] reported that hexanal was the major volatile oxidation compound found in oil and triacylglycerols rich in linoleic acid, and hexanal and heptanal equally were the most abundant compounds in oil and triacylglycerols rich in conjugated linoleic acid, analyzed by SPME-gas chromatography technique. Hexanal is known as a reliable indicator for oil and fat oxidation.[Citation17,Citation19,Citation20]
Kinetic analysis
As mentioned before, we observed a relation between peroxide degradation and hexanal formation during microwave heating. While peroxide showed a dramatical decrease at 9 min of microwave heating, hexanal conversely represented a sharp increase at the same time interval. The peroxides in oxidized oils are transitory intermediates that decompose into various carbonyl compounds.[Citation39] Unlike hydroperoxides, aldehydes do not decompose rapidly during oxidation. So aldehyde such as hexanal is considered as a reliable indicator for secondary oxidation products. In some oil samples the generation of secondary oxidation products begins almost simultanously with the generation of hydroperoxides, and in other, the degradation of hydroperoxides begins when the concentration of these compounds in appreciable.[Citation40] In this study, the PV changed in a narrow range up to 6 min and significantly decreased after 6 min up to 9 min while hexanal slightly formed up to 6 min and sharply increased up to 9 min. To the best of the author’s knowledge, such a relation between PV and hexanal was not reported before. To help clarify the situation we applied kinetic analysis to PV and hexanal during 9 min microwave heating. The results are summerized in .
Table 5. Peroxide and hexanal reaction rate constants (k1 and k2) and reaction orders (α and β).
The results showed that in the tested oils the reaction rate followed the order: SBO ˃ SFO ˃ HO ˃ OO for peroxide, and SFO ˃ SBO ˃ HO ˃ OO for hexanal. The reaction order (α) for peroxide was zero order and for hexanal was first order (β). The order of a reaction is important since it tells the functional relationship between concentration and rate. The order of a reaction determines how the amount of a compound speeds up or retards a reaction. The zero order of reaction for PV means that it has a rate which is independent of the concentration of reactant[Citation41] and it also showed that the reaction rate is controlled by the reaction rate constant.[Citation32] The first order reaction for hexanal formation shows that the rate of this reaction depends only on the first power of the concentration of a single reacting species.[Citation41] The cumulative effects of different parameters make oxidation of oil systems highly complex so a simple model does not express good enough the reactions in whole system.
Basturk et al.[Citation32] reported the reaction rates and orders of primary (PV) and secondary oxidation products (anisidine value) for SBO during storage at 45, 60, and 75°C. They noted that the reaction rates increased as the storage temperature increased for both primary and secondary oxidation products as well as reaction order of primary oxidation products. They also mentioned that during oxidation, primary and secondary oxidation products were simultanously generated.
Conclusions
Microwave heating promotes the oxidative deterioration of vegetable oils. Significant decreases (p < 0.05) were observed in linoleic and linolenic acids contents of SBO and tocopherol contents of tested oils after 9 min exposure to microwave heating. FFA of oils showed significant increase (p < 0.05) during treatment. PVs of samples increased up to 3 min, then sharply decreased at 9 min. The conjugated diene acid contents of samples initially increased and finally decreased at 9 min. The conjugated triene acids of HO, SBO, and SFO significantly increased (p < 0.05) during microwave heating. The simultanously evaluation of PV and hexanal levels showed that at 9 min of microwave heating, a considerable amount of peroxides were converted to hexanal. Since peroxides and conjugated dienes showed irregular variations, and triene acids increased in a narrow range during microwave heating, to evaluate the quality of microwaved oils, parameters such as hexanal which presents the secondary oxidation products should be taken into account. In conclusion, hexanal can be considered as an indicator to evaluate the adverse effects of microwave heating on quality of vegetable oils.
References
- Luo, Z.; He, X.; Fu, X.; Luo, F., Gao, Q. Effect of Microwave Radiation on the Physicochemical Properties of Normal Maize, Waxy Maize and Amylomaize V Starches. Starch/Starke 2006, 58, 468–474.
- De Pilli, T.; Giuliani, R.; Derossi, A.; Severini, C. Effects of Microwave Drying on Lipid Oxidation of Stuffed Pasta. Journal of American Oil Chemists Society 2008, 85, 827–834.
- Lampi, A.M.; Piironen, V.; Hopia, A.; Koivistoinen, P. Characterization of the Oxidation of Rapeseed and Butter Oil Triacylglycerols by Four Analytical Methods. LebensmittelWissenschaft und Technologie 1997, 30, 807–813.
- Vieira, T.M.F.S.; Regitano-D’arce, M.A.B. Stability of Oils Heated by Microwave: UV-Spectrophotometric Evaluation. Ciencia e Tecnologia de Alimentos 1998, 18, 1–9.
- Vieira, T.M.F.S.; Regitano-D’arce, M.A.B. Canola Oil Thermal Oxidation During Oven Test and Microwave Heating. LebensmittelWissenschaft und Technologie 2001, 34, 215–221.
- Dostalova, J.; Hanzlik, P.; Reblova, Z.; Pokorny, J. Oxidative Changes of Vegetable Oils During Microwave Heating. Czech Journal of Food Science 2005, 23, 230–239.
- Lukesova, D.; Dostalova, J.; El-Moneim, E.M.; Svarovska, M. Oxidation Changes of Vegetable Oils During Microwave Heating. Czech Journal of Food Science 2009, 27, 178–181.
- Chiavaro, E.; Barnaba, C.; Vittadini, E.; Rodriguez-Estrada, M.T.; Cerretani, L.; Bendini, A. Microwave Heating of Different Commercial Categories of Olive Oil: Part II. Effect of Thermal Properties. Food Chemistry 2009, 115, 1393–1400.
- Megahed, M.G. Effect of Microwave Heating of Linseed Oil on the Formation of Primary and Secondary Oxidation Products. Agriculture and Biology Journal of North America 2011, 2, 673–679.
- Yoshida, H.; Tatsumi, M.; Kajimoto, G. Influence of Fatty Acids on the Tocopherol Stability in Vegetable Oils During Microwave Heating. Journal of American Oil Chemists Society 1992, 69, 119–125.
- Jittrepotch, N.; Kongbangkerd, T.; Jojsuntornkitti, K. Influence of Microwave Irridiation on Lipid Oxidation and Acceptance in Peanut (Arachis Hypogaea L.) Seeds. International Food Research Journal 2010, 17, 173–179.
- Yoshida, H.; Hirakawa, Y.; Abe, S.; Mizushina, Y. The Content of Tocopherols and Oxidative Quality of Oils Prepared From Sunflower (Helianthus Annuus L.) Seeds Roasted in a Microwave Oven. European Journal of Lipid Science and Technology 2002, 104, 116–122.
- Yoshida, H.; Hirakawa, Y.; Tomiyama, Y.; Nagamizu, T.; Mizushina, Y. Fatty Acid Distributions of Triacylglycerols and Phospholipids in Peanut Seeds (Arachis Hypogaea L.) Following Microwave Treatment. Journal of Food Composition and Analysis 2005, 18, 3–14.
- Yoshida, H.; Tomiyama, Y.; Hirakawa, Y.; Mizushina, Y. Microwave Roasting Effects on the Oxidative Stability of Oils and Molecular Species of Triacylglycerols in the Kernels of Pumkin (Cucurbita Spp.) Seeds. Journal of Food Composition and Analysis 2006, 19, 330–339.
- Anjum, F.; Anwar, F.; Jamil, A.; Iqbal, M. 2006. Microwave Roasting Effects on the Physico-Chemical Composition and Oxidative Stability of Sunflower Seed Oil. Journal of American Oil Chemists Society 2006, 83, 777–784.
- Oomah, B.D.; Liang, J.; Godfrey, D.; Mazza, G. Microwave Heating of Grapeseed: Effect on Oil Quailty. Journal of Agricultural and Food Chemistry 1998, 46, 4017–4021.
- Shahidi, F. Indicators for Evaluation of Lipid Oxidation and off-Flavor Development in Food. In Food Flavors: Formation, Analysis and Packaging Influences; Elsevier Science: New York, NY, 1998.
- Abdalla, A.E.; Roozen, J.P. Effect of Plant Extract on the Oxidative Stability of Sunflower Oil and Emulsion. Food Chemistry 1999, 64, 323–329.
- Brunton, N. A Comparison of Solid-Phase Microextraction Fibers for Measurment of Hexanal and Pentanal in Cooked Turkey. Food Chemistry 2000, 68, 339–345.
- Chitsamphandhvej, W.; Phakdee, W.; Thanasan, W. A Headspace Solid Phase Microextraction Method for Using to Monitor Hexanal and Heptanal Content in Food Samples. Kasetsart Journal (Natural Science) 2008, 42, 206–212.
- Garcia-Martinez, M.C.; Marquez-Ruiz, G.; Fontecha, J.; Gordon, M.H. Volatile Oxidation Compounds in a Conjugated Linoleic Acid-Rich Oil. Food Chemistry 2009, 113, 926–931.
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of Volatiles to Rice Aroma. Journal of Agricultural and Food Chemistry 1988, 36, 1006–1009.
- Deng, C.; Zhang, X.; Li, N. Investigation of Volatile Biomarkers in Lung Cancer Blood Using Solid-Phase Microextraction and Capillary Gas Chromatography–Mass Spectrometry. Journal of Chromatography B 2004, 808(2), 269–277.
- Xue, R.; Dong, L.; Zhang, S.; Deng, C.; Liu, T.; Wang, J.; Shen, X. Investigation of Volatile Biomarkers in Liver Cancer Blood Using Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry. Rapid Communication in Mass Spectrometry 2008, 22, 1181–1186.
- IUPAC Standard. Methods for Analysis of Oils, Fats and Derivatives.International Union of Pure and Applied Chemistry, 7th Ed; Blackwell Scientific Publications: Oxford, Great Britain, 1991.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society. American Oil Chemists’ Society: Champaign, IL, 1989.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society. American Oil Chemists’ Society: Champaign, IL, 2003.
- Javidipour, I.; Qian, M. Volatile Component Change in Whey Protein Concentrate During Storage Investigated by Headspace Solid-Phase Microextraction Gas Chromatography. Dairy Science and Technology 2008, 88, 95–104.
- Bakkalbaşı, E.; Yılmaz, Ö.M.; Javidipour, J.; Artık, N. Effects of Packaging Materials, Storage Conditions and Variety on Oxidative Stability of Shelled Walnuts. LWT–Food Science and Technology 2012, 46, 203–209.
- Duncan, D.B. Multiple range and multiple F-test. Biometrics 1955, 11, 1–42.
- Crapiste, G.H.; Brevedan, M.I.V.; Carelli, A.A. Oxidation of Sunflower Oil During Storage. Journal of the American Oil Chemists’ Society 1999, 76, 1437–1443.
- Basturk, A.; Javidipour, I.; Boyacı, I.H. Oxidative Stability of Natural and Chemically Interesterified Cottonseed, Palm and Soybean Oils. Journal of Food Lipids 2007, 14, 170–188.
- El-Moneim, E.A.M.; Dostalova, J.; Pokorny, J.; Lukesova, D.; Dolezal, M. Oxidation of Olive Oils During Microwave and Conventional Heating for Fast Food Preparation. Czech Journal Food Science 2009, 27, 173–177.
- Kiralan, M.; Kiralan, S.S. Changes in Volatile Compounds of Black Cumin Oil and Hazelnut Oil by Microwave Heating Process. Journal of American Oil Chemists’ Society 2015, 92, 1445–1450.
- Megahed, M.G. 2011. Effect of Microwave Heating of Linseed Oil on the Formation of Primary and Secondary Oxidation Products. Agriculture and Biology Journal of North America 2011, 2, 673–679.
- Wagner, K.H.; Wotruba, F.; Elmadfa, I. Antioxidative Potential of Tocotrienols and Tocopherols in Coconut Fat at Different Oxidation Temperatures. European Journal of Lipid Science and Technology 2001, 103, 746–751.
- Lee, J.; Lee, Y.; Choe, E. Temperature Dependence of the Autoxidation and Antioxidants Of Soybean, Sunflower, and Olive Oil. European Food Research and Technology 2006, 226, 239–246.
- Cossignani, L.; Simonetti, M.S.; Neri, A.; Damiani, P. Changes in Olive Oil Composition Due to Microwave Heating. Journal of American Oil Chemists Society 1998, 75, 931–937.
- Rossell, J.B. Classical Analysis of Oils and Fats. In Analysis of Oils and Fats; Hamilton R.J.; Rossell, J.B.; Eds.; Elsevier Applied Science Publishers: New York, NY, 1986; 1–90.
- Guillen, M.N.; Cabo, N. Fourier Transform Infrared Spectra Data Versus Peroxide and Anisidine Values to Determine Stability of Edible Oils. Food Chemistry 2002, 77, 503–510.
- Göksunger, Y. Reaction and Fermentation Kinetics in Food Engineering. Sidas Medya Ltd. Şti. Publisher: İzmir, Turkey, 2011; 16–23.